Shadow area and partitioning influencing mortality, healthiness and growth of juvenile Red Swamp Crayfish Procambarus clarkii (Decapoda)
Abstract
Crayfish show both shelter-seeking behaviour and agonistic behaviour. Agonistic interactions among crayfish combatants can be triggered and released by the access of shelter, which is a necessary resource for crayfish. The use of shadow as a shelter has not been thoroughly tested in experiments. In this study, we provided the Red Swamp Crayfish Procambarus clarkii (Girard) with different shadow area and shadow partition to test if shadow can act as a solid shelter. Eleven different treatments designed with different shadow area and partition, and 2640 juvenile crayfish were used. The survival rate increased with the increase of shadow area ratio. The shadow area contributed less to the body weight gain and the number of the survivals without injury. The growth variance of the juveniles under shadow conditions was significantly lower than those maintained without providing shadow. Under the recent experimental settings, generally, more partitioned shadow resulted in lower mortality. With 60% shadow area, more partitioned shadow led to higher body weight gain. The more the partition was provided, the fewer were the injury events, and the lower body weight variance observed. Our experiments indicated that both area and partition of the shadow influenced the growth of juvenile Red Swamp Crayfish.
Introduction
The importance of shelters (refuges, hiding places) to crayfish is well documented (Figler, Cheverton & Blank 1999). The presence of shelters, which triggers longer and more intense inter-specific fight (Bergman, Kozlowski, McIntyre, Huber, Daws & Moore 2003), might be more limiting than even food in controlling population abundance and viability (Capelli & Hamilton 1984; Miller, Savino & Neely 1992). The completion of life cycle of the White-clawed Crayfish Austropotamobius pallipes relies on the availability of hiding places (Tricarico & Gherardi 2010). Shelter possession directly affects individual survival, especially through decreased risk of inter- and intra-specific predation (Blake, Nyström & Hart 1994; Söderbäck 1994; Steele, Skinner, Alberstadt & Antonelli 1997; Figler et al. 1999). Undoubtedly, decreasing mortality caused by fighting, following cannibalism and due to increasing number of crawfish harvested, will benefit the crayfish farmers. In crayfish aquaculture, the benefit of the provided refuge in a commercial production setting must be weighed against its cost and longevity, as well as the conditions of the particular management scenario into which it may be incorporated.
Crayfish are known to be agonistic intraspecifically (Nakata & Goshima 2003) and interspecifically (Capelli & Munjal 1982; Miller et al. 1992; Söderbäck 1994; Vorburger & Ribi 1999), fighting for crucial and limited resources including shelters and food. The winner lowers its aggression after it occupies a shelter. The resources are then partitioned based on the fight-determining hierarchy (Bovbjerg 1953; Issa, Adamson & Edwards 1999; Herberholz, McCurdy & Edwards 2007; Martin & Moore 2008). When the shelter is absent, the dominant Red Swamp Crayfish Procambarus clarkii (Girard) engages in aggressive behaviour and borrows shelters, whereas the subordinates behave submissively and do not borrow much (Herberholz, Sen & Edwards 2003), albeit with locomotor activities unimpaired (Herberholz et al. 2007). The occurrence of shelters increases the aggressive level among different crayfish species (Capelli & Hamilton 1984), and the hierarchy dominance order is established by the fierce form of fighting (Bovbjerg 1953; Issa et al. 1999). Moreover, in P. clarkii, the establishment of linear dominance hierarchy, which can be rapidly and stably formed (Copp 1986), is helpful to decrease the frequency of agonistic behaviour (Issa et al. 1999).
Crayfish use natural crevices and/or they build burrows for their shelter (Tricarico & Gherardi 2010). The shapeless darkness/shadow and the opaque solid shelters with a fixed shape are two major shelter types (Alberstadt, Steele & Skinner 1995; summarized by Steele et al. 1997), and both can arouse the shelter-seeking behaviour of crayfish (Alberstadt et al. 1995).The features of shelter include the darkness, the thigmotactic cues and their combination (Antonelli, Steele & Skinner 1999). In juveniles and adults of P. clarkii, darkness appears to be the controlling factor in the cover-seeking behaviour (Antonelli et al. 1999); however, the function contributed by the only factor of shadow/darkness in the settings, where other factors (e.g. thigmotactic cues) are excluded, was neglected in previous studies relevant to crayfish.
It is presumable that shadow reduced the intensity of within-group communication via untransformed chemical cues and visual cues, which along with combinations of different senses may play a role in mediating recognition in crustacean hierarchies (Skog, Chandrapavan, Hallberg & Breithaupt 2009). Recently, the role of the olfactory and visual pathway in the behaviour of crayfish has been abundantly researched (Ameyaw-Akumfi 1977; Copp & Watson 1988; Dunham & Oh 1992; Corotto, Bonenberger, Bounkeo & Dukas 1999; Schneider, Schneider & Moore 1999; Schneider, Huber & Moore 2001; Breithaupt & Eger 2002; Correia, Bandeira & Anastácio 2007). Olfactory pathway influences several aspects of fighting behaviour (Horner, Schmidt, Edwards & Derby 2008) in crayfish, and so does visual signals.
Presumably, shadow/darkness may play an important role in crayfish's growth. In this study, we aim to investigate the effect of shadow on healthiness, survival and growth of P. clarkii. We take interest in whether only darkness can work like the opaque solid shelter for crayfish P. clarkii or not, and hypothesize that, (1) Shadow works in a way similar to what the opaque solid shelter does in increasing the healthiness, survival rate and growth rate of P. clarkii; and an alternative hypothesis is, (2) Shadow works negatively on P. clarkii, which show increased injuries, mortality and declined growth rate. In contrast to hypothesis 1 and 2, the null hypothesis is that the shadow has neither positive nor negative effect on the experimental animals.
Material and methods
Experimental animals and maintenance
The specimens of Red Swamp Crayfish P. clarkii were provided by the Institute of Freshwater Aquaculture of Jiangsu Province (IFAJ). The experiments were performed in Lukou, the research base of the IFAJ. Before the experiments, about 3000 juveniles were maintained in eight cement aquariums (each 5.0 × 10.0 × 0.6 m deep, water depth 0.4 m) with natural pond water (pH 7.0–8.0, dissolved oxygen >4.0 mg L−1) for 7 days. A total of 2640 sexually immature animals of both sexes were chosen by visual judgment. All of them were active, undamaged (with both chelae and all the walking legs), in inter-moult (evaluated by hardness of the carapace) and of similar body size (mean body weight 2.29 g, mean length 3.50 cm). Exact sex ratio was ignored (Figiel, Babb & Payne 1991). The diet for crayfish during maintenance and experiments was prepared in our laboratory (fomula: fishmeal 14%, bean cake 6%, rapeseed cake 5%, peanut cake 5%, fish oil 1.7%, peanut oil 1.7%, starch 52%, zeolite powder 6%, monocalcium phosphate monohydrate 2.2%, sodium chloride 0.4%). Through direct observation throughout the experiments, the tested animals consumed this artificial diet well. During the experiments, the water temperature was ~20–28°C.
Design of experimental settings
We used 33 cement aquariums (each 2.0 × 2.0 × 0.6 m deep), where water was maintained at a depth of 0.40 m throughout the experiments. Aquariums were located on the exposed ground where sunlight could reach without interception. Every aquarium contained 80 individuals, which were randomly chosen from the 2640 experimental animals. The designed density of experimental animals, 20 ind. m−2, was considered as a safe density for P. clarkii rearing, and was used as a starting density in studying the density effect of this species (Ramalho, Correia & Anastácio 2008). We designed eleven treatments, each of which had three replicates (R1, R2 and R3, Table 1). Artificial shadow was produced by placing boards (40 mm thick and sunlight was completely sheltered) of different breadths (ten types 2.00, 1.60, 1.20, 0.80, 0.53, 0.40, 0.27, 0.27, 0.20 and 0.13 m respectively), placed on the top of the aquariums (Table 1) and the distance between board and water surface was 0.20 m. This provided both different shaded areas and shadow of the same area, but with different partition by re-arranging boards. At daytime, the transition between the shadow and the bright zone was clear-cut, and crayfish usually stayed in the shadow (through direct observation).
| Treatments | Shaded rate (%) | Number of shadow partition(s) | Number of unshaded zone(s) | Number and breadth of board(s) |
|---|---|---|---|---|
| 1 | 100 | 1 | 0 | 1, 2 × 2 m2 |
| 2 | 80 | 1 | 1 | 1, 2 × 1.6 m2 |
| 3 | 80 | 3 | 2 | 3, 2 × 0.53 m2 |
| 4 | 80 | 6 | 5 | 6, 2 × 0.27 m2 |
| 5 | 60 | 1 | 1 | 1, 2 × 1.2 m2 |
| 6 | 60 | 3 | 2 | 3, 2 × 0.4 m2 |
| 7 | 60 | 6 | 5 | 6, 2 × 0.2 m2 |
| 8 | 40 | 1 | 1 | 1, 2 × 0.8 m2 |
| 9 | 40 | 3 | 2 | 3, 2 × 0.27 m2 |
| 10 | 40 | 6 | 5 | 6, 2 × 0.13 m2 |
| 11 | 0 | 0 | 1 | Without board |
The experiment lasted for 4 weeks from June 15, 2003 to July 15, 2003. During the experiments, the diet mentioned above was provided every 2 days, and the rearing water was constantly aerated. No escaper was observed. The designations of treatments were showed in Table 1.
Data collection
The dead crayfish, if any, were checked, recorded and removed every day. By the end of the experiments, we counted, (1) The number of survivals; (2) The number of survivals with one or two chelae lost (abbreviated as SLC in the following text; one or two were not distinguished in data collection), the percentage of which is used to estimate the intensity of agonistic disputes in P. clarkii (Bechler, Deng & McDonald 1988; Holdich 1993); and measured (3) The body weight of survivors individually. Body weight (accurated to 0.1 g) of crayfish was measured after leaving it on dry tissue to dry for 10 min.
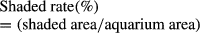
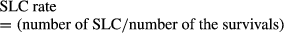
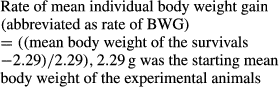


Data analysis
Data were analysed using software spss (version 13.0 for Windows, SPSS Inc., Chicago, IL, USA). Descriptive statistic: means ± standard deviation. The differences among the treatments were analysed using one-way anova. The Homogeneity of variance was tested using Levene's Test. Bonferroni correction was employed under equal variances situation; otherwise Tamhane's T2 was used. Differences were regarded as statistically significant at P < 0.05.
Results
In general, larger shadow area can significantly improve survival of juveniles (Fig. 1a). The significant differences of mortality occurred among treatments with shaded rates of 0%, 40% and the treatments with shaded rates larger than 60% (P0% vs. 40–100% < 0.001; P40% vs. 60–100% < 0.05). The treatment where no shadow was provided had the highest mortality. The treatments with shaded rates of 60%, 80% and 100% did not show pairwise significant difference (P60% vs. 80% = 0.409; P60% vs. 100% = 0.307; P80% vs. 100% = 1.000).
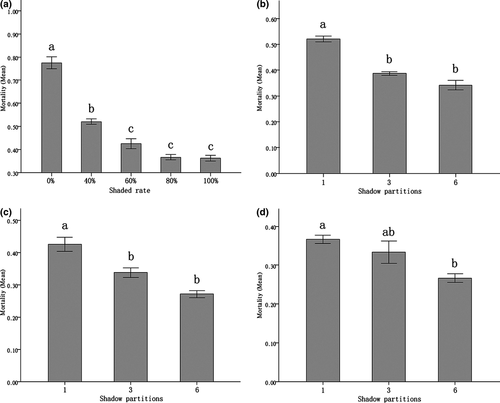
With the equal shaded areas, the occurrence of more shaded zones was better for reducing the mortality of juveniles with only one exception that when the shaded rate was 80%, the treatment of three-partitioned shadow did not significantly differ from that of the treatment of one shaded zone and that of six-partitioned shadow (P1 vs. 3 = 0.027; P3 vs. 6 = 0.027). When the shaded rate reached 40%, mortality of the treatment with one shaded zone was significantly higher (P1 vs. 3 < 0.005; P1 vs. 6 < 0.001) than the treatments equipped with three- and six-partitioned shadow, respectively, which did not show significant difference in mortality (P 3 vs. 6 = 0.139) (Fig. 1b). In the treatments of 60% shaded rate, the mortality most significantly declined with the increase in the number of partitioned shadow (P1 vs. 3 < 0.05; P1 vs. 6 < 0.005; F = 0.082, P3 vs. 6 = 0.083) (Fig. 1c). When the total shaded area reached 80%, the mortality of the treatment with one shaded zone was significantly higher than that with six-shaded zones (P1 vs. 6 < 0.05), and both the mortality difference between one-shaded zone treatment and three-partitioned treatment (P 1 vs. 3 = 0.790), and the difference between three- and six-partitioned treatment, were not significant (P3 vs. 6 = 0.146) (Fig. 1d).
In the case of one shaded zone, no significant difference in BWG occurred among treatments with different shaded areas (Fig. 2a). When the shaded rate reached 40%, the difference of BWG between one-shaded zone treatment and three-partitioned treatment was not significant (Fig. 2b). Under 60% shaded condition, the BWG differences between one- and three- and between one- and six-partitioned treatments were significantly different (P1 vs. 3 = 0.038; P1 vs. 6 = 0.046),whereas the BWG were not significantly different between three- and six- partitioned treatments (P3 vs. 6 = 1.000) (Fig. 2c). In the case of 80% shaded condition, the BWG values were significantly different among one-, three- and six-partitioned treatments (P1 vs. 3 = 0.005; P1 vs. 6 = 0.000; P3 vs. 6 = 0.023), and with a declined tendency (Fig. 2d).
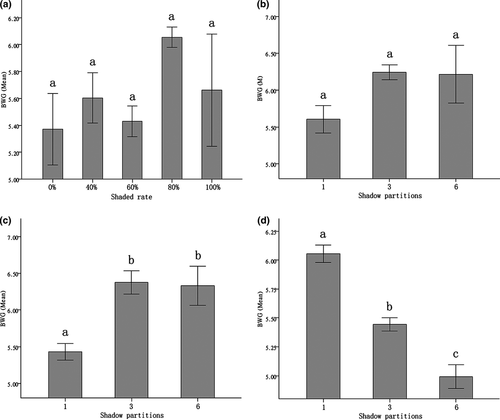
In the case of one shadow provided, no significant difference among SLC rates resulted from different shaded-ratio treatments as shown in the (Fig. 3a). When the shaded rate was 40%, the SLC rate of three treatments did not significantly differ (Fig. 3b). Under 60% shaded condition, one-shaded zone treatment produced a significantly higher SLC rate than those of three- and six-partitioned treatments (P1 vs. 3 = 0.008; P1 vs. 6 = 0.023), and the latter two treatments showed no significant difference (P3 vs. 6 = 1.000) (Fig. 3c). When the shaded area increased to 80%, the SLC rates only differed between of one- and six-partitioned treatments (P1 vs. 6 = 0.049) (Fig. 3d).
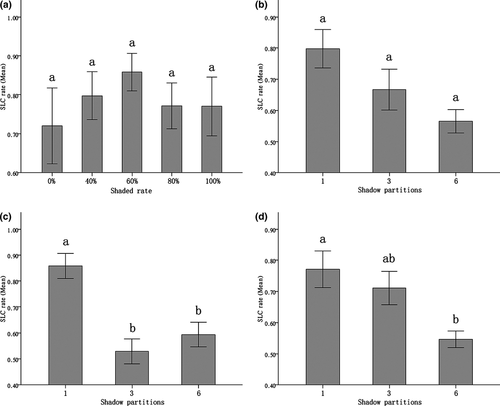
When only one shaded zone was provided, the variances of individual body weight, represented by CVSW, resulted from 40%, 60%, 80% and 100% shaded conditions, which did not significantly differ from each other, and were significantly lower than the variance resulting from the treatment with no shaded zone provided (P0% vs. 40–100% = 0.000~0.005 < 0.01) (Fig. 4a). In the case of 40% shaded condition, the variances of individual weight resulted from one- and six-partitioned treatments that were significantly different from each other (P1 vs. 6 = 0.033) (Fig. 4b). When more shaded area was provided, as in 60%, the variance of individual weight from treatment with one shaded zone was significantly larger than those from three- and six-partitioned treatments (P1 vs. 3 = 0.021; P1 vs. 6 = 0.002). Three- and six-shaded zone treatments showed not enough difference in individual weight variance (P3 vs. 6 = 0.097) (Fig. 4c). Under the condition of 80% shaded treatment, significant difference in CVsw did not occur (Fig. 4d).
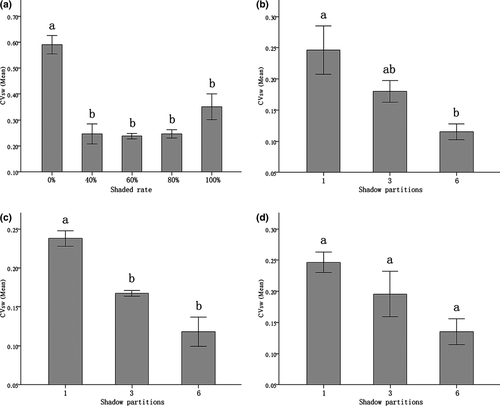
Summary of results
- The shadow significantly affected the survival rate of juvenile P. clarkii. Compared with unshaded condition, the survival rate increased with the increase in shadow area. The shadow area contributed less to the body weight gain and the SLC rate of the survivals. However, the growth variance of the juveniles under shadow conditions, although did not differ significantly among different treatments, under 40–100% shadow conditions, it was significantly lower than those maintained without shadow provided.
- Under recent experimental settings, generally, more partitioned shadow resulted in lower mortality.
- When 60% of shaded rate was provided, more partitioned shadow, in comparison with only one shadow, led to a higher body weight gain. When the shadow area reached 80%, the body weight gain decreased with the increased fragmentation/partition of the shadow.
- The more the shadow was fragmented/partitioned, the fewer were the injury events, represented by SLC rate, and the lower the body weight variance observed.
Discussion
The results rejected the null hypothesis proposed, i.e. there is a certain association between the availability of shadow and both the healthiness and the survival of the juvenile P. clarkii. The shadow partition contributed much to the decrease of injury events and body weight variance, both of which are important factors in crayfish farming. The significant decreases in mortality and SLC rate, against the increases of both individual body gain and survivals’ body weight gain indicates that the negative outcome of agonistic behaviour leading to limb/claw loss or death, which is commonly seen in crayfish, was reduced by the provision of shadow, which is an apparent condition increasing habitat complexity. A similar effect is seen in juvenile signal crayfish Pacifastacus leniusculus; the presence of shelter has a marginal probability in increasing the number of animals without injuries (Savolainen, Ruohonen & Tulonen 2003). Individual survival can also be directly affected by the ownership of solid shelter, especially through decreased risk of inter- and intra-specific predation (Blake et al. 1994; Söderbäck 1994; Steele et al. 1997; Figler et al. 1999). This result partially supports the ideas raised by Wall, Paterson and Mohan (2009) in the study of mud crab (Scylla serrata) that behaviour contributes to growth rate and might be a focus of genetic selection for more uniform growth.
The outcomes of CVSW under different shadow conditions were obviously different. This is an indication that shadow distribution can affect competition intensity among crayfish. Darkness is the controlling factor in the cover-seeking behaviour of both juvenile and adult P. clarkii (Antonelli et al. 1999). As a result of shelter-seeking behaviour, crayfish were relatively inactive when staying in shadow, which can also affect vision which is very important in agnostic communication (Bruski & Dunham 1987; Crook, Patullo & Macmillan 2004) and conspecific recognition through highly variable facial features (Van der Velden, Zheng, Patullo & Macmillan 2008). Hence in shadow, the fighting bouts of juveniles would be affected by the reduction of visionary communication. Previous study suggests that under dim light, both males and females fight more frequently when they are dark-adapted (Bruski & Dunham 1987). However, our study yielded the opposite result, where the significantly lower mortality of the crayfish maintained in darkness, especially in 100% shaded experiments, suggests less fiercer fighting. In addition, more partitioning resulted in smaller groups, which led to fewer fighting bouts. The shadow partitioning allowed the establishment and maintenance of small groups of crayfish within it and worked equivalently as a solid shelter in releasing agonistic interactions among crayfish individuals (Baird, Patullo & Mcmillan 2006; Patullo, Baird & Macmillan 2009), at least by providing heterogenous/fragmented habitats. Therefore, shadow and partitioning, if properly provided, can effectively alleviate the conflict among animals.
Crayfish are able to recognize the social status of potential fight opponents and avoid energetically costly and potentially injurious interactions (Issa et al. 1999; Breithaupt & Eger 2002; Horner et al. 2008), which were well avoided by providing and partitioning artificial shadow in this study. Another factor influencing the outcome of agonistic behaviour of crayfish is the relative size of shelter, as for crayfish Orconectes rusticus, animals in small shelter treatments initiate and win more fights, because they use differing shelter sizes to alter self-assessment of body size (Percival & Moore 2010). In this study, the shaded zone which was presumed to be the working shelter, in comparison with the body size of any individual crayfish, was large enough to nullify the possible effect of different shadow area working on crayfish.
It should also be noticed that our research just shows the limited similarity of shadow to real solid shelter. However, this study highlights the value and the practical possibility of the shadow in crayfish farming, where high survival rate, good health state and uniform growth are urgently required. Compared with the solid shelters traditionally supplied to the aquariums and settled on the bottom, the shadows are cheap and easy to be prepared and easier for daily maintenance. The proper arrangement of shadow incorporated in crayfish farming settings, could therefore benefit the crayfish farmers by direct gain in the reduction of the crayfish death rate, the injury rate and in reaching the uniform growth to meet the market needs, and also directly by reducing the cost in aquaculture equipments, daily maintenance and final harvest.
Acknowledgments
We thank colleagues from IFAJ for assistance in maintenance of crayfish, two anonymous referees for comments on the manuscript. Funding came from project of Ministry of Science and Technology of China (2006FY111000).




