Histological evidence of accumulation of iron in postlarvae of red abalone, Haliotis rufescens
Abstract
The effect of iron on abalone postlarvae (Haliotis rufescens) was investigated in a controlled-culturing system. Three iron concentrations (0.15, 1.5 and 15 mg L−1 of Fe) and a control (no iron added) were used to culture H. rufescens postlarvae while being fed the diatom Navicula inserta over 10 days. Results indicate that H. rufescens postlarvae accumulate iron granules in the stomach, digestive gland and mantle, but not in the gills or other tissues. The number and diameter of iron granules in tissues increased with increasing iron concentration in the culturing environment. The iron accumulation is assumed to have been acquired in the digestive system through the iron-enriched diatom feed and in the mantle through subcutaneous iron transfer. The lack of iron granules in the gills suggests that iron is not absorbed through the respiratory system, as is the case for many filter feeding bivalves. Exposure to the highest iron concentration (15 mg L−1) resulted in tissue abnormalities where granules accumulated, and may have significantly affected the health of H. rufescens postlarvae. These findings provide valuable information for the regulation of appropriate iron levels within aquaculture settings and highlights the importance of monitoring iron levels within abalone larval culturing environments.
Introduction
For most marine invertebrates, such as abalone, the larval stage is one of the most vulnerable times in their life cycle. After the first week in the water column, abalone larvae undergo initial settlement and metamorphosis. Once in their benthonic life stage, postlarvae begin to feed on microalgae, until they complete the development of the radula and digestive system to consume macroalgae. The post-larval period is a critical step in their development, and requires a good supply of biofilms with diatoms and bacteria (Searcy-Bernal 1996). However, during the first few weeks after settlement, massive mortalities (>90%) often are observed, especially if the complex of bacteria and diatom biofilms are too dense (Hahn 1989; Searcy-Bernal, Salas-Garza & Flores-Aguilar 1992a; Searcy-Bernal, Salas-Garza, Flores-Aguilar & Hinojosa-Rivera 1992b). This density-dependent mortality appears to be related to increased susceptibility to post-larval stresses, such as oxygen saturation and boundary layer conditions (Searcy-Bernal 1996). Postlarvae also may be exposed to high concentrations of environmental contaminants (e.g., heavy metals, pathogens), and chemical fluctuations in their surroundings, which often cause high mortalities. Within these stressful environments, heavy metals are particularly dangerous for marine invertebrate larvae and early juveniles, since these chemicals are easily incorporated and accumulated in soft tissues (Chang & Reinfelder 2002; Viant, Walton, TenBrook & Tjeerdema 2002; Ferrer, Andrade, Asteasuain & Marcovecchio 2006; Gorski & Nugegoda 2006a,b). For example, in crab larvae (Cancer magister) and in embryos of two bivalves (Crassostrea gigas and Mytilus edulis), Martin, Osborn, Billing and Glickstein (1981) found toxic and lethal effects of a range of heavy metals, including copper, mercury, silver, zinc, arsenic, cadmium, nickel, lead, chromium and selenium. In fact, all biologically essential (e.g., aluminium, arsenic, chromium, cobalt, copper, iron, zinc) and non-essential (e.g., cadmium, gold, lead, mercury, silver) metals have the potential to be toxic for marine invertebrates at a given concentration (Viarengo 1989; Rainbow 2002). The influence of heavy metals may be expressed at various levels of organization, including molecular, cellular, tissue, organism and community (Gagneten & Vila 2001; Kucuksezgin, Kontas, Altay, Uluturhan & Darilmaz 2006; Szefer, Fowler, Ikuta, Paez Osuna, Ali, Kim, Fernandes, Belzunce, Guterstam, Kunzendorf, Wolowicz, Hummel & Deslous-Paoli 2006), and their effects may extend to different biological activities, such as growth, morphology, behaviour, reproduction, settlement and recruitment (Rainbow 1995; Goodyear & McNiell 1999). Furthermore, since most metals cannot be metabolized, they are either accumulated in the body or are excreted at various rates depending on the organism and the tissues (Goyer 1992). Marine larvae may have specialized cells whose function is the sequestration of iron and its storage as a less toxic compound after binding it with other molecules (Tsioros & Youson 1997a). Iron-rich organs and tissues (e.g., kidneys, gills, adipose tissues, epidermal layer) may show distinctive granules where iron is accumulated. These granules usually indicate the deposition of iron in macrophages which may be eliminated through specialized tissues, such as kidneys, or through exfoliation of epithelial cells within the tissues (Tsioros & Youson 1997b). However, when the iron concentration is too high for it to be eliminated or stored effectively, toxicity may be apparent through degenerative tissue manifestations (e.g., fibrosis, cirrhosis).
Within culturing systems, such as hatcheries and nurseries, the concentration of heavy metals can be controlled to minimize or eliminate their effect on larval production. However, the range of non-lethal effects and accumulation mechanisms of various heavy metals on different invertebrate larvae remains unclear. Iron (Fe) is commonly added to culturing systems to enrich microalgae, which are fed to invertebrate larvae. Even though it is recommended that hatchery water contains <0.5 mg L−1 total iron concentration (Tucker 1991), microalgae may accumulate up to 200 mg L−1 of iron in various bound and unbound states (Richmond 2004). In addition, final concentrations available to larvae in a culturing environment will vary depending on physiochemical conditions, such as the redox potential, pH and dissolved CO2 (Olsson 1998). Iron also can be found as a part of chemical complexes, which may be incorporated into the larvae through various pathways. Some iron availability in the culturing environment is important since larvae will actively uptake iron to fulfil many physiological activities, including the production of cytochromes and other enzymes, but exceedingly high iron concentrations in their bodies may become toxic over time. For example, ionic (unbound or ‘free’) iron (either in the Fe2+ or Fe3+ state) may interact with oxygen-derived products, which may have physiological consequences in the respiratory system (Tsioros & Youson 1997a).
Limited information exists regarding the effect of iron on early life stages of marine invertebrates. Gorski and Nugegoda (2006b) showed that iron, among other metals, had a toxic effect (mortality or abnormal morphology) on veliger larvae of the abalone, Haliotis rubra, after 48 h of exposure to a dissolved iron concentration of 4102 mg L−1. Iron also was found to be lethal to embryos of the yellow crab, Cancer anthonyi, after 7 days of exposure at a concentration of 1000 mg L−1, and it was observed to suppress hatching at extremely low concentrations (10 mg L−1), compared with other heavy metals (Macdonald, Schields & Zimmer-Faust 1988).
One of the methods used to identify potential sublethal effects of heavy metals is to locate where these elements are accumulated in the body. Hyne, Smith and Ellender (1992) investigated the accumulation of iron in adult abalone (H. rubra) tissues through biochemical and microscopic techniques, and found an 80% (3130 μg g−1) accumulation of iron (as inorganic granules) in the digestive gland, and much lower concentrations in the gills, kidneys and other tissues. Few studies have focused on the accumulation of heavy metals in marine invertebrate larvae, even though this is of critical importance for the production of larvae in hatchery environments. Thus, this contribution is the first to clearly identify and characterize the accumulation of iron in post-larval tissues of the abalone Haliotis rufescens after short-term exposures to different iron concentrations.
Materials and methods
Source of larvae
Postlarvae of H. rufescens (2.4 mm in shell length) were obtained from the hatchery at Centro de Cultivo Pacífico Austral, Valdivia, Región de Los Ríos, Chile, where they were grown in a re-circulating water (2 μm filtration) system at 15°C with daily water exchanges. The postlarvae were transported to the pathology diagnostic laboratory BIOVAC, Puerto Montt, Región de Los Lagos, Chile, for further experimentation.
Macroscopic and microscopic analyses
The postlarvae were first measured and examined under a stereo microscope to identify possible abnormalities in shell and soft tissue morphology, and to observe tentacular and pedal retraction responses following physical contact with a thin brush. These tests were performed only to corroborate that the larvae were in healthy condition to start the experiment. To identify the histological condition of the postlarvae (pre-treatment or control condition), about 60 postlarvae were placed in each of eight histological cassettes and submerged in Davidson fixative at 10% glacial acetic acid for 6 h. The cassettes were imbedded in paraffin, from which histological sections (5–6 μm) were cut with a microtome. A haematoxylin–eosin stain (Luna 1968) was used to emphasize the biological tissue, structures and iron granules. An optical microscope was used to visualize the thin sections, which were photographed with a Leica DS-320 with IM-100 software.
Iron exposure assays
The lengths (anterior to posterior region) of 30 postlarvae were measured to obtain an initial size average and standard deviation. Then, 90 postlarvae were acclimatized for 7 days in cleaned aerated seawater at a salinity, temperature and pH of 31 ppm, 16–20°C and 7.2–7.8 respectively. The postlarvae were fed the diatom Navicula inserta during the acclimatization period. The N. inserta cultures were grown with F2 medium containing 100 μm L−1 of iron, with a photoperiod of 16 h light and 8 h darkness, and the same algal stock and light conditions were used for all treatments. After acclimatization, 24 postlarvae were randomly introduced into each of 12 plastic containers with 300 mL of clean seawater. The 12 containers were allocated into three treatments [0.15, 1.5 and 15 mg L−1 of ferrous ion, Fe2+ (added in the FeCl2 salt form)] and one control (no Fe2+ added) with three replicates each (three containers per treatment and control). The iron was dissolved in the water within each container, which already contained the diatom biofilms. The postlarvae were maintained in the containers for 10 days, during which time they continued to feed on N. inserta. Dead postlarvae were removed immediately and noted. The experimental larvae were transferred into new containers with clean filtered seawater and a new population of diatom biofilm with the appropriate iron concentration on day 4 to maintain the food supply and treatment conditions. On day 10, the postlarvae were removed and preserved for histological analyses. All histological sections were made as mentioned above. The total number of granules μm−2 (±SD) inside cells, and the mean diameter (±SD) of 10 randomly chosen granules were recorded for all samples examined under the microscope. The granules were clearly identified by their brown collocation. To avoid pseudo-replication, the data from seven to eight larvae within each replicate tanks were averaged to provide single measurements per replicate container (n = 3), which were then used to calculate means and standard deviations for each treatment and control. After checking that the data met parametric assumptions, two-way anovas with Tukey tests were performed to test differences in mean number and diameter of granules among treatments and tissues. Statistica 6.0 software (StatSoft Inc., Tulsa, OK, USA) was used for statistical analyses.
Results
Macroscopic analyses
The results from the macroscopic analysis of shell and soft tissue morphology indicate no differences within or between treatments and controls at the start of the experiment. Tentacular and pedal responses were rapid (healthy) and similar in all treatments and controls at the start of the experiment, and all animals appeared to feed well, as evidenced from grazing haloes around the postlarvae, and the presence of vast amounts of food in their digestive tracts. The experimental postlarvae had a shell length (±SD) of 2.4 ± 0.2 mm at the beginning of the assays. The level of mortality throughout the experiment was extremely low, with only one and two dead postlarvae in the 0.15 and 15 mg L−1 treatments respectively. No mortalities were observed in the other treatments or control.
Microscopic analyses
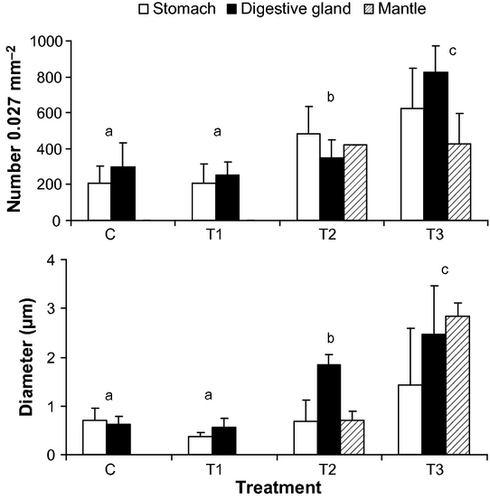
Based on the histological sections, there were no observable differences in the amount and location of iron accumulation, and the structure of the tissues in the initial postlarvae (evaluated upon arrival to the laboratory) compared with those used as control in the exposure experiment. Analyses of these sections indicate that inclusion of iron in H. rufescens postlarvae takes place as distinctive granules in the stomach, digestive gland and mantle, but not in the gills or other tissues in the postlarvae.
Results from the exposure experiment showed no statistical difference in the number of iron granules, or granule diameter accumulated in post-larval tissues within the control and 0.15 mg L−1 of iron treatment, but the number of granules and their diameters increased within treatments with 1.5 and 15 mg L−1 of iron (Fig. 1, Tables 1, 2). In addition, iron granules were absent in mantle tissues of postlarvae exposed to control and 0.15 mg L−1 of iron conditions, and only appeared within these tissues in the 1.5 and 15 mg L−1 of iron treatments. Significant interactions for anova tests on number of iron granules and granule diameter reflect the different effects of iron concentration across the different tissues (Tables 1, 2).
| Source | d.f. | MS | F | P | |
|---|---|---|---|---|---|
| Treatment | 3 | 812 315 | 43.19 | 0.001 | |
| Tissue | 1 | 41 034 | 2.18 | 0.146 | ns |
| Treatment×tissue | 3 | 71 933 | 3.83 | 0.015 | |
| Error | 52 | 18 806 | |||
| Tukey non-significant test | 0 versus 0.15 mg L−1 | ||||
- Non-significant test is indicated with ‘ns’. Non-significant Tukey test is presented.
| Source | d.f. | MS | F | P |
|---|---|---|---|---|
| Treatment | 3 | 68.51 | 126.29 | 0.001 |
| Tissue | 1 | 48.40 | 89.22 | 0.001 |
| Treatment×tissue | 3 | 14.21 | 26.19 | 0.001 |
| Error | 592 | 0.54 | ||
| Tukey non-significant test | 0 versus 0.15 mg L−1 | |||
- Non-significant Tukey test is presented.
Exposure to 0.15 and 1.5 mg L−1 of iron did not produce observable tissue abnormalities in the mantle, head region, or digestive system of postlarvae (Figs 2-4). However, exposure to 15 mg L−1 of iron resulted in tissue abnormalities in these areas. The high inclusion of iron granules in the mantle and head region (especially in the nervous cephalic ganglia) appeared to be responsible for an extensive disintegration of connective tissue and muscular fibres in both anatomical areas. At the same high iron exposure, extensive accumulation of iron granules was observed in the digestive system and digestive gland. This accumulation was associated with the disintegration of connective tissue and disorganization of epithelial tissue.
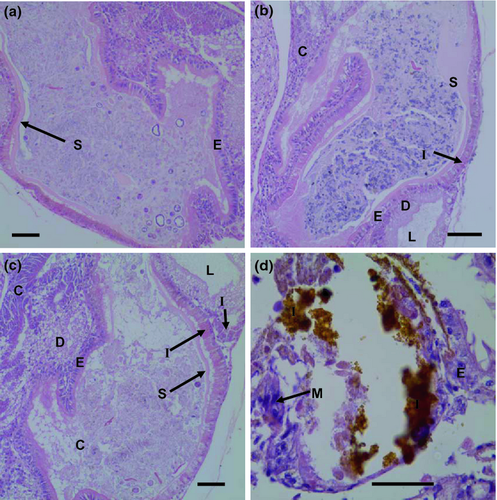
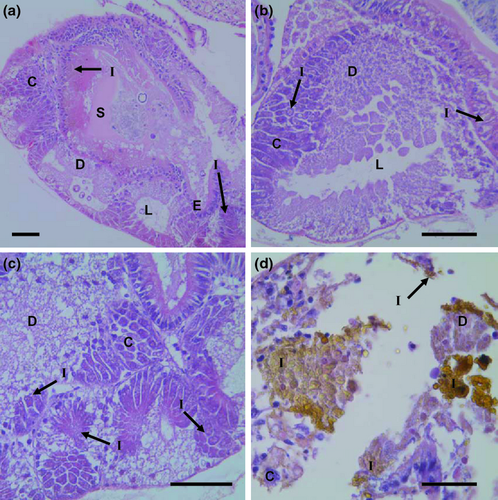
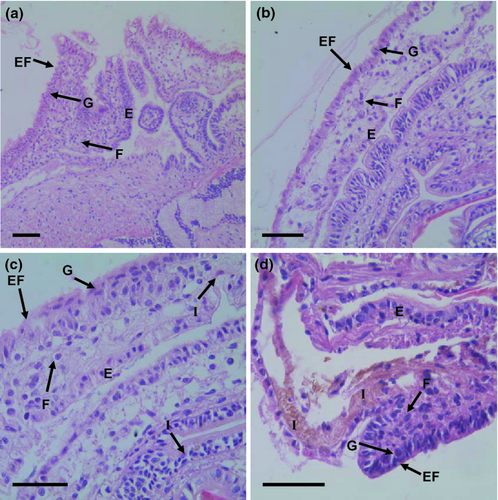
Discussion
This contribution is the first to report the accumulation of iron granules and subsequent tissue abnormalities in postlarvae of abalone. In this study, H. rufescens postlarvae exposed to elevated iron concentrations (>1.5 mg L−1) accumulated granules in the stomach, digestive gland and mantle, but not in the gills. Previous studies have reported iron granule accumulation in adult abalone (Campbell 1965; Bryan, Potts & Forster 1977; Bevelander 1988; Hyne et al. 1992), but not in postlarvae. Bevelander (1988) reported the accumulation of iron granules in epithelial tissues of the stomach and vacuoles in the digestive gland of H. rufescens adults. In addition, Hyne et al. (1992) described the presence of irregular granules in the digestive gland of adult H. rubra, which were composed of iron, calcium, copper, sodium, potassium and sulphur.
In the present study, H. rufescens postlarvae accumulated iron granules in the digestive system (i.e., digestive gland, stomach) and presented abnormalities in these tissues. The inclusion of iron granules is assumed to have been through the ingestion, digestion and assimilation of iron through its food (N. inserta), which was enriched with the iron added to the treatments. This is why iron granules were found only in the digestive system of the abalone postlarvae. Thus, the greater accumulation of iron granules in postlarvae maintained in treatments with 1.5 and 15 mg L−1 of iron reflect a greater incorporation of dissolved iron in the microalgae, which were then ingested by the postlarvae. Heavy metal assimilation through the diet has been shown to occur for various marine invertebrates (Rainbow 2002), and is thought to be one of the most important pathways of heavy metals incorporation in marine invertebrates (Weeks & Rainbow 1993). The presence of iron granules in mantle tissues suggests that since the mantle is in direct contact with the microalgae during the feeding process, subcutaneous transfer of iron may have occurred in these tissues. In addition, dissolved minerals, especially positive metal ions (e.g., calcium), are actively taken up by the mantle for shell deposition. Thus, it is possible that iron ions may have been more readily incorporated into mantle tissues in larvae exposed to treatments with high iron concentrations.
In the case of the gills, the acquisition of iron would have required a direct absorption of this metal from the water to this respiratory organ. Clearly, this absorption did not occur, even at the high iron concentration of 15 mg L−1 used in the experiment. Alternatively, it is expected that iron concentration in the treatment water would have decreased as the microalgae incorporated this nutrient into their tissues, thus reducing the availability of dissolved iron in the treatments. Although the final iron concentration in the treatment water was not measured at the end of the experiment, it is unlikely that the amount of dissolved iron would have decreased enough to eliminate the concentration gradient across gill membranes, which causes metal absorption. Given that no iron granules were observed in the gills, it is inferred that direct absorption does not take place through the respiratory system in postlarvae of H. rufescens. This conclusion is contrary to other marine invertebrates, such as filtering bivalves, which are known to absorb large quantities of metals through their gills (Cheung & Wong 1992). The filtration system of these organisms actively traps particles which are concentrated in the gill mucus, and heavy metal exposure is observed in the gill tissue (Cheung & Wong 1992). It is possible that the grazing activity of abalone, rather than the filtering method of many bivalves is responsible for the lack of iron accumulation in the gills of abalone postlarvae. However, further studies need to be conducted to investigate this difference.
The accumulation of iron granules in animals has been attributed to a mechanism designed to eliminate such toxins (Olsson 1998). Thus, marine invertebrates, such as abalone, tend to accumulate heavy metals in different organelles within cells to avoid direct interaction with the cytoplasm. These contaminants eventually are eliminated through specialized excretory systems, such as kidneys, digestive glands, intestine and mantle (Olsson 1998). Furthermore, Skinner, Turoczy, Jones, Barnett and Hodges (2004) found a concentration of iron (7–13 μg g−1 of dry weight) in adult H. rubra obtained from culturing systems, although natural populations of the same species contained 8–31 μg g−1 of dry weight. Such studies suggest that adult abalone can accumulate iron with little observable health consequences. However, the present study demonstrates that this is not the case for postlarvae, which developed abnormal tissues when exposed to iron concentrations of 1.5–15 mg L−1. Gorski and Nugegoda (2006a) also found abnormal development in veliger larvae of H. rubra after the fertilized eggs were exposed to 4.1 mg L−1 of iron. Abnormal tissue development through the digestive system appears to be related to the mechanisms of incorporation, accumulation and elimination of heavy metals, which vary from species to species (Cheung & Wong 1992).
Finally, this contribution highlights the fact that even low concentrations of dissolved iron (<0.15 mg L−1) can have a health effect on abalone postlarvae. This is especially important in hatchery environments where iron is routinely used to enrich microalgae, which are then fed to postlarvae, and where steel fittings and pipes may be used in the water circulation system. Although iron concentrations up to 15 mg L−1 may not produce massive mortalities in the culturing environment, it is likely that abnormal tissue development may result from exposure to these concentrations, and may significantly reduce the abalone's natural ability to eliminate these potentially toxic chemicals. Abalone hatcheries may use this information as a guide to regulate iron levels within their facilities to improve the general health conditions of the culturing environment.
Acknowledgments
We are thankful to the hatchery of the Centro de Cultivos Pacifíco Austral for providing abalone postlarvae, and the Pathology Laboratory at BIOVAC S.A. for making their laboratory, equipment and technical staff available for this research. This project was supported by a FONDEF grant D04I1067-2004. We also thank two anonymous reviewers who improved this manuscript with their constructive comments.




