Inherited lipodystrophies and the metabolic syndrome
Summary
Lipodystrophies represent a heterogeneous group of diseases characterized by an abnormal subcutaneous fat distribution, the extent of which can vary from localized, to partial, to generalized lipoatrophy. Whereas partial and generalized lipodystrophies are each associated with metabolic abnormalities, the localized form is not. These metabolic changes include insulin resistance with type 2 diabetes, acanthosis nigricans, dyslipidaemia predominantly consisting of hypertriglyceridaemia (associated with the onset of pancreatitis) and depressed HDL cholesterol, liver steatosis and hypertension. Affected women are often hirsute and this can be associated with the presence of polycystic ovarian syndrome (PCOS). Most of these clinical features are present to some extent in patients with the common metabolic syndrome. As the prevalence of metabolic syndrome far outweighs that of lipodystrophy, the diagnosis of this rare disorder may often be overlooked with the affected patient diagnosed as merely being ‘yet’ another case of metabolic syndrome. In this article, we draw attention to the importance of recognizing patients with lipodystrophy who present with metabolic abnormalities, as both the diagnostic as well as the therapeutic approach of these patients differ profoundly from patients with the metabolic syndrome.
Introduction
Since the first description of lipodystrophy associated with metabolic abnormalities by Berardinelli,1 major advances have unravelled the molecular genetic basis of these disorders. Several classifications have been proposed during the past decade. Whereas the initial classifications were based predominantly on clinical phenotype (generalized vs. partial), later classifications were based on mode of inheritance (dominant vs. recessive). More recently, with discovery of the genetic basis for several types and subtypes of lipodystrophies, diagnosis and classification has been based on the causal molecular genetic defect.
For a physician the clinical phenotype is the most important and expedient diagnostic tool; thus we will discuss the clinical classification (i.e. generalized vs. partial, summarized in Table 1), based on the OMIM database (Online Mendelian Inheritance in Man, http://www.ncbi.nlm.nih.gov/entrez), taking into account recently published articles on lipodystrophy. Acquired lipodystrophies will not be discussed, as these have been the subject of an excellent recent review.2
| Genetic defect | OMIM # | |||
|---|---|---|---|---|
| A) Generalized | Congenital generalized lipodystrophy (CGL) | CGL type 1 (BSCL1) | AGPAT2 (encoding 1-acylglycerol-3-phosphate o-acyltranferase2), BSCL1 locus op chromosome 9q34 | 603100608594 |
| CGL type 2 (BSCL2) | seipin | 269700 | ||
| BSCL2 locus op chromosome 11q13 | 606158 | |||
| CGL due to other mutations | ||||
| Generalized lipodystrophies with dysmorphic features | MADB | ZMPSTE24 metalloproteinase | 608612 | |
| Wiedemann–Rautenstrauch syndrome | 264090 | |||
| Hutchinson–Gilford progeria | LMNA (encoding lamin A/C) on chromosome 1q21·2 | 176670 | ||
| B) Partial | Familial partial lipodystrophy | Kobberling type (FPLD1) | 608600 | |
| Dunnigan type (FPLD2) | LMNA (encoding lamin A/C) on chromosome 1q21·2 | 151660 | ||
| Dunnigan type (FPLD3) | PPARG (peroxisome proliferator activated receptor gamma) on chromosome 3p25 | 604367 | ||
| Other lipodystrophies | MADA | LMNA (encoding lamin A/C) on chromosome 1q21·2 | 248370 | |
| Mutation in a protein kinase | AKT2 (V-AKT murine thymoma viral oncogene homolog 1) on chromosome 14q32·3 | 164731 |
Generalized lipodystrophy
Congenital generalized lipodystrophy (CGL)
CGL was first described in 1954 by Berardinelli and was further characterized by Seip, hence the eponym Berardinelli–Seip congenital lipodystrophy (BSCL).1,3 Because the characteristic features of these patients are present at birth, CGL is usually diagnosed by neonatologists and paediatricians. Male and female subjects are affected in equal proportions. Because of the lack of subcutaneous fat, affected individuals have a masculinized appearance and soon develop acromegaloid features. Despite an excessive appetite, they usually remain lean, but frequently develop fatty liver disease that can progress to fibrosis. Early in childhood they develop pronounced insulin resistance, often leading to diabetes with concomitant dyslipidaemia. The invariably high triglyceride levels can result in recurrent episodes of pancreatitis. Approximately 300 patients with CGL have been reported to date.4,5
In 1999, by using a genome-wide linkage analysis of 17 pedigrees with CGL, a locus on chromosome 9q34 called BSCL1 was identified.6 A few years later, AGPAT2 (1-acylglycerol-3-phosphate O-acyltransferase 2) was identified as the causative gene among the positional candidate genes at this locus.7 This protein is a member of the acyltransferase family of enzymes that are involved in glycerolphospholipids and triacylglycerols. Therefore, it has been suggested that mutations in AGPAT2 could cause lipodystrophy through a direct effect on lipid biosynthesis.8
In 2001, Magre et al. discovered a new locus for CGL on chromosome 11q13. They analysed 29 families from different geographical regions and in 11 families found a linkage with markers from this new locus that they called BSCL2; they showed that the causative gene within this region was GNG3L1 encoding a novel protein coined ‘seipin’.9 The function of seipin is still under investigation. In a joint effort both groups analysed 45 families with CGL:10 26 had a mutation in APGAT2 while 11 had a mutation in BSCL2 (seipin), with the result that the conditions were renamed CGL1 and CGL2, respectively. Because eight families with CGL had no underlying genetic defect, it became clear that there must be additional genes underlying this phenotype. Before the detection of the genetic basis of these disorders, CGL1 and CGL2 were not considered to be distinguishable. But with molecular classification, a slight difference in clinical presentation was found: CGL2 patients appeared to have increased prevalence of mental retardation and cardiomyopathy, an observation that was subsequently confirmed by another group.11 This clearly demonstrates the importance of collecting more CGL families for genetic analyses that could potentially lead to discovery of new causative genes.
Generalized lipodystrophies associated with dysmorphic features
Mandibulo-acral dysplasia (MAD) was first described in 1971 and is a rare autosomal recessive disorder characterized by mandibular hypoplasia, acroosteolysis, stiff joints, and cutaneous atrophy.12 Other clinical features include postnatal growth retardation, progeroid features, and lipodystrophy with metabolic abnormalities. Because of the rarity of this disorder, little is known about the natural course or history of this disease. Following extensive clinical and biochemical analysis, as well as MRI fat measurements in four patients with MAD, Simha et al. found that three of the patients had a partial lipodystrophy characterized mainly by the loss of subcutaneous fat in the extremities, which they referred to as MAD type A.13 The fourth patient, in contrast, had a generalized lipodystrophy that they called MAD type B; this patient was subsequently found to have compound heterozygote mutations in a zinc metalloproteinase called ZMPSTE24.14 This enzyme plays a critical role in proteolytic cleavage of prolamin A to lamin A. Novelli et al. investigated nine individuals from five different consanguineous families with MAD and found homozygous mutations in LMNA, the gene encoding lamin A/C.15 All MAD patients with LMNA mutations had the type A pattern of lipodystrophy. The LMNA gene encodes several nuclear lamin isoforms that play a key structural role in the nuclear envelope. Various mutations in this gene underlie a very wide range of clinical phenotypes that have been called ‘laminopathies’, which have been the subject of recent comprehensive reviews.16,17
Lipodystrophies as a component of progeroid syndromes
Hutchinson–Gilford progeria syndrome (HGPS) is a rare disorder characterized by accelerated ageing. These patients are normal at birth, but age quickly, with an estimated 10 years of biological ageing per one calendar year. The average life span of these patients is approximately 13 years and they often die of cardiovascular disease. One of the characteristic features of these patients is profound atrophy of subcutaneous fat stores. There are more than 100 case reports and the incidence has been estimated to be around 1: 4–8 million.18 Although HGPS was first described in 1886, the underlying genetic defect was found in 2003 to be a spontaneous mutation in LMNA, affecting splicing.19
Neonatal progeria syndrome (also called Wiedemann–Rautenstrauch syndrome) was first described by Rautenstrauch in 197720 and there are more than 20 case reports in the literature.21 It is an autosomal recessive syndrome that is characterized by aged appearance at birth, with triangular face, natal teeth, hypotrichosis of the scalp hair, eyebrows and eyelashes, relative macrocephaly, micrognathia and generalized lipodystrophy. All these patients died at a young age. One patient had hyperinsulinaemia at the age of 16 months but no diabetes was reported. These syndromes are not associated with metabolic abnormalities, presumably because the patients do not survive long enough to develop the features of metabolic syndrome.
Partial lipodystrophy
In 1974, Dunnigan reported a dominantly inherited partial lipodystrophy with metabolic abnormalities.22 One year later, Kobberling described another type of partial lipodystrophy that was limited to the extremities.23 More than a decade later, they proposed the first classification of familial partial lipodystrophy (FPLD).24 Diabetes mellitus, hyperlipoproteinaemia, and acanthosis nigricans are present to a variable degree in patients with FPLD, and the abnormal distribution of subcutaneous fat is the essential hallmark of the syndrome. Most clinical detected FPLD cases were female patients and, therefore, Kobberling and Dunnigan proposed an X-linked dominant mode of transmission. Ever since, numerous case reports have been described and it has now become clear that, whereas female patients are most readily recognized clinically, male subjects are also affected.
Kobberling type of lipodystrophy, FPLD1
In type 1 familial lipodystrophy, loss of subcutaneous fat is confined to the limbs, sparing the face and trunk (Fig. 1). To date, no genetic mutation has been found that underlies this disease. It is generally agreed that there are more FPLD1 phenotypes than are observed. Herbst et al. elegantly demonstrated this by specifically looking for the FPLD1 phenotype.25 They were able to identify 13 female subjects with FPLD1 within tertiary care outpatient clinics over the course of a few months by skinfold measurement of fat. This suggests that this syndrome is indeed more common than previously thought. No male subjects were identified as having FPLD1. There was a similar finding for FPLD2 until more detailed cross-sectional imaging of fat was performed.26 These data imply that both men and women with FPLD1 are often misdiagnosed. A combination of careful clinical examination with additional imaging is required for definite recognition of these syndromes.
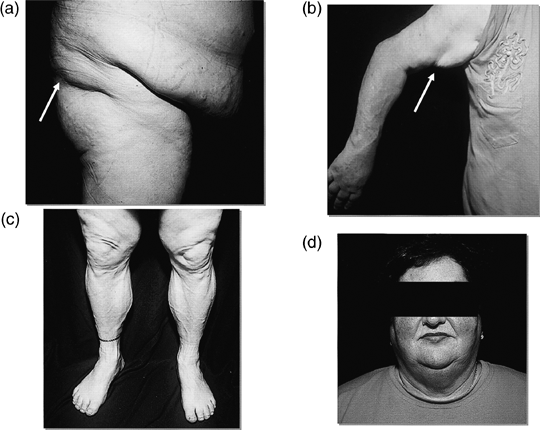
Anatomical features of FPLD1 in an obese subject. (a) Gluteal view demonstrating central adiposity and the ledge of fat above the gluteal area (arrow). (b) Upper and lower arm demonstrating ledge of fat over triceps area (arrow). (c) Lower extremities demonstrating prominent musculature and veins. (d) Face and neck demonstrating Cushingoid appearance of the face, with moon facies and fat under chin. (Illustrated by Herbst et al. Diabetes Care 2003; 26: 1819–1824: with permission of the copyright owner.)
Dunnigan type of lipodystrophy, FPLD2
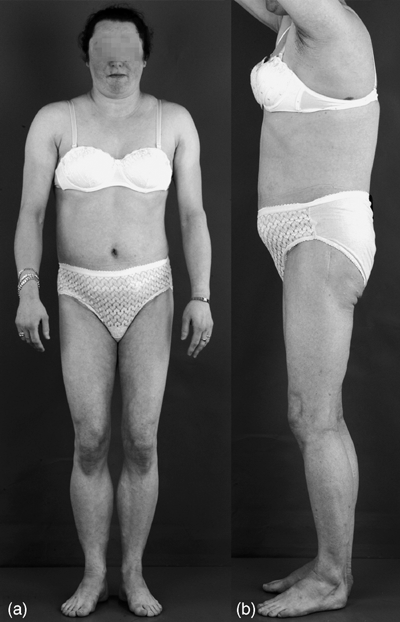
In type 2 familial lipodystrophy the trunk is also affected, with the exception of the vulva, giving an appearance of labial hypertrophy (Fig. 2). There is usually fat hypertrophy in the neck and head region, giving the patient a Cushingoid appearance.27 Often patients have been evaluated for Cushing's disease before lipodystrophy is considered. In 1998 Peters et al. carried out a genome-wide scan with a set of highly polymorphic short tandem-repeats (STR) in individuals from five well-characterized pedigrees and mapped the FPLD2 locus to chromosome 1q21–22.28 Two years later, after genetic analysis of five FPLD2 probands, the causative gene was found to be LMNA,29 again implicating nuclear lamin A in adipose metabolism.
Anatomical features of FPLD2. The lipoatrophy in these patients is not confined to the extremities but also involves the trunk (face-sparing lipodystrophy). The skinfold measurements of this proband are similar to other FPLD2 subjects, falling in the low range (usually less than fifth percentile) when compared with the normal population. This explains the extreme masculine look of the proband. Increased subcutaneous fat in the neck and face creates a Cushingoid appearance. (Illustrated by Morel et al. Journal of Clinical Endocrinology and Metabolism 2006; 91: 2689–2695: with permission of the copyright owner, Copyright 2006, The Endocrine Society.)
Dunnigan type of lipodystrophy, FPLD3
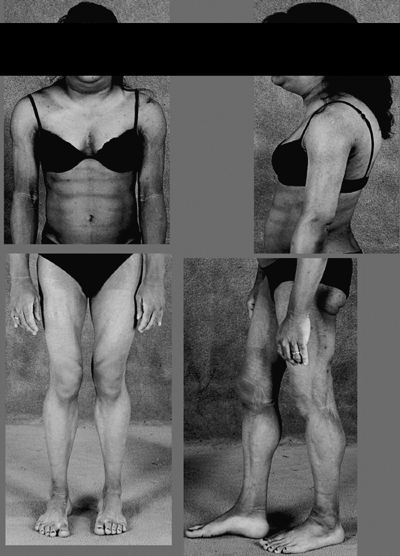
In 1999, by analysis of 85 unrelated subjects with severe insulin resistance, all coding exons of PPARγ1 and PPARγ2 were studied and new missense mutations in the ligand-binding domain of PPARγ were found in only two subjects.30 The lipodystrophic features of those individuals were not reported at that time. In 2002, the direct link between FPLD and PPARγ was reported by two groups.31,32 In 2003, a close evaluation of patients with the first PPARγ mutations revealed the presence of a partial lipodystrophy phenotype,33 demonstrating how lipodystrophy can be missed during routine physical examination. Additional techniques for fat measurements (e.g. MRI) are needed to improve diagnostic accuracy, particularly in men. Since the association of PPARG with lipodystrophy, this syndrome has been annotated as FPLD3. To date 14 patients have been reported to have mutations in PPARG gene causing FPLD3.34 Interestingly, it was recently shown that use of MRI measurements and quantification of lower extremity subcutaneous fat depots in patients with FPLD2 and FPLD3 distinguished between these two forms.35 This corroborated the impression that there is a difference between the two and showed that subcutaneous fat loss in FPLD2 subjects was greater than in FPLD3 individuals (Fig. 3).
Anatomical features of FPLD3. Although these patients also have a masculine appearance, the subcutaneous fat loss in the extremities is less obvious than in FPLD2 subjects. Furthermore, the trunk is not affected, hence the name trunk-sparing lipodystrophy. Note the Cushingoid appearance.
Other FPLDs
Recently, by screening genomic DNA from 104 unrelated subjects with severe insulin resistance for mutations in genes that are implicated in insulin signalling, George et al. identified a missense mutation in the serine/threonine kinase gene AKT2 in one Caucasian proband who had a lipodystrophic phenotype.36 This finding is consistent with the recent finding that AKT2 knockout mice develop severe diabetes and age-dependent loss of subcutaneous fat.37 Although AKT2 can cause lipodystrophy, the prevalence of AKT2 mutations in a cohort of lipodystrophic patients was shown to be insignificant.38
Therapeutic strategies
With respect to therapeutic strategies, current options are largely limited to managing metabolic abnormalities with drugs for treatment of hypertension, diabetes and dyslipidaemia. However, targeted interventions are emerging. First, administration of a specific adipokine, such as leptin, in lipodystrophic patients was shown to induce sustained improvements in glycaemia, dyslipidaemia, and hepatic steatosis.39,40 Long-term effect of this treatment modality is currently being addressed in several centres and is very promising. A second option may be transplantation of subcutaneous fat. In fact, white adipose tissue transplantation has been shown to be effective in leptin-deficient mice.41,42 These mice were prevented from becoming obese and had normal insulin levels. It is too soon to determine whether adipose transplantation will be effective in lipodystrophic patients. Finally, an attractive option for treating lipodystrophic patients might be the use of gene therapy. However, this therapeutic area remains unexplored at this time.
Concluding remarks
Generalized lipodystrophies are easily detected clinically and are usually diagnosed by paediatricians because of the characteristic features from birth onwards. Partial lipodystrophies, on the other hand, are more difficult to recognize, only causing metabolic abnormalities later in life (Table 2). As many of the metabolic features of partial lipodystrophy resemble those of the metabolic syndrome and/or type 2 diabetes mellitus, patients with FPLD are often misdiagnosed. The only clinical signs that are unique to such patients are lipoatrophy and the onset of severe metabolic abnormalities. Because of the marked difference in natural courses of the metabolic derangements in lipodystrophic patients vs. metabolic syndrome subjects, more attention needs to be paid to improving correct identification of lipodystrophic subjects.
| Partial lipodystrophies | Clinical features | Metabolic abnormalities |
|---|---|---|
| Kobberling type (FPLD1) | Lipoatrophy is confined to the extremities | • Severe insulin resistance often combined with only modestly increased BMI |
| Dunnigan type (FPLD2) | Severe lipoatrophy of the extremities and the trunk, with exception of the vulva | • Cushingoid appearance without hypercortisolism |
| Lipohypertrophy of the head and neck region | • Acanthosis nigricans | |
| Dunnigan type (FPLD3) | Like FPLD2, but with less severe lipoatrophy | • Recurrent episodes of pancreatitis related to hypertriglyceridaemia |
| • Liver steatosis | ||
| • Hypertension | ||
| • Hirsutism due to PCOS |




