IKK inhibitor bay 11-7082 induces necroptotic cell death in precursor-B acute lymphoblastic leukaemic blasts
Based upon preclinical studies using small molecule inhibitors in animal cancer models, the IκB kinase (IKK) complex has emerged as a potential cancer therapeutic target (Lee et al, 2006). The IKK complex is activated by multiple signalling cascades and is best known as a positive regulator of nuclear factor (NF)-κB activity. In this role, IKK phosphorylates the ‘inhibitor of κB’ (IκB), triggering its ubiquitination and proteosomal degradation. Dissociation of IκB unmasks the nuclear localization signal of NF-κB, permitting nuclear import and transcription activity (Perkins, 2007). NF-κB participates with receptor-interacting protein 1 (RIP1), a death domain-containing kinase that regulates activation of NEMO (Hitomi et al, 2008), and also plays a critical role in necroptosis (Degterev et al, 2008). Necroptosis represents an alternative programmed cell death path, which can be rescued by necrostatins that target RIP1 (Degterev et al, 2008). At least 32 genes were recently implicated in the necroptosis pathway, seven of which are also shared within apoptosis pathways (Hitomi et al, 2008). Signatures of necroptosis include activation of Nox-1 NADPH oxidase (Kim et al, 2007) to generate reactive oxygen species (ROS), mitochondrial dysfunction and other distinctive morphological changes (Galluzzi & Kroemer, 2008). Because of the potential importance of IKKβ inhibitors in treating acute leukaemias (Cilloni et al, 2006), we evaluated IKK inhibition as a potential strategy in treating patients with precursor-B acute lymphoblastic leukaemia (ALL). We showed that IKK inhibition induces rapid necroptotic cell death in pre-B ALL cells via a ROS-dependent mechanism.
Cell lines (from DSMZ-Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH) were cultured in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) with 10% fetal bovine serum. Bone marrow samples were acquired at diagnosis with informed consent (Human Research Review Committee #05-435). Mononuclear cells were enriched by Ficoll-Paque centrifugation. Peripheral blood B cells were isolated from a normal donor.
The IKK inhibitors Parthenolide, BMS-345541, and PS1145 were from Sigma-Aldrich (St Louis, MO, USA), and Bay 11-7082, (E)-3[(4-methylphenylsulfonyl]-2-propenenitrile, was obtained from Calbiochem (Gibbstown, NJ, USA). IKKγ NBD peptide was from IMGENEX (San Diego, CA, USA) and the TransAM™ NFκB enzyme-linked immunosorbent assay (ELISA) kit was from Active Motif (Carlsbad, CA, USA). Antibodies to caspase 9(Asp330) and caspase 3(8G10) were from Cell Signalling (Danvers, MA, USA).
Lysates (5–10 × 106 cells) were prepared in ice-cold 1% Nonidet P-40 buffer (Yang et al, 2007) and clarified. Protein levels were measured prior to loading for sodium dodecyl sulphate polyacrylamide gel electrophoresis. Blocked membranes were sequentially incubated with primary and horseradish peroxidase-conjugated secondary antibodies (Jackson Immunoresearch, West Grove, PA, USA) and bands on film visualized by chemiluminescence.
Lymphoblast viability was measured by using 7-Aminoactinomycin D (7-AAD) exclusion methods. Cells were loaded with CM-H2DCFDA (Invitrogen) to measure ROS. Mitochondria membrane potential was measured by Mitoprobe™ JC-1 assay (Molecular Probes, Eugene, OR, USA). Data were acquired on a FACSCalibur flow cytometer.
The IKK inhibitor, Bay 11-7082, was tested for the ability to kill four cell lines (Nalm6, 697, Sup B15, RS4:11); these lines represent important pre-B ALL subclasses based upon cytogenetic characterization. Fig. 1A compares viability after Bay 11-7082 treatment over a range of doses, as measured by 7-AAD uptake. Nalm6 and 697 cells had similar responses, with >90% cell death after overnight treatment with 1 μmol/l Bay11-7082. Two other IKK inhibitors, Parthenolide and BMS-345541, gave similar results to Bay 11-7082, but PS-1145 had no effect (Fig 1B). We next evaluated cell death after uptake of the cell permeable NEMO binding domain (NBD) peptide (May et al, 2000), which disrupts the interaction between NEMO with IKKα/IKKβ. NBD peptide induced significant cell death in 697 cells compared with control cell-permeable peptide (Fig 1C). These results showed that IKK regulates pro-survival pathways in leukaemia cells. The time course of 697 cell-death was essentially complete within 4 h of treatment with 2·5 μmol/l Bay 11-7082 (Fig 1D). Importantly, sensitivity extended to freshly isolated patient blasts. Overnight treatment with 1 μmol/l Bay 11-7082 induced 70–99% cell death in CD19+ pre-B ALL cells isolated from six patients (Fig 1E). Peripheral blood B cells from a normal blood donor were c. 85% viable after overnight treatment at this same dose, but showed significant loss of viability (c. 50%) at a 10× concentration (Fig 1E).
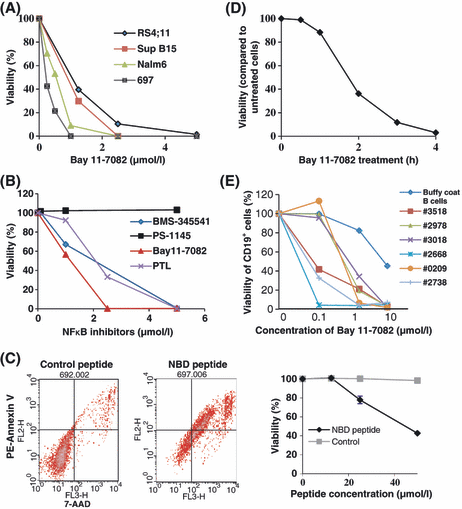
IKK inhibition causes rapid cell death in pre-B ALL cell lines and blasts. (A) The effect of Bay 11-7082 on the viability of four pre-B ALL cell lines after 20 h treatment was tested by flow cytometry. Representative data from three experiments is shown. (B) Comparison of IKK inhibitors Bay 11-7082, PS1145, BMS-345541 and Parthenolide (PTL) on the viability of 697 cells. (C) Effect of the cell-permeable NEMO (NFκB essential modifier) binding domain (NBD) peptide and control cell-permeable peptide on the viability of 697 cells was detected. Cell viability was tested by flow cytometry after PE-Annexin V (FL2H) and 7-AAD (FL3 -H) double staining in two experiments. (D) Time course of cell death in 697 cells after treatment with 2·5 μmol/l Bay 11-7082. Representative data from three experiments is shown. (E) The effect of Bay 11-7082 on the viability of CD19+ blasts from six children and young adults compared to B cells isolated from the buffy coat of a healthy donor. Cellular viability for each dosage point was assessed for each patient sample using the average of duplicate flow cytometric analyses. Viability assays were performed at the time of receipt of each patient sample.
Inhibition of NFκB DNA binding was one important outcome of Bay 11-7082 treatment (Fig 2A), occurring within 30 min as measured by the TransAM™ NFκB p65 ELISA kit. Consistent with this, loss of IκBα phosphorylation was complete within 1 h of Bay 11-7082 treatment (Fig 2B). NFκB-independent effects of IKK inhibition are also suggested. Bay11-7082 treatment resulted in rapid ROS accumulation, which occurred within 30 min in 697 cells loaded with CM-H2DCFDA and peaked at 1 h. The oxidative scavenger, NAC, completely blocked Bay 11-7082-induce cell death even at high concentrations (20 μmol/l Bay 11-7082, 20 h; Fig 2C, D). To distinguish between apoptotic and necroptotic programmed cell death pathways, cells were treated with Z-VAD (a caspase inhibitor) and necrostatin-1 (inhibitor of RIP1 kinase). Only necrostatin-1 was effective at blocking cell death (Fig 1E). Consistent with a necroptotic mechanism, loss of mitochondria membrane potential was rapidly detected in Bay11 7082-treated cells using the fluorescent reporter JC-1 (Fig 2F). Cleavage of the mitochondrial matrix protein, NDUFS3, was also detected with 30 min of Bay 11-7082 treatment (Fig 1G). Cleavage of caspase 3 and caspase 9 was not marked in treated cells (Fig 2G), showing that induction of apoptotic pathways is not critical for Bay 11-7082-induced cell death. Figure 2H shows transmission electron micrographic changes in Bay 11-7082-treated cells, demonstrating a distinctive loss of integrity of intracellular organelles, suggestive of necroptotic cell death.
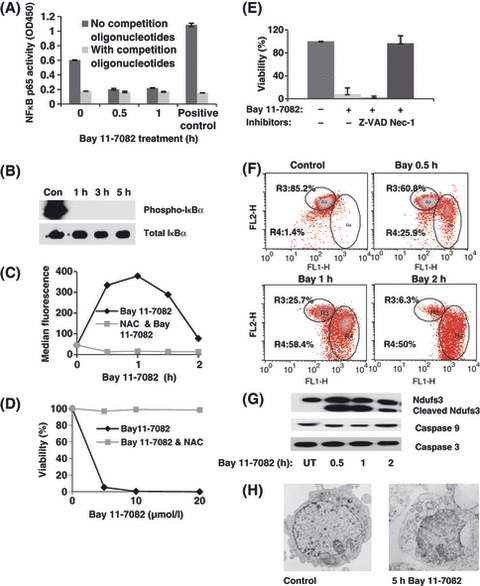
IKK inhibition causes loss of NFκB DNA-binding, induction of reactive oxygen species (ROS), loss of mitochondria membrane potential and necroptosis. (A) Inhibition of NFκB DNA binding was detected by TransAMTM NFκB p65 ELISA kit at different time points after 2·5 μmol/l Bay 11-7082 treatment. In all cases, the data shown are drawn from three experiments. (B) Western blots show that IκBα phosphorylation is ablated in 697 cells within 1 h of treatment with 2·5 μmol/l Bay 11-7082; total IκBα is shown as a loading control. (C) Induction of ROS over the time course of drug treatment (697 cells; 2·5 μmol/l Bay 11-7082), which is blocked by addition of the ROS scavenger, N-acetylcysteine (NAC). (D) NAC also protects 697 cells from Bay11-7082-induced cell death, measured after 20 h with indicated doses. (E) 697 cells were protected from Bay 11-7082-induced cell death by 60 μmol/l necrostin-1, but not 100 μmol/l z-VAD. (F) Loss of mitochondria membrane potential was measured in 697 cells at different time points after 2·5 μmol/l Bay 11-7082 treatment. In this flow cytometry assay, mitochondria depolarization is indicated by the change in red/green fluorescence intensity ratio for JC1 (gate R4). (G) Cleavage of the mitochondrial protein NDUFS3 was observed in Bay11-7082-treated 697 cells. There was little or no decrease in full-length caspase-9 and caspase 3 in Bay 11-7082 treated cells, indicating lack of caspase cleavage and activation. (H) Electron micrographs comparing the morphology of control 697 cells (left) to 697 cells treated for 5 h with 2·5 μmol/l Bay 11-7082 (right). Note the largely intact nucleus in the treated cell but marked loss of cell integrity and disruption of internal organelles.
The data presented here implicate IKK in constitutive regulation of an NF-κB-independent pathway that suppresses necroptotic cell death. IKK inhibition leads to activation of RIP1 kinase, upregulation of ROS production, mitochondria dysfunction and necroptosis. This programmed cell death pathway may represent a new therapeutic target in pre-B ALL.
Acknowledgements
This work was funded by the Leukaemia Lymphoma Society SCOR program (LLS 7388-06). We acknowledge the UNM Cancer Center Cytometry Core and UNM-SOM EM Core. The UNM Pediatric Hematology/Oncology division acquired consented samples.
Authorship contributions
XM, MM and M S-M performed the work; XM and BSW designed the study; SSW provided clinical samples; XM, SSW and BSW wrote the manuscript, and SSW is the corresponding author.
Disclosures
The authors have no conflicts of interest to disclose.




