Feeding selectivity of the seahorse, Hippocampus kuda (Bleeker), juveniles under laboratory conditions
Abstract
This study examined the feeding selectivity of Hippocampus kuda juveniles under captive conditions and evaluates different food organisms that could be used to improve hatchery-rearing of this species. Newly born H. kuda were reared for 10 days in 60-L capacity tanks and fed rotifers (Brachionus rotundiformis), zooplankton (mostly Pseudodiaptomus annandalei and Acartia tsuensis) alone or both food sources. The size and amount of food ingested increased as seahorses grew. Selective feeding of seahorses appeared to change as they develop, preferring copepod adults over nauplii and rotifers. A. tsuensis was highly selected by juveniles over P. annandalei. Specific growth rate in terms of body weight (SGR-BW, 15% day–1) was the highest and mortality rate (9% at day 10) the lowest in seahorses fed a mixed food sources. Slowest growth rate (0.3% day–1) and highest mortality rate (60% at day 7) were observed in seahorses fed rotifers alone. These results indicate that copepods are suitable food for seahorse juveniles, but a mixture of food organisms in the rearing tank environment enhances survivorship and growth of H. kuda, thus potentially providing a source of cultured rather than wild specimens for characterizing the life history of this threatened species.
Introduction
Seahorses are widely distributed. They are found worldwide in tropical and temperate shallow coastal habitats including sea grass beds, coral reefs, mangroves and estuaries (Lourie , Vincent & Hall 1999a). In general, seahorses are voracious carnivores preying mainly on zooplanktonic organisms such as copepods (Al-Baharna 1986; Tipton & Bell 1988; Whitfield 1995; Kanou & Kohno 2001), amphipods and mysids (Kitsos, Tzomos, Anagnospoulou & Koukouras 2008).
Wild seahorse populations are at risk globally. Their low reproductive rates and low mobility and population density make them remarkably vulnerable to fishing pressure (Vincent et al. 1996). Hippocampus kuda is one of the 32 existing species native in Indo-Pacific waters (Lourie et al. 1999a). It is a highly valued seahorse species, and is one of the most heavily traded seahorse species in the Philippines, because it possessed a smooth appearance and usually exhibit a pale yellow colour that are preferred by overseas markets of both traditional Chinese medicine and patent medicinal preparations, live aquarium and curio trade. The International Union for the Conservation of Nature included H. kuda in its lists of vulnerable species. Threatened to near extinction by habitat destruction and heavy exploitation for use in traditional Chinese medicine and aquarium trade, seahorses have gained some protection as a threatened species in several countries (reviewed in Lourie et al. 1999a; Lourie, Pritchard, Casey, Truong, Hall & Vincent 1999b). The present supply of seahorses cannot meet the increasing market demand, especially with the increasing affluence of Chinese consumers, the world's biggest market for seahorses (Vincent 1996). Wild H. kuda and other seahorses from Southeast Asia are the only source of these consumer products. Hence, there is an increasing interest to culture seahorses to alleviate their depleted wild populations (Foster & Vincent 2004). Culture of H. kuda and other seahorse species is becoming increasingly important, as captive fisheries have failed in the past. This warrants researches to establish the feeding ecology of seahorses.
Captive H. kuda adult preferred mysids to Artemia (Do, Truong & Hoa 1998). Under captive condition, Hippocampus trimaculatus diet consists of copepod, rotifers and Artemia (Sheng, Lin, Chen, Gao, Shen & Lu 2006; Olivotto, Avella, Sampaolesi, Piccinetti, Ruiz Navarro & Carnevali 2008; Murugan, Dhanya, Sreepada, Rajagopal & Balasubramanian 2009). Difficulties in long-term rearing of adult seahorses have been attributed primarily to inadequate diet. Similarly, survivorship of juveniles largely depends on the availability of appropriate prey to newly born seahorses. The use of Artemia nauplii in many earlier attempts to rear newly born seahorses have resulted in poor survival rates in many species (Payne & Rippingale 2000) including H. kuda (Hilomen-Garcia, pers. com.). In addition, as juveniles are released as independent young, they require very specialized diets. Thus, determination of food selection and appropriate food types for the first exogenous feeding period is essential to ensure growth and survival of the young in captivity and in the natural environment during restocking.
Over the last few years, the interest in seahorse aquaculture projects around the world has increased dramatically, and commercial aquaculture of seahorses has been repeatedly proposed as one solution to provide alternative source for trade, research and conservation efforts while ensuring natural stocks (Koldewey & Martin-Smith 2010). Although a number of studies on breeding and juvenile culture of seahorses have been conducted (Woods 2000, 2003, 2005; Lin, Lu, Gao, Shen, Cai & Luo 2006; Lin, Gao, Sheng, Chen, Zhang & Lu 2007; Lin, Lin & Zhang 2008; Murugan et al. 2009), there were only few studies that precisely described an appropriate feeding regime which can improve survivorship of early juveniles and can be consequently applied to a large-scale rearing of juvenile seahorses. Current functional approach for seahorse aquaculture is a small-scale intensive system (i.e. having the most extreme levels of human control with high stocking density, extensive use of artificial feeds (often supplemented with vitamins, essential elements, antibiotics and close environmental control) culturing seahorses for the aquarium market (Koldewey & Martin-Smith 2010). A recent study by Garcia and Hilomen-Garcia (2009) demonstrated some success in large-scale rearing of seahorse juveniles (10–15-day- and 0.7–1.5-month-old) in trialing arrays of floating bamboo and nylon. However, it should be considered that success in large-scale rearing of late juveniles would not be possible if a sufficient supply and improved survivorship of early juvenile (0–10 days) are lacking. Limited early juvenile production remains the major bottleneck that must be overcome to improve the efficacy and commercial viability of seahorse aquaculture.
This article reports changes in food selectivity among early juvenile (day 0 to day 10) H. kuda fed various types and stages of food organisms, namely the rotifer Brachionus rotundiformis and nauplii and adult copepods Acartia tsuensis and Pseudodiaptomus annandalei. A suitable feeding regime for the first feeding period of seahorse juveniles is suggested.
Materials and methods
Live food culture
Mixed zooplankton (mostly P. annandalei) was collected from a nearby brackishwater pond (Oton, Iloilo, Philippines; 10°41′35″N, 122°28′25″E), using a 60 μm mesh-size net (larger organisms were initially removed by larger-sized mesh net, 150 μm). Rotifers (B. rotundiformis) were mass produced on marine Chlorella sp., at the Aquaculture Department of Southeast Asian Fisheries Development Center Aquaculture Department (SEAFDEC/AQD) in Tigbauan, Iloilo, Philippines, where this experiment was undertaken.
Ten days prior to anticipated spawning of seahorses, circular 60 L capacity replicate tanks with 50 L green seawater (approximately 5–10 × 104 cells mL−1 marine Chlorella sp. or 105 cells mL−1 Tetraselmis tetrahele) were inoculated with either ~15 000 B. rotundiformis L−1, mixed zooplankton (density, 50 female copepods L−1) or their combination (~15 000 rotifers L−1plus 50 female copepods L−1). Each of these stocking strategies was followed in three replicate tanks.
Dead and faecal materials were siphoned out from the bottom of each tank before 50–70% of the water volume was changed daily. To drain the water, 40 μm mesh-sized filters were used to keep food organisms in the tanks. Green water in each tank was maintained throughout the day by addition of Chlorella sp. or T. tetrahele after water change to keep the quantity of alga at approximately 1−3 × 105 cells mL−1. Moderate aeration was provided.
Abundance, size and composition of zooplankton were monitored daily after once a day water change. A total of 500 mL of rearing water was sampled at three sampling sites from each tank using a PVC pipe water sampler. Samples were concentrated to 5 mL and preserved in 5% seawater formalin. Developmental stages of copepods were categorized according to morphology and size as shown in Table 1.
| Food organism | Body length (mm) | Body width (mm) | Proportion (%)a |
|---|---|---|---|
| Brachionus | 0.09 ± 0.001 | 0.065 ± 0.001 | 29.10 |
| 0.198 ± 0.002 | 0.135 ± 0.002 | 60.00 | |
| Acartia nauplii | |||
| n1–n3 | 0.116 ± 0.0013 | 0.063 ± 0.0013 | 0.04 |
| Pseudodiaptomus nauplii | |||
| n1–n3 | 0.158 ± 0.00003 | 0.090 ± 0.00003 | 7.75 |
| n4–n6 | 0.228 ± 0.0009 | 0.112 ± 0.0009 | 0.76 |
| Copepod adults | |||
| c1–c3 | 0.504 ± 0.002 | 0.200 ± 0.002 | 0.31 |
| c4m–c4f | 0.694 ± 0.0002 | 0.231 ± 0.0002 | 0.80 |
| c5m–c5f | 0.831 ± 0.0004 | 0.253 ± 0.0004 | 0.71 |
| c6m–c6f | 0.900 ± 0.00005 | 0.264 ± 0.00005 | 0.50 |
- a Proportion (%), proportion of developmental stages of copepods based on the total copepod stages observed in the rearing tanks.
Seahorse feeding
Newly born H. kuda juveniles (n = 810–882) with mean initial body weight (BW) and stretched height (SH, vertical distance from the tip of the coronet to the tip of the tail) of SEM 1.749 ± 0.78 mg and 6.918 ± 0.1091 mm, respectively, were obtained from brood of a breeding pair of H. kuda. The initial BW and SH were obtained from H. kuda juveniles sacrificed before the start of experiment (n = 90). As it is difficult to obtain juveniles from more than one brood at the same time, this experiment was repeated at least two times on separate occasions using juveniles from another breeding pairs (n = 2). The spawning period of the breeders have been determined, which usually occurs every 12 days from the first spawning (Hilomen-Garcia and Celino, unpublished data). Upon spawning (10th day of the live food culture), juvenile seahorses (n = 90–98 for each replicate tank) were stocked in three replicate tanks of the live food culture, which were designated as Treatment 1, rotifers alone; Treatment 2, mixed copepods alone [mostly P. annandalei (Sewell)and A. tsuensis, (Ito)]; and Treatment 3, a combination of rotifers and copepods. The stocking density of juvenile seahorses is within the optimal stocking range for seahorse juveniles (Hilomen-Garcia, unpublished data). Each of the rearing tanks was provided with a 584 lux of light intensity, which is considered to be within the range of optimal light intensity observed in other species (Murugan et al. 2009). No substrate was used in any tanks. Water temperature, salinity, dissolved oxygen and pH ranged from 26.0 to 28.1°C, 30 to 31 ppt, 6.1 to 8.3 ppm and 7.7 to 8.1 respectively. The average pH between tanks was not statistically different (P < 0.05).
Hippocampus kuda juveniles were maintained at 12L:12D photoperiod. Live food was added as necessary to maintain densities of not lower than 5 000 rotifers L−1 and 50 adult copepods L−1 in respective tanks with seahorses. Initial counts of food organisms were computed after food adjustment.
Three to five seahorses were sacrificed daily from each tank 1–2 h after food adjustment (about 14.00–15.00 hours). We initially observed that juvenile H. kuda tend to excrete the body of live diets after 3–4 h of feeding (pers. observation), and thus, the sampling time is optimum for assessment of gut content. The samples were preserved in 5% seawater formalin for growth and morphological measurements, and gut content analysis. Seahorse mortalities were monitored daily. The total number of H. kuda juveniles were counted at the end of experiment for each treatment, and hence, all dead juveniles were accounted for.
SH, BW and snout depth at the narrowest point (SDN, vertical measurement of the snout at its narrowest point) were determined according to Lourie et al. (1999a). Digestive tracts of seahorses were dissected, and food organisms were removed. The composition and size of food organisms in the gut were then examined under a microscope.
Data presentation and analysis
Body width (W) of live foods was preliminarily determined based on body length (BL) of nauplii and adult copepods, and lorica length of rotifer using the following equations: for B. rotundiformis, W = 0.634 BL + 0.0066 (n = 200); for nauplii of P. annandalei, W = 0.301 BL + 0.0429 (n = 200); where BL excludes length of posterior spine; and for nauplii of A. tsuensis, W = 0.366 BL + 0.0210 (n = 200) (Toledo, Golez, Doi & Ohno 1997). Body width of copepod adults, however, was computed following the formula: W = 0.16 BL + 0.12, which was determined using regression analysis of W and BL from a total of 51 samples (r2 = 0.77).
Food selectivity indices (E) of seahorses at various ages (day 0 to day 10) were calculated from Treatment 2 and Treatment 3 using Ivlev's (1961) formula: E = (r% − p%)/(r% + p%), where r% is the percentage of the prey species or size in the gut and p% is the percentage of the prey species or size in the tank water.
Specific growth rate (SGR) was computed as follows: SGR = [log (BWf or SHf) – log (BWi or SHi)/T] × 100; where the f refers to the final BW or SH, and the i and T refer to the initial BW or SH, and days of culture respectively.
Daily cumulative mortality rate is the percentage of the cumulative number of dead seahorses based on initial number of seahorses in each tank. This was arcsine transformed prior to statistical analysis. Linear regression analysis was used to show the changes in the total amount of food in the gut and growth. All treatment means were compared using Duncan Multiple Range Test (DMRT) following Analysis of Variance (anova, α = 0.05) using spss/pc statistical package.
Results
Live food culture
Copepods naturally propagated in culture tanks. Pseudodiaptomus annandalei and A. tsuensis nauplii were produced 1 day after stocking of copepod adults.
The total amount of food items in the rearing tanks did not significantly differ between Treatments 1 and 3 throughout the culture period. In Treatment 2, however, the total amount of food items was lower than those in Treatments 1 and 3 (P < 0.05). Moreover, there is no apparent decrease in the total amount of zooplankton in the tanks without seahorses (control) unlike in treatments tanks with seahorses, wherein a decrease in zooplankton was observed based on the initial and final counts of prey items.
Acartia tsuensis nauplii observed in the rearing tanks were smaller (0.116 ± 0.0013 mm BL) than P. annandalei nauplii (0.158 ± 0.00003 − 0.228 ± 0.0009 mm BL, mostly 0.158 ± 0.00003 mm BL). Body length of copepod adults ranged from 0.504 ± 0.002 − 0.900 ± 0.00005 mm (mostly 0.694 ± 0.0002 mm BL). Size of rotifers ranged from 0.09 ± 0.001 to 0.198 ± 0.002 mm BL (Table 1).
Seahorse feeding
Spawning of seahorses occurred 10 days after the initial stocking of live food organisms. Hence, stocking of newly born seahorses (day 0) was on the tenth day (day 10) of the live food culture.
All seahorse juveniles started feeding in all treatment groups at day 0 (i.e. immediately after stocking in experimental tanks).
Composition and amount of food in the gut
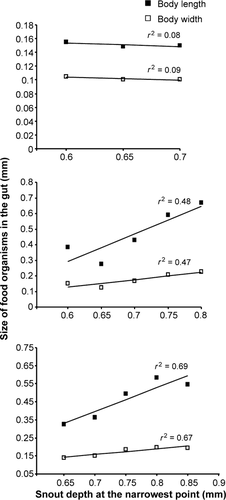
The mean size of food items ingested in terms of BL (r2 = 0.08; P < 0.05) and BW (r2 = 0.09) in Treatment 1 remains the same throughout the culture period. The mean sizes of food ingested in Treatment 2 and Treatment 3 increased significantly along with increasing SDN [r2 = 0.48 (BL), r2 = 0.47, (BW) and r2 = 0.69 (BL), r2 = 0.67 (BW)] (P < 0.05; Fig. 1).
The amount of nauplii ingested decreases significantly with growth (r2 = 0.48; P < 0.05), whereas the amount of copepod adults ingested significantly increased with growth (r2 = 0.64; P < 0.05; Fig. 2).
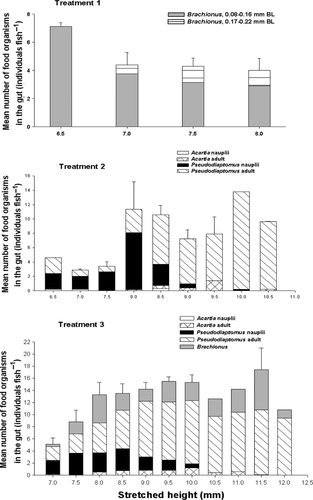
In Treatment 1 and 2, there was no significant increase in the amount of food ingested by seahorses. On the other hand, the total number of food in the gut of seahorses in Treatment 3 significantly increased with growth (r2 = 0.27; P < 0.05; Fig. 2). The size of food organisms ingested by juvenile seahorses in the different classes of SH did not differ between Treatments 2 and 3. Moreover, the total amount of food ingested by H. kuda juveniles in Treatment 3 was significantly higher than those in Treatment 1 and 2, starting day 4 and onwards (P < 0.05, data not shown).
Food preference
Ivlev's food selectivity indices for Acartia nauplii and adult, P. annandalei nauplii and adults and B. rotundiformis in Treatments 2 and 3 are shown in Fig. 3.
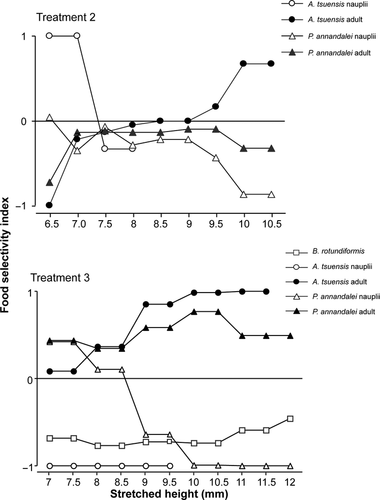
The selectivity index values for A. tsuensis and P. annandalei nauplii decreases with increasing SH, whereas it increases for both species of copepod adults with increasing SH. B. rotundiformis had consistently negative selectivity index values from day 0 to day 10.
With regard to selectivity between different species of nauplii, H. kuda juveniles have higher preference to A. tsuensis nauplii than P. annandalei nauplii at low SH values. As SH increased, seahorses showed higher preference to P. annandalei nauplii than A. tsuensis nauplii. Although A. tsuensis and P. annandalei adults have relatively the same BL and BW (Table 1), food selectivity index of seahorses for A. tsuensis adult become increasingly higher than P. annandalei adult through an increasing SH.
Growth and survival
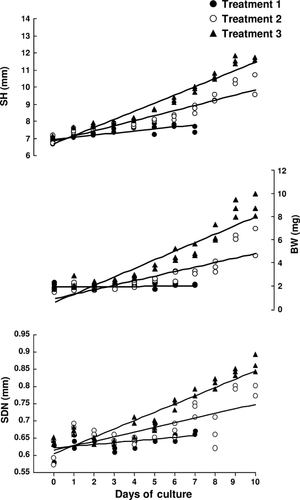
Changes in SH, BW and SDN of seahorses throughout the culture period are shown in Fig. 4. SH of seahorses in Treatment 1 increased only slightly, but not significantly, whereas BW remained the same throughout their survival period of 7 days. Seahorses in Treatment 2 and Treatment 3 increased in size in terms of SH (r2 = 0.86 and r2 = 0.95; P < 0.05) and BW (r2 = 0.72 and r2 = 0.87; P < 0.05) during the culture period of 10 days (Fig. 4). SDN increased with age in all treatments (r2 = 0.88, r2 = 0.90 and r2 = 0.94 respectively; P < 0.05). Seahorses in Treatment 3 had significantly higher SH and BW at the middle of the culture period and onwards among the treatments (P < 0.05). Moreover, SGR was significantly highest in Treatment 3 (SH, 4.6 ± 0.1% day-1; BW, 14.8 ± 0.2% day-1) followed by Treatment 2 (SH, 3.2 ± 0.3% day-1; BW, 10.5 ± 1.2% day-1), and the lowest in Treatment 1 (SH, 1.5 ± 0.2% day-1; BW, 0.27 ± 0.7% day-1) (P < 0.05).
Mortality
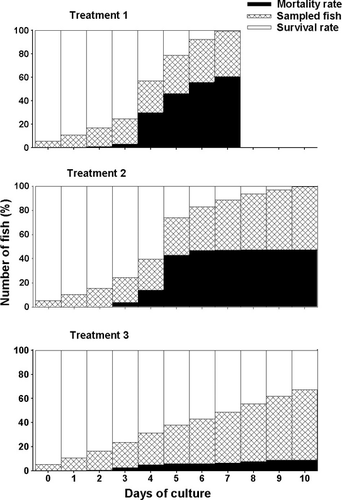
Cumulative mortality rate of seahorses is shown in Fig. 5. At day 4, mortality of seahorses was highest in Treatment 1 (30%), followed by Treatment 2 (14%), and lowest in Treatment 3 (5%) (P < 0.05). Moreover, mortality rate of seahorses in Treatment 1 further increased at day 5 (46%), consequently decreasing survival rate (P < 0.05). Mortalities then continued until the stock was totally depleted at day 7. In Treatment 2, however, mortality rate substantially increased only at day 5 (43%) (P < 0.05), and no significant mortalities were observed thereafter. Finally, lowest mortality rates were observed in Treatment 3 having a cumulative mortality of only 9% at the end of the culture period. The total mortality in Treatment 3 (8.33 ± 1.08 SEM) was significantly lower compared with Treatment 1 (56.67 ± 2.16 SEM) and Treatment 2 (45.67 ± 2.16 SEM) (P < 0.05).
Discussion
Brachionus rotundiformis and calanoid copepods (mostly P. annandalei and A. tsuensis) can successfully proliferate in green water in small laboratory tanks (60-L capacity) for up to at least 20 days. The production of copepod nauplii, a day after stocking of copepod adults suggests that copepod culture should start at least a day before anticipated spawning of seahorses provided that copepod adults are gravid. The population densities of these food organisms, however, fluctuate and may fall below the desired levels during culture. Hence, it is necessary to monitor the densities of food organisms in the laboratory.
The upper limit of prey size is set by the mouth size of the predator (Hunter 1981). Regardless of age class, H. kuda have a larger mouth size (in terms of SDN) than the body width of most prey types (Table 1), and thus, newly born seahorses are capable of swallowing all types of food in the tanks. This supports our observation of 100% feeding incidence at day 0 in all treatment groups. Nonetheless, H. kuda preferred relatively smaller prey during the first few days of exogenous feeding, where SDN is smaller. As in other fishes, (Hunter 1981) food size ingested increased with the increase mouth size in seahorses fed copepods alone (Treatment 2) or with rotifers (Treatment 3). Previous research in feeding selectivity suggests that many fish larvae choose the largest prey items within the size range bounded by their mouth-gape (Detwyler & Houde 1970), which is approximately two-thirds to three quarters of their gape. Seahorses therefore fit into this pattern. There was also an increase in the amount of copepod adults ingested with increasing stretched height in both treatments, which may be attributed to the increasing energy requirements of seahorses with growth. Hence, our study supports observation of Govoni, Ortner, Al-Yamani and Hill (1986) that the proportion of large prey would increase in the diet as the fish grow.
Copepods were highly selected over rotifers by H. kuda in this study. As copepods were abundantly present in their natural habitat, it is possible that seahorses have evolved to recognize them as prey (Payne & Rippingale 2000), and like most marine fish larvae, copepods are their typical food items in the sea (Hunter 1981). In their natural habitat, the diets of H. mohnikei (Kanou & Kohno 2001) and H. zosterae (Tipton & Bell 1988) consist mostly of copepods. Similarly, under captive condition, H. trimaculatus juveniles preferred to feed on copepod nauplii rather than rotifers (Sheng et al. 2006; Murugan et al. 2009). Seahorses may also feed according to optimal foraging theory (Werner & Hall 1974) similarly with pipefish–Syngnathus fuscus (Ryer & Orth 1987), where they concentrated their feeding on more profitable prey (i.e. copepods), and even rotifers were of higher density. Copepods are relatively bigger, and thus, more valuable prey for seahorses. Hora and Joyeux (2009) reported that H. reidi juveniles seemed to ignore smaller zooplankton that are less energetic prey (Payne & Rippingale 2000), which may be possibly due to overall negative recurrent cost of capture. This may also be true in the case of the present study, wherein rotifers were less preferred by seahorse juveniles. Foraging theory predicts that animals will choose feeding alternatives to maximize energy gained relative to energy lost while acquiring food (Stephens & Krebs 1986; Schoener 1987). On the other hand, B. rotundiformis may only be utilized when copepods are present in low quantity. This observation coincides with the studies of Houde and Schekter (1980) which shows that, at lower nauplii levels, linedsole switched from a specialist to a generalist with alternate, less valuable prey being increasingly important in the diet, although nauplii remained the most important prey.
Furthermore, H. kuda may become more effective predators as they grow. With an increase in snout depth and length, and presumably maturity and learning, and consequently improved prey capture efficiency, H. kuda may have been able to select larger prey, resulting in an increase in the selectivity index for both species of copepod adults with an increase in SDN (Fig. 3). This observation indicates an ontogenetic shift in the utilization of prey items as maturity progressed, which was similarly observed in wild (Teixeira & Musick 1995) and laboratory-reared syngnathids (Joba, Dob, Meeuwigc & Halla 2002; Sheng et al.2006; Olivotto et al. 2008; Hora & Joyeux 2009; Murugan et al. 2009).
In both copepods alone and mixed zooplankton treatments, at lower SH values, juveniles have higher preference for A. tsuensis and P. annandalei nauplii than both species of copepod adults, whereas it increases for both species of copepod adults with increasing SH. Moreover, at 6.5–7.0 mm SH, juveniles have higher preference for A. tsuensis than P. annandalei nauplii. At 7.5 mm, SH juveniles preferred P. annandalei nauplii. over A. tsuensis nauplii. Considering that A. tsuensis nauplii present in the tanks were of smaller size than P. annandalei, this may imply that at this stage, seahorses may discriminate food items by size. However, at later stages of growth (from SH = 9.0 to > 9.0 mm SH), seahorses continuously preferred adult A. tsuensis than P. annandalei adults. As the A. tsuensis adult is relatively of the same size as the P. annandalei adult, it is unlikely that prey size is the only factor affecting food selection of seahorses especially at the later stages. It may be that juveniles discriminate between A. tsuensis and P. annandalei adults by other factors. A. tsuensis may be preferred due to physiological and biochemical differences between the two species. Studies of Toledo, Golez, Doi & Ohno (1999) indicated that A. tsuensis sp. has higher n3-highly unsaturated fatty acid (HUFA) and docosahexaenoic acid and eicosapentaenoic acid (DHA:EPA) ratio than than P. annandalei and their nauplii have been observed to be suitable food organisms for first-feeding fish larvae (Toledo, Golez, Doi, Bravo & Hara 1996; Toledo et al. 1997). Thus, in H. kuda, prey size is the first ‘filter’ in prey selectivity, with prey species becoming important only after sorting for prey size. Moreover, although A. tsuensis have spiny appendages (Hunter 1981), H. kuda still preferred them over non-spiny P. annandalei, suggesting that protective spines appear to have little effect on prey selection of seahorses. Food preference of seahorses in this study therefore seems to have been determined by a combination of predator size, nutritive value and size of prey, as well as prey type and capture efficiency.
As in most studies of fish culture, i.e. Milkfish (Chanos chanos) fry (Pantastico, Baldia & Reyes 1986) and Siganids (Siganus guttatus) larvae (Duray 1986), seahorses showed better growth performance when offered mixed diet. In the natural environment, prey diversity has been found to be significantly and positively correlated with the daily growth of the spotted sea trout, Cynoscion nebulosus (Baltz, Fleeger, Rakocinski & McCall 1998). Previous reports also suggest that the total energy gained increased by the greater number of prey items processed (Ryer & Boehlert 1983). The increased feeding rate of seahorses on mixed diet may have led to increased energy consumption, and hence, faster growth. Moreover, copepods preying on rotifers and green water may be more nutritious than copepods on green water alone, thereby enabling seahorses on mixed diet to optimize growth and survival. Rotifers may have supplemented the nutritional need of seahorses directly or indirectly through the copepods. Predation of copepods on rotifers (Toledo pers. com.), enriches the copepods. Furthermore, a similar result has been observed in H. reidi in which feeding formula that gave the best results consisted of mixed diet of nauplii of the harpacticoid copepod Tisbe spp. with rotifers, and copepodites/copepods and Artemia nauplii (Olivotto et al. 2008). When offered rotifers alone, seahorses showed very high mortality rates and did not grow or survive beyond day 7. This may also have been a result of lower DHA:EPA ratio in B. rotundiformis than in calanoid copepods such as Acartia and Pseudodiaptomus spp. (Toledo, et al. 1999; Olivotto et al. 2008). It is well established that larvae and juveniles of most marine fish species require live prey with a high nutritional content, especially at the first feeding stage when HUFAs are essential in the diet (Sargent, McEvoy, Estevez, Bell, Bell, Henderson & Tocher 1999). Deficiencies in HUFA result in a decline in animal health and condition, causing detrimental effects on central nervous system development (Sargent et al. 1999), growth (Furuita , Konishi & Takeuchi 1999, Coperman, Parrish, Brown & Harel 2002; Olivotto, Holt, Carnevali & Holt 2006), stress tolerance (Vagelli 2004), pigmentation, swimming and feeding activity (McEvoy, Naess, Bell & Lie 1998; Coperman et al. 2002, Bell, McEvoy, Estevez, Shields & Sargent 2003). The HUFA requirement of young seahorses is unknown; however, it is likely that this requirement is similar to that of other marine fish larvae. Hunter (1981) reported that although plaice larvae are able to survive through metamorphosis, growth is slow when larvae were fed Brachionus plicatilis alone. Moreover, unlike in marine fish larvae (Pryor & Epifanio 1993; Toledo et al. 1996; Toledo et al. 1997), it may be possible that seahorse juveniles cannot digest or fully utilize Brachionus during the earlier stages of development. Furthermore, the variability in growth and mortality among treatments may not only be solely due to dietary value of the prey regimes but also due to the size and energy value of the different prey. One benefit of fish larvae selecting for increasingly larger prey items during development is that energy gain to energy loss ratio increases with prey size. Rotifers therefore may be unsuitable as food because of its low energetic yield. Although the total amount of food items in the rearing tank did not significantly differ between Treatments 1 and 3 throughout the culture period, and the total amount of food items in Treatment 2 was slightly lower than in Treatment 1 throughout the culture period, the amount of food ingested which may consequently contributed to higher survival and growth of juvenile seahorses in Treatments 2 and 3 were still higher than those in Treatment 1. Nevertheless, in the case of Treatment 2 and 3, in which the former has lower amount of prey in the tank, which may be due to lower nutrient source for copepods, it may result in the lower amount of prey ingested, and hence, in the variability of growth and survival between the two treatments. The presence of a variety of prey in Treatment 3 may have supplied the diversity in prey size required to satisfy the changing preferences of growing juveniles (Sheng et al. 2006).
The availability of a diversity of food organisms in the rearing tank environment increases survival and growth rates of H. kuda. Thus, employing a mixed diet of copepods and rotifers supplemented with green water supports the viability of rearing newly born H. kuda juveniles in captivity, providing a suitable feeding and culture strategy for seahorse juveniles than can be potentially applied in large-scale rearing of seahorse juveniles. Several authors have suggested that calanoid copepods are ideal food items for marine fish larvae and seahorses (Payne & Rippingale 2000, Job , Buu & Vincent 2006) because they are pelagic, and hence, more available as prey (Payne & Rippingale 2001; Olivotto et al. 2006). However, unlike Tisbe spp., they are considered to be good candidates only for experimental scale feeding studies and not for large-scale production (Olivotto et al. 2008), because they are difficult to culture on a continuous basis and can only be cultivated in large tanks (Holt 2003). In our study, however, we demonstrated that calanoid copepods (P. annandalei and A. tsuensis) with B. rotundiformis can be propagated in small laboratory tanks (i.e. 60-L capacity tanks) provided with green water. This feeding regime has raised some H. kuda stocks to market-sized individuals, and sufficient numbers of hatchery-bred stocks of H. kuda were produced.
These results highlight the importance of the biodiversity of zooplankton prey in the culture of threatened teleost species such as H. kuda. The information from this present study would be important for following aquaculture work on seahorse and other Syngnathid species. In addition, the present success in rearing newly born H. kuda juveniles opens an array of possibilities to examine behavioural and physiological characteristics in the life history of this threatened species without additional pressure on wild stocks.
Acknowledgements
This study was funded by SEAFDEC Aquaculture Department (study code Br-04-F95T of GV Hilomen-Garcia). Thanks to Dr. T. Gonzales for laboratory assistance. The authors also thank Dr. L.M.B Garcia (University of the Philippines, Diliman, Philippines) and Dr. Richard Hanna (North Carolina University, USA) for critically reading an early draft of this paper.




