The use of molecular markers of bone turnover in the management of patients with metastatic bone disease
Summary
Biochemical markers of bone turnover are widely used in clinical practice. These indices have been shown to be associated with the occurrence, prognosis and therapeutic response of malignant bone lesions. For example, markers of bone resorption are often elevated in patients with established bone metastases and while this may point to a role of these markers in the diagnostic workup of cancer patients, the available evidence does not permit any final conclusions as to the accuracy and validity of the presently used markers in the early diagnosis of bone metastases. Many bone turnover markers appear to respond to antiresorptive and antineoplastic therapies, and recent evidence from prospective trials suggests that the aim of bisphosphonate therapy should be to normalize rates of bone remodelling to optimize therapeutic and prognostic outcomes. However, it remains unknown whether the use of bone markers in the routine clinical setting has any defined beneficial effects on overall outcome in cancer patients. Clearly, bone turnover markers have insufficient diagnostic or prognostic value to be used in isolation; however, the combination of these markers with other diagnostic techniques may improve clinical assessment of patients with bone-seeking cancers. This article reviews the available evidence (as of August 2007) on the clinical use of bone turnover markers in the management of patients with metastatic bone disease.
Introduction
Bone metastases are frequent and seen in many malignancies, but occur preferentially in patients with breast, prostate, thyroid, kidney, bladder and lung cancers. Although usually not lethal in themselves, bone metastases often cause refractory pain, hypercalcaemia, fractures, neurological symptoms and, as a result, profound morbidity and a reduction in quality of life.1 In a long-term follow-up series, over 60% of breast cancer patients with skeletal metastasis had skeletal complications, averaging to four such events per year of follow-up.2 Of note, newer bisphosphonates (BPs) are able to significantly reduce the rate of skeletal events in cancer patients, particularly when used early in the disease process.3 Consequently, there is a clear clinical requirement to identify patients with bone metastases as early as possible, and to develop sensitive and specific means to monitor treatment efficacy and predict outcomes.
The diagnostic approach to metastatic bone disease traditionally focuses on the localization and characterization of the lesion, using an array of different imaging techniques such as radiographs, computed tomography (CT), magnetic resonance imaging (MRI) 99Tc bone scans, and positron-emission tomography (PET). All of these procedures are of value in case-finding studies, where the goal is to identify patients with established metastatic spread. At earlier stages of the disease process, however, changes in skeletal morphology or radionuclide uptake may be discrete, nonspecific or absent. In addition, not all imaging techniques are equally well suited for the monitoring of skeletal disease progression or therapeutic response, particularly in patients with short survival times. Tumour markers such as prostate specific antigen (PSA) have been found to correlate closely with the extent of neoplastic tissue and may therefore be useful in the clinical monitoring of tumour behaviour and treatment response.4 With scant exceptions, however, these markers do not provide information specific to the skeleton's involvement.
Under normal conditions, the bone remodelling process is a balanced, lifelong continuum of resorbing old bone (through the action of osteoclasts) and replacing the removed tissue by an equal amount of newly formed bone (through the action of osteoblasts). The presence of bone metastases greatly perturbs this balance. Driven by a number of tumour-derived factors, the osteoclasts surrounding cancer metastases become activated to resorb bone. By contrast, bone formation may be increased or decreased, but is usually inadequate to compensate for the escalation in bone resorption. Radiographically, this results in predominantly lytic or mixed lytic–sclerotic lesions, as typically seen in breast cancer metastases to bone. Prostate cancers, by contrast, usually cause sclerotic lesions characterized at the cellular level by a relative excess of bone formation compared to bone resorption. However, even the skeletal metastases of prostate cancer are characterized by an increase in the rate of both bone resorption and formation. High bone turnover with excess bone resorption is therefore an archetypal feature of most, if not all, bone metastases. (The pathophysiology of this process is beyond the scope of this article, but was reviewed in 2005 by Yoneda and Hiraga5 and Chung et al.6)
Biochemical markers of bone turnover (Fig. 1) are noninvasive and relatively inexpensive tools that, if applied and interpreted correctly, can be used effectively in assessing changes in bone remodelling associated with metastatic bone disease. Table 1 summarizes the biological and technical details of the currently used bone markers. For an in-depth review of the basic biochemistry of bone markers, refer to my 2003 article.7 The present review focuses on the clinical application of biochemical markers of bone turnover in metastatic bone disease, with specific emphasis on the clinically important topics of diagnosis, prognosis and monitoring.
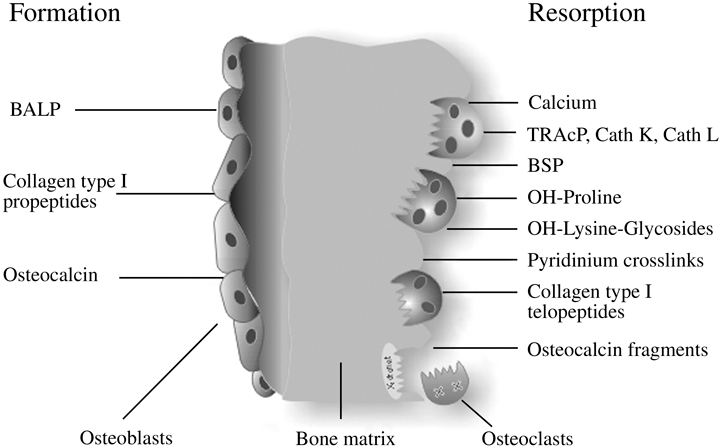
Biochemical markers of bone remodelling.
| Marker (abbreviation) | Tissue | Specimen required | Remarks |
|---|---|---|---|
| Markers of bone formation | |||
| Bone-specific alkaline phosphatase (BAP) | Bone | Serum | Specific product of osteoblasts. Some assays show up to 20% cross-reactivity with liver isoenzyme (LAP) |
| Osteocalcin (OC) | Bone, platelets | Serum | Specific product of osteoblasts; many immunoreactive forms in blood; some may be derived from bone resorption |
| C-terminal propeptide of type I procollagen (PICP) | Bone, soft tissue, skin | Serum | Specific product of proliferating osteoblasts and fibroblasts |
| N-terminal propeptide of type I procollagen (PINP) | Bone, soft tissue, skin | Serum | Specific product of proliferating osteoblast and fibroblasts; partly incorporated into bone extracellular matrix |
| Markers of bone resorption | |||
| Collagen-related markers | |||
| Hydroxyproline, total and dialysable (Hyp) | Bone, cartilage, soft tissue, skin | Urine | Present in all fibrillar collagens and partly collagenous proteins, including C1q and elastin. Present in newly synthesized and mature collagen; both collagen synthesis and tissue breakdown contribute to urinary hydroxyproline |
| Hydroxylysine-glycosides | Bone, soft tissue, skin, serum complement | Urine, serum | Hydroxylysine in collagen is glycosylated to varying degrees, depending on tissue type. Glycosylgalactosyl-OHLys in high proportion in collagens of soft tissues, and C1q; galactosyl-OHLys in high proportion in skeletal collagens |
| Pyridinoline (PYD) | Bone, cartilage, tendon, blood vessels | Urine, serum | Collagens, with highest concentrations in cartilage and bone; absent from skin; present in mature collagen only |
| Deoxypyridinoline (DPD) | Bone, dentin | Urine, serum | Collagens, with highest concentration in bone; absent from cartilage or skin; present in mature collagen only |
| Carboxyterminal cross-linked telopeptide of type I collagen (ICTP, CTX-MMP) | Bone, skin | Serum | Collagen type I, with highest contribution probably from bone; may be derived from newly synthesized collagen |
| Carboxyterminal cross-linked telopeptide of type I collagen (CTX-I) | All tissues containing type I collagen | Urine (α/β) Serum (αα/ββ) | Collagen type I, with highest contribution probably from bone. Isomerization of aspartyl to β-aspartyl occurs with ageing of collagen molecule |
| Aminoterminal cross-linked telopeptide of type I collagen (NTX-I) | All tissues containing type I collagen | Urine, serum | Collagen type I, with highest contribution from bone |
| Collagen I alpha 1 helicoidal peptide (HELP) | All tissues containing type I collagen | Urine | Degradation fragment derived from the helical part of type I collagen (α1 chain, AA 620–633). Correlates highly with other markers of collagen degradation, no specific advantage or difference with regard to clinical outcomes |
| Non-collagenous proteins | |||
| Bone sialoprotein (BSP) | Bone, dentin, hypertrophic cartilage | Serum | Acidic, phosphorylated glycoprotein, synthesized by osteoblasts and osteoclastic-like cells, laid down in bone extracellular matrix. Appears to be associated with osteoclast function |
| Osteocalcin fragments (ufOC, U-Mid-OC, U-Long-OC) | Bone | Urine | Certain age-modified OC fragments are released during osteoclastic bone resorption and may be considered an index of bone resorption |
| Osteopontin (OPN) | Bone, kidney, placenta, dentin, cartilage, brain, muscle, blood vessels | Serum | Synthesized by a variety of tissue types. Synthesis in bone is stimulated by 1,25-dihydroxy-vitamin D3 |
| Osteoclast enzymes | |||
| Tartrate-resistant acid phosphatase (TRAcP) | Bone, blood | Plasma, serum | Six isoenzymes found in human tissues (osteoclasts, platelets, erythrocytes). Band 5b predominant in bone (osteoclasts). Enzyme identified in both the ruffled border of the osteoclast membrane and the secretions in the resorptive space |
| Cathepsins (e.g. K, L) (Cath K, Cath L) | K: primarily in osteoclastsL: macrophage, osteoclasts | Plasma, serum | Cathepsin K, cysteine protease, plays an essential role in osteoclast-mediated bone matrix degradation by cleaving helical and telopeptide regions of collagen type I. Cathepsin K and L cleave the loop domain of TRAcP and activate the latent enzyme. Cathepsin L has a similar function in macrophages. Tests for measurement of cathepsins in blood are under evaluation |
Diagnostic use
Most studies in this area have compared biochemical markers of bone turnover between groups of cancer patients with and without established bone metastases. While this is a sensible and straightforward approach, its validity largely depends on a correct diagnosis in the ‘negative’ group, that is the group of cancer patients declared to be free of skeletal disease. Given the different techniques used to prove the absence of malignant bone lesions in the more than 100 studies reported so far, and their specific limitations particularly in early stage bone disease, the assumption of a ‘negative status’ may not always be correct. In addition, many studies have investigated different types of cancers in a heterogeneous mix of subjects, and almost as a rule, data on estimates of tumour burden are missing. Not surprisingly, the available information on the diagnostic use of bone markers in metastatic bone disease is heterogeneous and often incongruent. The picture becomes more consistent when comparisons are made between a marker of bone turnover and specific imaging techniques, namely bone radioisotopes scans, or surgical outcomes in well-defined groups of patients.8–11
Of the available bone formation markers (Table 1), serum total (TAP) and bone-specific alkaline phosphatase (BAP) usually exhibit the most pronounced changes in response to metastatic bone involvement. In most cases of advanced cancers metastatic to bone, serum TAP and BAP levels are elevated, pointing to either a strong osteoblastic component or, in lytic lesions, to active repair.12–14 However, a number of studies reported no differences in serum TAP or BAP levels when patients with and without cancer metastases to bone were compared (e.g. Jung et al.15). In patients with prostate cancer, the combined measurement of PSA and BAP in serum seems to increase the diagnostic sensitivity for bone lesions compared to healthy subjects or patients with benign prostate hyperplasia.16–18
In general, serum osteocalcin (OC) levels are more variable compared to other bone formation markers, and in advanced, untreated metastatic bone disease, serum OC levels may be low in the presence of high BAP levels.19 The reasons for this dissociation are unclear, but possibilities include proteolytic cleavage of OC, changes in gene expression or disturbed osteoid maturation in the presence of active tumour osteopathy. In patients with multiple myeloma (MM), several studies have reported low serum OC values in the presence of high bone resorption markers. Suppressed serum OC concentrations are thought to reflect impaired osteoblast activity and have been associated with poor survival.20 Consequently, some authors have postulated a myeloma cell-derived factor that would specifically inhibit osteoblast activity. Tian et al.21 demonstrated increased expression of dickkopf1 (DKK1), an inhibitor of osteoblast differentiation, in myeloma cells from lytic tumours. Myeloma cells express the receptor activator of nuclear factor κB ligand (RANKL), a major driver of osteoclastogenesis. Thus, simultaneous overexpression of RANKL and DKK1 by myeloma cells would increase bone resorption while inhibiting osteoblast differentiation and bone formation; that is, repair.
The serum concentrations of both carboxyterminal (PICP) and aminoterminal propeptide of type I procollagen (PINP) have been found to be elevated in patients with breast, prostate or lung cancer metastatic to bone.22–24 In patients with breast cancer, a decreased PICP/PINP ratio seems to signify a more aggressive phenotype with a higher propensity to metastasize to bone.22
Once established in the bone microenvironment, viable cancer cells are thought to initiate bone resorption through the activation of resident osteoclasts. This process rapidly creates space for further growth of the new settlement, and ultimately leads to widespread destruction of skeletal structures.5,6 Hence, it is not surprising that bone resorption markers are considered prime candidates for the diagnosis of such lesions.
The majority of patients with breast, prostate, lung or oral squamous cell cancers metastatic to bone exhibited abnormally high urinary levels of the collagen crosslink, deoxypyridinoline (DPD).11,25–28 In some studies (e.g. Pecherstorfer et al.26), a significant proportion of cancer patients without evidence for malignant bone involvement also had elevated urinary crosslink levels (Fig. 2). This observation may be attributable to the presence of undiagnosed bone metastases in the ‘negative’ control group, and may point to an inherent problem in study design (discussed earlier). However, these observations may alternatively reflect systemic, cytokine-mediated acceleration of bone turnover.29
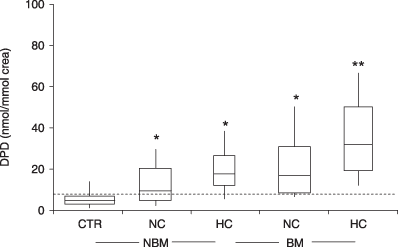
Urinary deoxypyridinoline (DPD) in patients with and without bone metastases. A normal range was established by measuring urinary DPD in healthy young adults (broken line: upper limit of normal). Cancer patients were then stratified by calcaemic status (HC, hypercalcaemic patients; sCa > 2·6 mmol/l; NC, normocalcaemic patients) and by the presence or absence of neoplastic bone involvement (BM, patients with bone metastases; NBM, patients with no bone metastases). Box and whisker plot; horizontal lines depict the medians. *P < 0·001, **P < 0·0001. (From Pecherstorfer et al.,26 with permission.)
Pecherstorfer et al.30 reported significantly higher levels of urinary DPD in patients with MM as compared to healthy adults, patients with monoclonal gammopathy of undetermined significance (MGUS) or patients with postmenopausal osteoporosis. Although urinary DPD correctly identified patients with advanced MM (stage III), the test did not discriminate between patients with MGUS, or with early (stage I) MM or osteoporosis. This result, even though disappointing from a clinical point of view, is not surprising; in fact, it reiterates that bone markers reflect bone turnover, not underlying pathologies. As bone resorption rates are similarly low in MGUS and early stage MM, bone markers would not be expected to discriminate between these entities.
The higher molecular weight (‘telopeptide’) markers of collagen type I degradation (ICTP, CTX-I, NTX-I) (Table 1) have also been used in the evaluation of metastatic bone disease, although with varying results. For example, one study compared urinary NTX-I, serum ICTP and serum BAP in 106 breast cancer patients with and without bone metastases. With a clinical specificity of 91%, serum ICTP was found to be the marker with the highest sensitivity for established bone metastases.31 Similar results were reported for a comparison of serum ICTP, tartrate-resistant acid phosphatase (TRAcP), urinary NTX-I and serum BAP in a study of 156 breast cancer patients.32 Other studies indicate that urinary NTX-I has a higher predictive value for the diagnosis of bone metastatic progression than serum ICTP and BAP.33 While there is no general agreement on which telopeptide marker yields the best results, most studies indicate that these peptide markers are sensitive tools in patients with skeletal lesions attributable to breast14,34,35 and prostate cancer.36–39
The CTX-I telopeptide is present in two isoforms: α and β CTX-I. The α form is found in newly synthesized collagen whereas the epitope associated with β-CTX-I is thought to represent older, more mature collagen. More recently, a new assay for the measurement of the α-CTX-I isoforms in serum (termed ααCTX) and its use in cancer patients has been described.40 It has been suggested that the separate measurement of these isoforms, and calculation of the αα/β-CTX-I ratio, may help to identify patients with benign or malignant bone diseases.35 While intriguing as an idea, the clinical relevance of this concept remains unclear and further studies are warranted before definitive recommendations can be made.
Serum levels of TRAcP, an enzyme released by active osteoclasts, have been reported to be elevated in patients with established bone metastases.41–43 A study comparing serum TRAcP, urinary calcium, pyridinoline (PYD) and DPD levels found that PYD in urine had the highest diagnostic validity to distinguish between patients with and without bone metastases.26 However, the assay used in the study measured total TRAcP instead of the osteoclast specific isoenzyme, TRAcP 5b. Newer studies, making use of assays specific for the 5b band of TRAcP in serum, found this marker to be highly sensitive to the presence of metastatic bone disease.41–43 Furthermore, a study in patients with renal cell cancer reported no differences in serum TRAcP 5b levels when comparing patients with and without bone metastases.15
Bone sialoprotein (BSP) has emerged recently as a new marker of bone resorption in metastatic bone disease. This glycoprotein is a product of active osteoblasts, is incorporated into the bone matrix during bone formation and released from bone during osteoclastic bone resorption. Importantly, BSP is also synthesized and secreted by breast, prostate and thyroid cancer cells. Expression of BSP in these tumours has been proposed to play a role in the homing of tumour cells to bone, and in the enhanced survival of tumour cells in the bone microenvironment.44 We have demonstrated that serum BSP levels correlate with markers of bone resorption in metabolic or malignant bone disease and are often elevated in patients with tumours metastatic to bone.45 Of interest, the highest levels seemed to occur in patients with bone metastases from cancers that are known to express BSP ectopically, such as breast, prostate or thyroid cancers.46 In another study, serum BSP levels were closely related to serum PSA levels.47 High serum BSP values are also found in patients with untreated MM, and measurement of the protein's serum concentration seems to distinguish between patients with MM and benign osteoporosis.48 In general, patients with osteolytic lesions often have higher levels than individuals diagnosed with nonlytic bone disease.
In summary, most markers of bone remodelling, and particularly those of bone resorption, are elevated in patients with established bone metastases. The newer serum assays may be more sensitive to malignancy-induced changes in bone turnover than the older urine-based assays.49 While evidence suggests that bone markers may be useful diagnostic tools in cancer patients, the currently available data do not allow final conclusions regarding the accuracy and validity of any of the presently used markers in the early diagnosis of bone metastases.
Prognostic use
Whether or not bone markers are useful for the prediction of skeletal events in cancer patients with or without prevalent malignant spread is a controversial question. The results of recent studies seem to favour such an association.
Brown et al.50 reported on the association between baseline serum BAP and urinary NTX-I levels and subsequent skeletal event rate in 203 patients with prostate cancer and 238 patients with non-small cell lung cancer (NSCLC) followed in the placebo arms of two phase III BP trials. High levels of both bone markers were associated with poor prognosis, as defined by greater rates of skeletal events and shorter survival times. In patients with prostate cancer, for example, elevated NTX-I levels carried a relative risk of 3·25 (95% CI 2·26–4·68) for skeletal events (Fig. 3). The authors concluded that, in cancer patients, a rise in urinary NTX-I values should prompt more aggressive treatment to prevent skeletal-related morbidity. Costa et al.33 and Vinholes et al.51 demonstrated that an increase of 130–150% in urinary NTX-I or serum ICTP was a valid indicator of clinical disease progression.
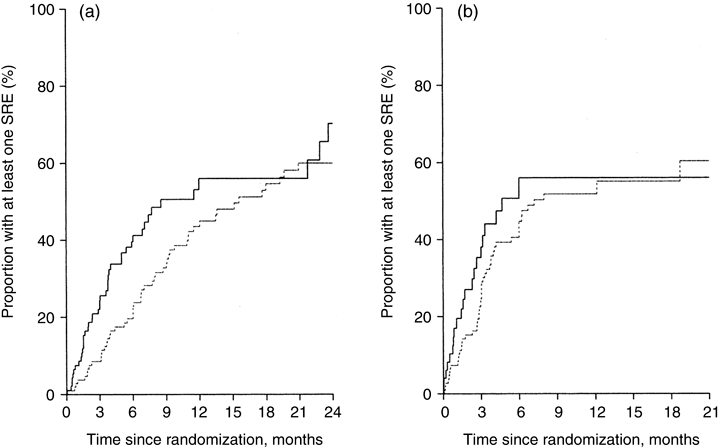
Baseline urinary NTX-I levels predict future skeletal-related events (SREs). Cumulative proportion of SREs in patients with (a) prostate cancer and (b) other malignancies (including NSCLC) according to urinary NTX-I concentrations at baseline (broken line: urinary NTX < 100 nmol/mmol Cr; solid line: urinary NTX > 100 nmol/mmol Cr. (From Brown et al.,50 with permission.)
Earlier studies in breast cancer patients indicate that postoperative serum PINP52,53 and NTX-I51,54,55 levels are predictive of poor survival and shorter time to progression (Fig. 4). In lung cancer patients, only markers of bone resorption, and not markers of bone formation, were associated with survival time.23 Similarly, higher urinary56 and serum (ICTP)57 crosslink concentrations were associated with the incidence of skeletal-related events and poor survival in patients with prostate cancer.39 However, other studies have not confirmed these findings,58,59 possibly because of the high variability of the bone markers studied60 and the small number of cancer patients developing bone metastases.
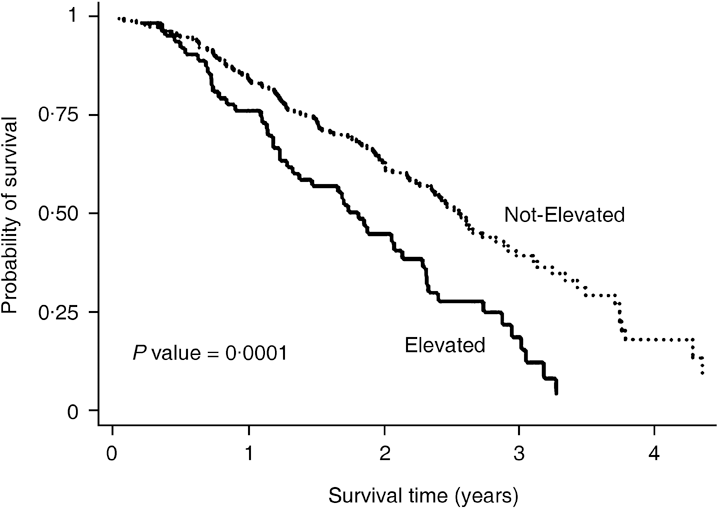
Serum NTX-I levels and survival in patients with breast cancer bone metastases after commencement of hormonal therapy according to serum NTX-I concentrations. (From Ali et al.,54 with permission.)
Using a retrospective study design, Bellahcene et al. demonstrated that the amount of BSP expressed in breast cancer tissues (as assessed by semiquantitative immunohistochemistry) correlated with the propensity of the cancer to metastasize to bone.46 Similarly, tissue expression of BSP in prostate cancer may enable the identification of subgroups of patients who are at risk of bone metastasis or recurrence.61 Our clinical 2-year prospective study demonstrated that, in women with newly diagnosed breast cancer, serum BSP concentrations were highly predictive of future bone metastases.62 Women with breast cancer and elevated serum BSP levels at baseline (i.e. before the operation) had a significantly increased risk of developing bone metastases than similar patients with normal baseline BSP concentrations.
In patients with NSCLC who subsequently develop bone metastases, the expression of BSP protein in the primary cancer is reported to be associated with the progression of distant bone metastases.63 This suggests that measuring BSP expression levels in lung cancers may be helpful in identifying patients at risk to develop bone metastases.63
A recent study by Ramankulov et al. suggests that the plasma concentrations of another noncollagenous bone protein, osteopontin, alone or in combination with other bone markers, may be useful as a diagnostic and prognostic marker in the detection of bone metastases in patients with prostate cancer.18
In MM, reduced serum OC levels seem to be associated with rapid disease progression and poor survival.20 However, this association was not confirmed in other studies,64,65 and more recent studies indicate that serum ICTP levels are a better prognostic marker in MM than most other biochemical indices.66,67 In patients with MM, serum BSP concentrations increase with disease progression and higher levels of the protein are associated with shorter survival time.
Monitoring of antitumour therapy
In addition to newer antineoplastic therapies, BPs have evolved as first-line agents in the treatment of patients with bone metastases. BPs have been demonstrated to alleviate pain and to decrease the incidence of skeletal-related events such as fractures.3,68,69 However, the introduction of these agents has not only improved clinical outcomes in patients with bone metastases but also precipitated the requirement for clinically useful, simple-to-use and inexpensive tools to determine therapeutic efficacy and monitor response in individual patients. Today, there is little doubt that markers of bone remodelling are useful for assessing the effect of BP on bone, as they have been shown to reflect therapy-induced changes earlier than most other techniques currently in use in clinical practice.
In general, bone markers respond to BPs with a rapid and pronounced fall in circulating and urinary concentrations. In most patients, markers of bone resorption react first (often within days) whereas markers of bone formation follow suit several weeks or months later38,45,67,68,70–73 (Fig. 5). This sequence is not unexpected, as osteoclasts are the primary target of BPs. The later reduction in osteoblast activity is considered an effect of the normal coupling between anabolic and catabolic actions in bone. However, as this balance may be perturbed in metastatic bone disease, ‘paradoxical’ effects are often observed. For example, in patients with MM or metastatic breast cancer, chemotherapy-induced remissions20,74 or treatment with BPs75 may lead to a normalization or even an increase of serum OC or BAP levels. In this context, it has been suggested that calculating the ratio of two bone markers may be more useful for identifying therapeutic responders than following changes in individual markers.22,36,76
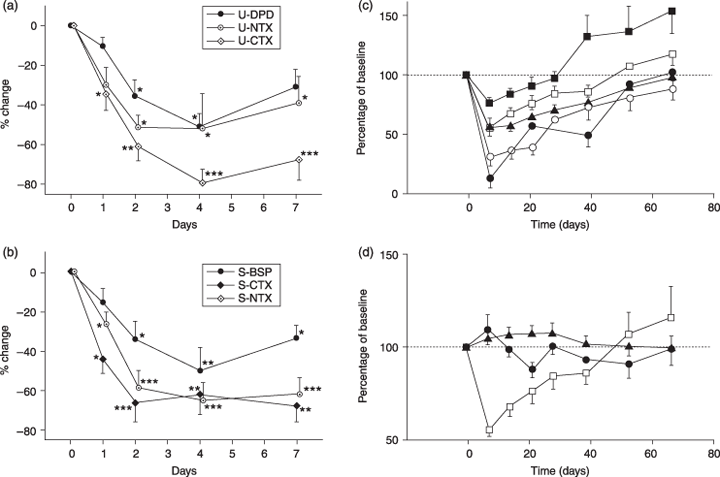
Changes in markers of bone turnover after treatment with IV pamidronate. (a) Short-term changes in urinary bone resorption markers: deoxypyridinoline (U-DPD), C-terminal (U-CTX), N-terminal (U-NTX) telopeptide of type I collagen. (b) Short-term changes in serum markers of bone resorption: bone sialoprotein (S-BSP), C-terminal (S-CTX), N-terminal (S-NTX) telopeptides of type I collagen. *P < 0·05, **P < 0·01, ***P < 0·001 vs. healthy controls. (a,b from Woitge et al.,49 with permission.) (c) Long-term changes in urinary markers of bone resorption ( , DPD;
, DPD;  , PYD;
, PYD;  , CTX-I;
, CTX-I; 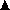 , hydroxyproline;
, hydroxyproline;  , calcium). (d) Long-term changes in serum markers of bone formation (
, calcium). (d) Long-term changes in serum markers of bone formation (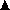 , total alkaline phosphatase;
, total alkaline phosphatase;  , osteocalcin). Changes in urinary DPD (
, osteocalcin). Changes in urinary DPD ( ) are shown again for comparison. (c,d from Body et al.,94 with permission.)
) are shown again for comparison. (c,d from Body et al.,94 with permission.)
Blomqvist et al.77 were the first to demonstrate that, after 6 months of therapy, the percentage change in bone markers vs. baseline was a good predictor of therapeutic outcome. Later studies indicate that pretherapeutic bone resorption rates predict the response to BP treatment. In a short-term, double-blind study on the effects of pamidronate on bone turnover and clinical outcomes, Vinholes et al. observed that cancer patients with high urinary NTX-I levels were less likely to respond to treatment than patients with a normal result. Furthermore, normalization of urinary NTX-I excretion was associated with better clinical outcomes.78 Lüftner et al.79 reported similar results for serum BAP and PINP. In a placebo-controlled, 6-month study of pamidronate in cancer patients, Lipton et al. found urinary NTX-I to be a sensitive marker for monitoring antiresorptive treatment. A reduction in urinary NTX-I levels was associated with reduced pain, fewer fractures and slower tumour progression.72 Measuring the post-therapeutic changes in collagen telopeptide markers and urinary calcium achieved the best results when monitoring cancer patients treated with zoledronate.80 Brown et al. demonstrated that the number needed to treat (NNT) to avoid one skeletal-related event equalled 2 when baseline urinary NTX-I concentrations were clearly abnormal (above 200 nmol/mmol creatinine), whereas the NNT was 31 with normal urinary NTX-I values.81 In patients with breast cancer, serum concentrations of TRAcP 5b fall significantly in response to BP treatment when no progression is detectable, but rise again with disease progression.41
In a posthoc exploratory analysis of three large, randomized clinical trials, Coleman et al.82 confirmed that bone turnover markers might prove valuable prognostic tools in patients with bone metastases receiving BPs. Specifically, the authors demonstrated that, compared to subjects with normal bone turnover, cancer patients with high levels of urinary NTX (and, to a lesser extent, serum BAP) had a significantly increased risk of skeletal complications, disease progression and death. This was particularly true for values measured during therapy with zoledronate, supporting the notion that the goal of BP therapy in cancer patients with metastatic bone disease should be to suppress bone resorption. However, this clinical concept is still awaiting final proof in appropriately designed studies.
The effects of antioestrogens such as tamoxifen on markers of bone remodelling are very different to those of the BPs. Given as an adjuvant therapy in patients with breast cancer metastatic to bone, tamoxifen induced an increase in pyridinium crosslinks83 and either a suppression84 or no change85 in bone formation markers. Noguchi et al.86 found that in patients with prostate cancer metastatic to bone, serial measurements of serum ICTP and PINP were superior to the measurement of PSA in monitoring the effects of hormonal treatment (median follow-up: 29 months). More recently, Johansen et al. demonstrated that serial monitoring of serum PINP, BAP and CTX-I provided prognostic information in 106 patients with prostate cancer metastatic to bone who had undergone hormonal treatment (i.e. total androgen ablation or parenteral oestrogen).55
The effect of chemotherapy on bone markers seems to vary depending on the type of chemotherapy and whether or not glucocorticoids are being used. It seems that most markers of bone formation change slowly following several cycles of chemotherapy as long as no glucocorticoids are involved. By contrast, serum levels of OC suppress profoundly and rapidly once cortisone is introduced.87 In patients with breast cancer, the progression of bone metastases after chemotherapy appears to be closer associated with changes in serum TAP than with carcinoembryonic antigen (CEA) and cancer antigen 15-3 (CA 15-3). In this study, however, measurements of serum TAP were unable to distinguish between responders and nonresponders to chemotherapy.12 In patients with breast cancer and osteolytic bone lesions, a rise in serum OC or TAP/BAP following chemotherapy has in some studies been associated with focal recalcification and therefore interpreted as a sign of therapeutic success.88 However, the significance of these observations needs to be shown in further and larger studies. In patients with MM, high-dose chemotherapy with autografting normalized bone turnover, although these effects were slow to appear.89 Serum BSP seems to reflect the response to chemotherapy in patients with MM, as the treatment-induced changes in serum BSP values correlate with the changes in the monoclonal protein.48 In patients with breast cancers, therapeutic responders exhibited a significant fall in both serum TRACP 5b activity and NTX levels, while in nonresponding patients, serum NTX increased significantly over time. In this recent study, serum TRACP 5b activity was reported to be a valuable tool to monitor post-therapeutic changes in breast cancer patients with bone metastasis.90
It should be noted that absolute changes in marker values are often misleading if the respective marker's analytical and biological variability is not taken into account. Numerous biological factors affect bone turnover and therefore bone marker levels.91,92 As a rule, markers demonstrating large changes in response to disease processes or interventions also exhibit substantial degrees of nonspecific variability. A comparison of two widely used resorption markers, urinary CTX-I and DPD, best illustrate this fact; therapy-induced changes in CTX-I are always more pronounced than those seen with DPD, a fact that is often misinterpreted as a sign of greater sensitivity on the part of CTX-I. However, the short- and long-term variability of CTX-I is also far greater than that of DPD.60 In the clinical setting, variability of bone markers should be of particular concern when it comes to serial measurements, for example during therapeutic monitoring. A moderate reduction in a bone resorption marker is frequently thought to be the effect of antiresorptive treatment, when it should in fact be attributed to nonspecific variability or to a regression to the mean (RTM).91 However, a true response can only be assumed when, within a single individual, the change in signal is greater than the imprecision of the measurement. Based upon the available evidence, a change in bone formation markers of more than 40% is likely to be significant. By contrast, changes below 60–80% in most bone resorption markers are within the range of nonspecific variation (‘background noise’).
In summary, markers of bone resorption respond promptly and profoundly to BP and antineoplastic therapy, and this response seems to be associated with a favourable clinical outcome in patients with bone metastases. Although the available evidence suggests that the aim of BP therapy should be to normalize increased rates of bone remodelling, it is currently unknown whether the use of bone markers in the routine clinical setting has any defined beneficial effects on overall outcome in cancer patients.
Outlook and summary
Most bone turnover markers are abnormal in patients with active bone metastases. In these unfortunate patients, the diagnostic validity of bone markers appears close to that of other diagnostic tools such as radioisotope bone scans, CT and MRI techniques. Further studies are required, however, to determine the diagnostic accuracy and validity of these markers in patients at early or uncertain stages of the disease. The crucial point in this undertaking will be to overcome the notorious variability of present-day bone markers92 and efforts will be required to reduce technical variability and to standardize the available assays.93
Further data will become available on the prognostic use of bone markers. While the available information is indeed promising, larger and longer-term studies are required to strengthen the observed associations between high levels of bone turnover markers and future skeletal events and/or poor survival in cancer patients. It will be of interest to see whether such associations are a general biological phenomenon or a feature typical for some, but not for other, tumours, patients or situations. Again, overcoming the problem of variability will be crucial.
Finally, bone turnover markers will increasingly be used to monitor antitumour therapies. It already seems that high bone turnover markers following treatment are indicative of an unfavourable therapeutic response and prognosis. Vice versa, a reduction in bone markers after antiresorptive treatment is often associated with reduced pain, fewer fractures and slower tumour progression. However, all of the supportive evidence is short term and we need longer-term studies. Nevertheless, in the future, it may be standard procedure to attempt normalization of bone turnover markers to achieve better clinical outcomes for our cancer patients.
In summary, the available evidence indicates that bone markers are useful in established and active metastatic disease, but does not permit any final conclusions as to the accuracy and validity of these indices in the early diagnosis of bone metastases. Clearly, bone turnover markers have insufficient diagnostic or prognostic value to be used in isolation; however, the combination of these markers with other diagnostic techniques may improve clinical assessment of patients with bone-seeking cancers. While many bone turnover markers respond reliably to antiresorptive and antineoplastic therapies, it remains unclear whether the use of these markers in the clinical setting has any defined beneficial effects on overall outcome in cancer patients.




