Growth performance of European whitefish [Coregonus lavaretus (L.)] under a constant light and temperature regime
Abstract
Growth, feed intake and maturation were monitored in a fast-growing benthivore morph of European whitefish [Coregonus lavaretus (L.)] held under constant light (LD 24:0) and temperature conditions (10°C) for 415 days. Their growth was best described by polynomial regression model and no indication of seasonal patterns (e.g. winter depression) in growth rates was found. There was no significant difference in food conversion ratio (FCR) between periods during the experiment and the FCR was favourable (0.84). Furthermore, there were no differences in growth between males and females. None of the fish became sexually mature by the time the experiment was terminated, although they had reached a mean size of about 444 g. The European whitefish seems to be a promising aquaculture species as the growth rate was relatively high with a remarkable homogenous growth performance between individual fish within each experimental unit, a relatively favourable FCR, a low degree of agonistic behaviour and negligible growth depression due to internal biological rhythms or gonad maturation. These results were obtained at relatively low temperatures that should be beneficial for sustainable cost-effective farming conditions in cold climate condition in northern Scandinavia.
Introduction
European whitefish, Coregonus lavaretus, is a highly valued cold-water fish that has the potential to become an important commercial species in fresh and brackish water aquaculture in northern Europe (Koskela, Jobling & Pirhonen 1997; Lehtonen, Peltonen, Heikinheimo, Saarijärvi, Saulamo, Vinni & Nurmio 1998; Ruohonen, Koskela, Vielma & Kettunen 2003; Känkänen & Pirhonen 2009; Siikavuopio, Knudsen & Amundsen 2010). The commercial aquaculture production of European whitefish is low, but has increased from about 50–1000 tonnes over the last 10 years and is predicted to reach 4000 tonnes in near future (Jobling, Arnesen, Befey, Carter, Hardy, LeFrancois, Keefe, Koskela & Lamarre 2010). Finland is at present the only country that has a significant production of farmed whitefish with an estimated value of €2.3 million for 2004 (source: Statistics, Finnish Game and Fisheries Research Institute). Thus, the whitefish farming industry is at its infancy in Norway as well as in the rest of northern Europe.
For new aquaculture initiatives to be viable and profitable, it is important to obtain basic information related to feed utilization and growth performance of a potentially new farming species. Fish growth and feeding are affected by a large number of abiotic and biotic factors (Brett 1979; Jobling 1994). The northern lakes in Norway have large seasonal variations in environmental conditions, with the temperature varying substantially during an annual cycle and the photoperiod changing from almost complete darkness in winter to continuous light in summer. The primary production, and hence the food availability, varies accordingly. Pronounced annual fluctuations in growth rates of fish are induced by these seasonal environmental variations (Sumpter 1992), mediated by factors such as photoperiod (Björnsson, Thorarensen, Hirano, Ogasawara & Kristinsson 1989), temperature (McKay, Friars & Ihssen 1984; Jobling 1994) and food availability (Brett 1979; Simpson & Thorpe 1997). In addition, growth rate also depends on physiological factors related to the onset of maturation (Le Bail 1988) and internal biological rhythms (Sæther, Johnsen & Jobling 1996; Tveiten, Johnsen & Jobling 1996). Generally, there is a marked increase in growth rate in spring and a depression during the autumn (Brown 1946; Higgins & Talbot 1985; Sæther et al. 1996; Tveiten et al. 1996).
Even though there exists only a single ancestor genetic linage of native whitefish in Fennoscandia (Bernatchez, Colombani & Dodson 1991; Partti-Pelinen 1991; Østbye, Bernatchez, Næsje, Himberg & Hindar 2005), the individual growth rates and other life-history traits are highly variable between different populations of whitefish (Lehtonen et al. 1998). The European whitefish is furthermore a highly polymorphic species and lakes in northern Fennoscandia may house monomorphic, dimorphic or trimorphic populations with large variation in biology, ecology and life-history traits including growth patterns (Lehtonen et al. 1998; Kahilainen & Østbye 2006; Siwertsson, Knudsen, Kahilainen, Præbel, Primicerio & Amundsen 2010). In northern Norway, several lakes harbour a fast-growing benthivore and a slow-growing planktivore whitefish morph (Amundsen 1988; Amundsen, Knudsen, Klemetsen & Kristoffersen 2004; Østbye, Amundsen, Bernatchez, Klemetsen, Knudsen, Kristoffersen, Næsje & Hindar 2006).
Perhaps the most important single factor that determines success in fish farming is the growth rate of the fish produced. For commercial aquaculture of whitefish, the fast-growing benthivore morph appears to have the most beneficial growth potential, compared with the slow-growing planktivore whitefish morph. There is a general lack of knowledge related to the growth potential of whitefish and the impacts of for e.g. heritability, maturation, endogenous rhythms and seasonality. The main objective of this study was to explore the growth capabilities of a subarctic European whitefish population and to survey the aquaculture potential of this species. The prime goal was to document the growth capacity of whitefish at a cost effective sub-optimal temperature (10°C). Secondly, we wanted to explore the possible seasonal variability in feed intake and growth rate, and growth variations related to sex and maturation status.
Materials and methods
Holding conditions and origin of fish
Eggs and milt were stripped from wild caught brood stock of a fast-growing benthivore morph of C. lavaretus (Stuorajavri, 69°04′N, 22°47′E, Northern Norway) in November 2002. The eggs were placed in 20 L incubators at ambient temperatures of 1–3°C at Tromsø Aquaculture Research Station, northern Norway (69°50′N, 18°55′E). After hatching, the larvae were transferred to start-feeding units and weaned on a formulated dry feed (AGLONORSE®, Trofi - Odd Berg Gruppen, Stakkevollveien, Tromsø, Norway) containing 59% protein and 21% crude oil. Following weaning the fish was fed on a commercially formulated feed until the end of the growth study (Skretting salmon feed), containing 50% protein and 23% crude oil. Water temperature was measured daily and kept stable at 10.0°C (SD ±0.5) during the trial period. The oxygen saturation was measured daily throughout the trial (OxyGard®, Handy Delta, OxyGard International A/S, Blokken, Denmark), and at no point were values below 80% recorded. The tank was cleaned once a week by flushing. Dead fish were removed, but not substituted. The fish were exposed to a continuous light regime (fluorescent lamp) with a light intensity of approximately 150 lx at the water surface.
Experimental design
On September 17th 2003, 225 fish were distributed randomly into six tanks of 0.084 m3 volume. On March 8th, 105 fish (35 fish in each tank) were anaesthetized (benzocaine, 0.05 g L−1) and individually tagged. Tags specially designed for small salmonids (Fingerling tag, Floy Tag Inc., Union Bay Place NE, Seattle, WA, USA), were sewn through the musculature just anterior to the dorsal fin. At the start and at monthly intervals throughout the trial the fish were anaesthetized, individually weighed and total length measured to the nearest 0.1 g and 0.1 cm respectively. The initial mean weight was 6.5 g (SD ±0.1) and did not differ significantly between replicated tank units. The growth study lasted for 415 days to end of October 2004.
From the start (day 1) until day 210, the fish were fed continuously (24 h) and in excess. At day 210 the fish were large enough for monitoring feed intake. The feed was then provided daily in excess for 6 h (03:00–09:00 hours) except for Saturday and Sundays (not fed). The reduced feeding period was an adaptation to the method used to measure feed intake on tank level, as it depends on retention of uneaten feed collected from the water outlet. The rations fed to the separate tanks were weighed and distributed from automatic feeders. Uneaten feed was collected 30 min after feeding in the morning and weighed immediately to measure feed intake according to Bendiksen, Jobling and Arnesen (2002).
All growth estimates in this study are based on individually tagged fish, whereas results on food conversion efficiency and daily feed consumption are based on the total biomass. The condition factor (CF) was defined as: CF = 100 W LT−3 where W is the weight of the fish and LT the corresponding total length. Food conversion ratio (FCR) was calculated as: FCR = (W2−W1)/C, where C is total feed intake between to sampling period.
Statistical methods
All statistical analyses were performed using Systat 12 (Systat software, San Jose, CA, USA). Growth trajectory was estimated using GLM, adding single variable using partial F-test (P < 0.05), resulting in a tree level polygonal fit of the line (Kleinbaum, Kupper & Muller 1988). Data of weight, length and CF are presented as tank mean ± standard deviation between tanks (SD) in the table. Possible changes in FCR during separate trial periods were tested by repeated measures anova (Zar 1996). Significance was assumed when P < 0.05.
Results
There were no significant differences in growth between replicate treatments at any time during the experimental period of 415 days. The basic characteristics for the experimental animals are shown in table (Table 1). The mean weight increased from 6.5 g (1.4 SD) at the beginning of the experiment to a mean of 443.8 g (85.9 SD) at day 415 when the experiment was terminated. A polynomial regression model account for more than 99% of the variation (r2 = 0.998) and the growth of the whitefish can be described by the growth equation Wt (g) = 9E−6 ×X3−0.003 × X2 + 0.605X + 0.048 (where Wt is fish body weight in grams and X is time in days from first feeding (start of the growth study; Fig. 1). There was in general a low variability in growth and size-structure between individuals, with a coefficient of variation (CV) between individuals below 20% at termination of the experiment.
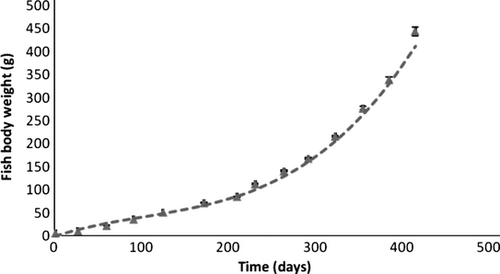
| n = 210 | Weight (g) | Length (cm) | CF | FCR |
|---|---|---|---|---|
| Day 0 | 6.5 (1.4) | 8.6 (0.5) | 1.0 (0.1) | |
| Day 90 | 36.4 (7.5) | 14.3 (0.9) | 1.2 (0.2) | |
| Day 210 | 93.8 (19.56) | 19.6 (1.2) | 1.3 (0.2) | |
| Day 320 | 185.4 (51.4) | 26.9 (1.5) | 1.4 (0.2) | |
| Day 415 | 443.8 (85,9) | 29.9 (2.3) | 1.7 (0.3) | |
| Day 210–415 | 0.84 (0.15) |
- a Results are given as mean (SD); n denotes number of fish in each experimental group.
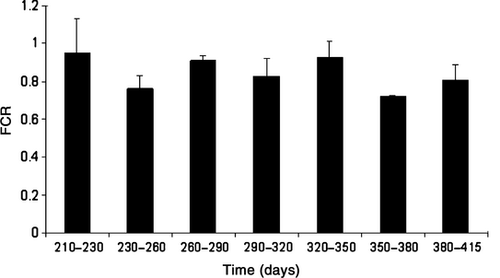
Condition factor increased from 1.0 at start to 1.7 at the end of the experiment (Table 1). The average FCR for the experimental period of 205 days was 0.84. There were no significant differences in FCR between subsequent time-intervals of 30 days (F6,14 = 0.914, P = 0.513; Fig. 2). At the end of the experiment the gonad index of all the fish was assessed as being immature and there was no significant difference in the weight of different sexes (F1,40 = 0.130, P = 0.720). Mortality was low during the whole study period as totally only 2.3% died. No visual injuries neither on fins nor on the body of the fish were observed, indicating low agonistic social interactions. This was also corroborated by visual inspections of the fish tanks.
Discussion
Until the present study, long-term experimental growth studies of the European whitefish held under constant temperature and light regimes have not been performed. Based on our findings, the whitefish seems to be a promising aquaculture species as the growth rate was relatively high and also very homogenous between individual fish within each tank, even though the temperature (10°C) most likely was suboptimal. Based on these experimental data the first growth model for whitefish at constant temperature and light was developed. Furthermore, in commercial aquaculture the FCR-ratio is the ultimate measure of growth-production and was found to be relatively high (0.84) during the experimental period (day 210–415). The observed FCR is comparable with other studies of whitefish (Känkänen & Pirhonen 2009), and nearly as high as for the commercial coldwater-adapted freshwater fish species, Arctic charr, that is regarded as a good food converter with a FCR that typically falls between 0.9 and 1.6 (Glebe & Turner 1993).
Throughout the experimental period, no indications of seasonal variation in feed intake or growth rate were observed, although the experiment was running for more than 1 year under constant light and temperature conditions. Seasonal effects on feed intake and growth are well documented for many fish species including several salmonid species (Houlihan, Boujard & Jobling 2001). Such temporal cycling in food intake and body condition seem to be most pronounced for those species that live at high latitudes and are adapted to large, but predictable seasonal environmental fluctuations (Loudon 1994; Tveiten et al. 1996; Jobling, Tveiten & Hatlen 1998). Furthermore, even when environmental cues such as photoperiod and temperature are kept constant; there appears to exist a persistent endogenous rhythm regulating the somatic growth of salmonids, including the cold-water-adapted Arctic charr (Sæther et al. 1996). The growth performance of whitefish recorded in this study contradicts these earlier results. Thus, as whitefish seem to lack seasonal fluctuations in growth rate under constant light and temperature conditions, but rather utilize feed to grow whenever available, the species appears to be favourable for aquaculture production. Growth depressions will hamper an economically viable production and put extra challenges to optimal management of the farmed fish.
In addition to seasonal depression in growth rates of cultured finfish, early maturation is considered disadvantageous in the commercial farming of salmonids, because the onset of sexual maturation often is accompanied by increased mortality, decreased growth rates and a reduction in flesh quality (Jobling, Jørgensen, Arnesen & Ringø 1993a). There were however, no indications of sexual maturation or growth differences between the genders at the end of this growth experiment in late autumn when this subarctic stock normally have their spawning season. This suggests that the experimental whitefish may have continued to grow without any growth depression due to maturation for at least one more successive year, and presumably achieved a much bigger size before harvest.
Our findings showed very homogenous size distribution between individuals at any time period of the year and indicated low agonistic behaviour and the absence of a strong dominance hierarchy within each tank, which also agrees with the lack of observed indices of aggression (bite marks, injuries). Similar results of homogenous growth and size-structure patterns between individual fish were also found in a recent study of whitefish at low winter temperatures (1°C, 3°C and 6°C; Siikavuopio et al. 2010) and in a previous study by Koskela et al. 1997;. Agonistic behaviour is commonly observed in other salmonids (e.g. Arctic charr) and is highly density-dependent (Jobling et al. 2010). The low variability in growth between individual whitefish is probably related to species-dependent behavioural traits such as the whitefish being regarded as a schooling species (Jobling, Koskela & Winberg 1999). To achieve a large size before maturation, low agonistic behaviour is a beneficial trait for the commercial industry and in addition, supports the appraisal of whitefish as a well-suited aquaculture species. These promising results were obtained at relatively low temperatures (10°C) which represent a beneficial quality for farming under the cold climatic conditions in northern Scandinavia and may reduce the heating cost of water considerably.
These promising growth results are obtained from the fast-growing benthivore morph which is ecologically diverged and genetically different to a co-occurring slow-growing planktivore morph in Stourajavri and elsewhere in northern Scandinavia (Amundsen et al. 2004; Østbye et al. 2006; Siwertsson et al. 2010). To our knowledge no such experimental growth data exist for slow growing morphs of whitefish. Thus, experimental growth studies of a slow-growing morph of Arctic charr with specific adaptations to forage in lean deep water environment (Klemetsen, Knudsen, Primicerio & Amundsen 2006; Knudsen, Klemetsen, Amundsen & Hermansen 2006) had higher growth rate (Klemetsen, Elliot, Knudsen & Sørensen 2002) then a genetically separated and ecologically distinct fast growing co-occurring morph (Westgaard, Klemetsen & Knudsen 2004; Amundsen, Knudsen & Klemetsen 2008). Klemetsen et al. (2002) suggested that the higher growth rate of the slowest growing morph is caused by genetic differences in the growth potential related to for e.g. improved feed-efficiency adaptations to lean foraging conditions in the wild. This suggests that experimental studies of the slow growing planktivore whitefish morph should be performed to test if they potentially could have a more beneficial growth potential for commercial aquaculture than that found from the fast-growing benthivore morph.
In conclusion, European whitefish seems to be a well suited candidate for intensive freshwater fish farming industry in northern Europe compared with other species that are associated with problems connected to early maturation, antagonistic behaviour and depressed winter growth (see e.g. Jobling, Jørgensen & Siikavuopio 1993b). Intensive rearing of European whitefish seems to give satisfactory results with relatively high growth, lack of seasonal fluctuations in growth rate, late maturity and low agonistic behaviour. However, knowledge about the optimum environmental conditions (e.g. temperature) in relation to growth of European whitefish is still limited. Further research is therefore needed to recognize the full growth potential, including differences between morphs and stocks, of the polymorphic European whitefish.
Acknowledgments
This work was financed by University of Tromsø and Nofima–Marin. We thank Laina Dalsbø, Cesilie Lien, Ivar Nevermo and Hugo Tøllefsen for their technical contribution to this work and Dr Philip James for comment on the article.




