Apparent digestibility coefficient of poultry by-product meal (PBM) in diets of Penaeus monodon (Fabricius) and Litopenaeus vannamei (Boone), and replacement of fishmeal with PBM in diets of P. monodon
Abstract
Nutrient apparent digestibility coefficients (ADCs) of pet food grade poultry by-product meal (PBM) were determined for black tiger prawn, Penaeus monodon and Pacific white shrimp, Litopenaeus vannamei by the indirect method (reference diet and test diet at 7:3 ratio). Subsequently, an 8-week growth trial was conducted to evaluate the effects of substitution of fishmeal (FM) with PBM in diets of P. monodon (initial weight = 0.21 ± 0.01 g). In the growth trial, six isonitrogenous and isoenergetic diets PBM0, PBM25, PBM50, PBM75, PBM100 and PBMA100, containing a gradient of PBM 0, 88.7, 177.4, 266, 354.7 and 354 g kg−1 to replace 0, 92.5, 185, 277.5, 370 and 370 g kg−1 FM were fed to four replicate groups respectively. The diet PBMA100 was supplemented with DL-Met to be similar to PBM0. The results showed that both P. monodon and L. vannamei had relatively high ADC of crude protein (77.6% and 84.2% respectively) and gross energy (72.8% and 84.0% respectively) for PBM. Litopenaeus vannamei showed significantly higher digestion ability for PBM than P. monodon (P < 0.05). In growth trial, no significant difference in growth performance was observed among shrimp fed the experimental diets. DL-Met supplementation did not improve the growth of P. monodon. PBM is a suitable protein ingredient for P. monodon feeds and can be used up to 354.7 g kg−1 to totally replaced FM.
Introduction
World shrimp production in 2007 was 6.529 million tons (MT) out of which 3.276 MT (50.2%) was from aquaculture production (http://www.fao.org/docrep/010/ah876e/ah876e10.htm). Black tiger prawn, Penaeus monodon (Fabricius) and Pacific white shrimp, Litopenaeus vannamei (Boone), are the two main species cultured in the world. Fishmeal (FM) is the most important protein source for shrimp feed because of its known nutritional and palatability characteristics. Commercial shrimp feeds typically contain above 25% of FM in formula (Tacon & Barg 1998); this meant that approximately 819 thousand tons of FM was used in the production of shrimp feeds just in 2007. The demand for fish feeds is rising by some 5% per year because production from aquaculture is rising (Tacon & Barg 1998). Given the growing demand by aquaculture industries for FM and its limited supply, prices are likely to continue to increase. Therefore, it is necessary to find alternative sources of high-quality protein using in shrimp feed.
Quantity and quality of dietary protein are primary factors influencing shrimp growth and feed costs. Knowledge of the nutrient requirements of grass shrimp and white shrimp nutrition has been improved greatly in recent years (Samocha, Davis, Saoud & DeBault 2004; Amaya, Davis & Rouse 2007). Considerable efforts had been aimed at defining the various vegetable or animal feedstuffs that might replace FM in shrimp feeds such as, soybean meal (Lim & Dominy 1990); lupin meal (Sudaryono, Tsvetnenko & Evans 1999); soy protein concentrate (Paripatananont, Boonyaratpalin, Pengseng & Chotipuntu 2001); solvent-extracted cottonseed meal (Lim 1996); rendered meat and bone meals (Forster, Dominy, Obaldo & Tacon 2003); and some blend of vegetable and/or animal proteins (Davis & Arnold 2000; Amaya et al. 2007). Poultry by-product meal (PBM) consists of terrestrial rendered clean parts of the carcass of slaughtered poultry such as necks, feet, undeveloped eggs and intestines (Yu 2007). The protein level and amino acids profile of pet food degree PBM are close to FM. There have been a few studies carried out on L. vannamei to test the replacement of FM with PBM, and general research studies showed that dietary FM could be partially replaced w/w by PBM without loss in performance (Samocha et al. 2004; Amaya et al. 2007; Cruz-Suárez, Nieto-López, Guajardo-Barbosa, Tapia-Salazar, Scholz & Ricque-Marie 2007). There is only one report on utilization of PBM in P. monodon reported by Menasveta and Yu (2002).
Apparent digestibility coefficients (ADCs) of nutrients, which provide estimated nutrient availability in diets, can be used to evaluate the nutritional value. However, the ingredients’ nutrient digestibility data for shrimp are deficient, especially for comparison among different species. Cruz-Suárez et al. (2007) reported that ADCs of nutrients for FM and 80% PBM replacement diets of L. vannamei were very high (>80%) and there was no difference between them.
In this study, two experiments were conducted to compare the ADCs of PBM by P. monodon and L. vannamei, and to evaluate the potential of replacing FM by PBM in P. monodon.
Materials and methods
Experiment 1: Apparent digestibility coefficients of PBM
Fishmeal was supplied by Copeinca (Lima, Peru); Pet food grade PBM was supplied by National Renderers Association (NRA) (Hongkong, China). The proximate nutrients and amino acid compositions of FM and PBM are presented in Table 1. The ADCs of PBM were determined using the 70:30 reference diet techniques with 0.5% chromic oxide as the inert marker.
| FM | PBM | |
|---|---|---|
| Moisture | 73.70 | 74.90 |
| Crude protein | 678.72 | 672.55 |
| Crude lipid | 119.29 | 128.19 |
| Ash | 182.88 | 133.11 |
| Total phosphorus | 33.47 | 21.95 |
| Lysine (Lys) | 47.61 | 45.33 |
| Methionine (Met) | 17.27 | 16.31 |
| Cystine (Cys) | 6.37 | 7.59 |
| Met + Cys | 23.64 | 23.89 |
| Valine (Val) | 28.39 | 32.10 |
| Isoleucine (Ile) | 25.91 | 27.59 |
| Leucine (Leu) | 47.61 | 49.74 |
| Phenylalanine(Phe) | 26.23 | 27.69 |
| Histidine (His) | 17.49 | 17.64 |
| Arginine (Arg) | 34.55 | 41.12 |
| Serine (Ser) | 23.53 | 27.18 |
| Tyrosine (Tyr) | 20.62 | 19.69 |
| Proline (Pro) | 30.55 | 47.69 |
| Total amino acids | 567.53 | 648.55 |
Juvenile L. vannamei and P. monodon (initial bodyweight, IBW: 0.28 ± 0.01 and 0.21 ± 0.01 g, respectively) were obtained from Donghai Island shrimp farm (Yuehai Feed Co. Ltd., Zhanjiang, Guangdong, China). After 3-weeks acclimatization in laboratory culturing system, 240 L. vannamei and 240 P. monodon were stocked into 16 flow-through system (256 L) at a density of 30 shrimp per tank with four replicate for each treatment. Tanks were covered with black plastic sheets, and were supplied with flowing filtered seawater with adequate aeration, and kept at a flow rate at approximately 1.0 L min−1 in each tank. Temperature was 22–23°C, salinity was 25–32 g L−1, dissolved oxygen was above 6.0 mg L−1, pH = 7.5–8.5 and ammonia below 0.5 mg L−1. The photoperiod was 12L:12D and the light intensity was 7 lx.
Shrimp groups were fed the test diets to visual satiety four times at 06:00, 11:00, 17:00 and 22:00 daily. Intact faeces were collected from the net connected to the outlet after 4 weeks of normal feeding. Half an hour after each meal, the rearing tanks and collection column were brushed out to remove uneaten feed and faecal residues. Then faecal matter was collected from each collection column four times a day (07:30, 12:30, 18:30 and 23:30), gently rinsed with distilled water, dried on filter paper and frozen at −20°C immediately (Merican & Shim 1995). Daily faecal samples from each tank were pooled through the course of the experiment until a sufficient sample (about 10 g per pool) for chemical analysis.
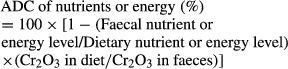
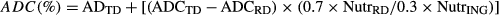
Experiment 2: Growth trial
Six isonitrogenous and isoenergetic diets PBM0, PBM25, PBM50, PBM75, PBM100 and PBMA100, containing a gradient of PBM 0, 88.7, 177.4, 266, 354.7 and 354 g kg−1 to replace 0, 92.5, 185, 277.5, 370 and 370 g kg−1 FM were prepared. The diet PBMA100 was supplemented with DL-Met to meet the contents of PBM0. The control diet PBM0 was the same as formulation of reference diet in experiment 1. Ingredients and chemical compositions of diets are shown in Table 2, and the amino acid profiles are presented in Table 3.
| Ingredients | Reference/PBM0a | PBM25 | PBM50 | PBM75 | PBM100 | PBMA100 |
|---|---|---|---|---|---|---|
| FMb | 370.0 | 277.5 | 185 | 92.5 | 0.0 | 0.0 |
| PBMb | 0.0 | 88.7 | 177.4 | 266.0 | 354.7 | 354.0 |
| Soybean meal | 280.0 | 280.0 | 280.0 | 280.0 | 280.0 | 280.0 |
| Squid liver meal | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 |
| Zeolite | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Lecithin | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| Fish oil | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Soy oil | 10.0 | 9.0 | 8.0 | 7.0 | 6. | 6.0 |
| Wheat flour | 235.0/240.0 | 244.8 | 249.6 | 254.5 | 259.3 | 258.4 |
| Ca(H2PO4)2 | 16.0 | 16.0 | 16.0 | 16.0 | 16.0 | 16.0 |
| DL-Met | 1.6 | |||||
| Premixc | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 |
| Cr2O3 | 5.0/0.0 | |||||
| Chemical composition of diet (g kg−1 dry matter) | ||||||
| Moisture | 107.5 | 103.2 | 104.4 | 103.2 | 100.5 | 102.6 |
| Crude protein | 450.9 | 449.2 | 448.7 | 450.6 | 449.9 | 452.1 |
| Crude lipid | 96.0 | 95.6 | 95.7 | 95.6 | 95.3 | 95.4 |
| Crude fibre | 31.4 | 38.9 | 26.6 | 36.2 | 41.5 | 35.4 |
| Ash | 118.1 | 114.0 | 108.8 | 104.6 | 99.1 | 110.1 |
| Total phosphorus | 17.3 | 17.1 | 17.4 | 16.6 | 16.7 | 17.2 |
| Gross energy (MJkg−1) | 20.3 | 20.6 | 20.8 | 20.9 | 21.2 | 21.2 |
- a PBM0 diet was also used as the reference diet for measuring the ADCs of the PBM with 70% of PBM0 diet and 30% of PBM.
- b FM: steam dried fishmeal (COPENCA Group, Peru); PBM: poultry by-product meal (petfood grade), supplied by National Renderers Association, Hong Kong.
- c Premix (g kg−1 diet): vitamin premix: thiamine hydrochloride 25.5 g, riboflavin 25.0 g, pyridoxine hydrochloride 50.0 g, cyanocobalamin 0.1 g, menadione 5.0 g, vitamin E 99.0 g, retinyl acetate 10.0 g, vitamin D 50.0 g, nicotinic acid 101.0 g, D-Ca-pantothenate 61.0 g, biotin 25.0 g,folic acid 6.3 g, inositol 153.1 g and cellulose 1389.0 g; mineral premix: ferric citrate 13.7 g, ZnSO4.7H2O 28.3 g, MgSO4.7H2O 0.1 g, MnSO4.H2O 12.4 g,CuSO4.5H2O 19.8 g, CoCl2.6H2O 4.1 g, KIO4 0.03 g, KCl15.3 g,Na2SeO3 0.02 g and zeolite 806.25 g), l-ascorbyl-2-monophosphate-Ca, 0.7, choline chloride 2, citric acid 3.2 and BHT 0.1 supplied by Beijing Enhalor Co. Ltd., Beijing, China.
| Reference/PBM0 | PBM25 | PBM50 | PBM75 | PBM100 | PBMA100 | |
|---|---|---|---|---|---|---|
| Essential amino acids | ||||||
| Asn | 48.1 | 45.3 | 45.6 | 42.4 | 46.6 | 47.9 |
| Lys | 32.3 | 30.1 | 29.5 | 25.9 | 28.3 | 29.1 |
| Met | 11.7 | 11.8 | 10.5 | 11.2 | 9.6 | 12.0 |
| Cys | 6.3 | 6.4 | 6.6 | 6.7 | 6.9 | 6.9 |
| Met+Cys | 18.0 | 18.2 | 17.1 | 17.8 | 16.5 | 18.9 |
| Thr | 19.5 | 18.2 | 18.4 | 17.2 | 18.9 | 19.2 |
| Val | 23.5 | 22.1 | 22.8 | 21.7 | 23.5 | 23.8 |
| Ile | 21.4 | 20.1 | 20.0 | 18.7 | 20.0 | 20.8 |
| Leu | 39.1 | 37.0 | 37.5 | 35.3 | 38.2 | 39.0 |
| Phe | 23.9 | 22.3 | 22.6 | 21.7 | 23.1 | 23.3 |
| Tyr | 17.7 | 16.7 | 17.2 | 16.6 | 16.8 | 16.7 |
| Phe+Tyr | 41.6 | 39.0 | 39.7 | 38.4 | 39.9 | 40.0 |
| His | 16.1 | 14.5 | 14.1 | 11.7 | 12.7 | 13.0 |
| Arg | 35.5 | 34.6 | 36.0 | 35.2 | 38.2 | 39.7 |
| Nonessential amino acids | ||||||
| Ser | 21.1 | 20.1 | 20.3 | 19.6 | 21.9 | 22.5 |
| Glu | 77.5 | 74.3 | 75.6 | 73.0 | 81.6 | 84.5 |
| Pro | 12.5 | 12.8 | 13.3 | 14.3 | 14.9 | 15.5 |
| Gly | 28.2 | 28.7 | 31.0 | 34.6 | 37.1 | 39.4 |
| Ala | 26.4 | 25.2 | 25.7 | 25.1 | 27.2 | 28.1 |
| Total | 460.8 | 440.0 | 446.5 | 431.0 | 465.6 | 481.5 |
All dietary ingredients were ground and passed through a 0.2-mm mesh sieve. All ingredients were mixed well and extruded using a twin-screw extruder (TSE65; Yanggong, Beijing, China) into 1- and 1.5-mm diameter pellets. Pellets were air dried to about 10% moisture and stored at 4°C during the experiment.
The growth trial of P. monodon was carried out in the same system for 8 weeks. Thirty shrimp with a mean IBW of 0.21 ± 0.01 g were randomly allocated to 24 tanks (256 L) allowing four replicates per treatment after 1-day fasting. Water quality and photo condition were the same as in experiment 1. During the trial, shrimp were fed by hand four times a day to apparent satiation at 6:00, 11:00, 17:00 and 22:00. The daily feed supplied was recorded and uneaten feed was removed 1 h after feeding. At the end of the growth trial, the shrimp of each tank were counted and batch weighed after 1-day fasting. Ten shrimp of each tank were sampled randomly for whole body and muscle chemical analysis.
A crystallized amino acid (CAA) leaching experiment was conducted after the growth trial. Leaching losses of free methionine (Met) in diet PBMA100 were determined by placing five weighed feed samples in aquaria without shrimp for 30, 60, 90 and 120 min and then collecting, drying, weighing and determining the free Met content.
Chemical analysis
Proximate analyses on feedstuffs, diets, faeces and shrimp body composition were performed. Briefly, crude protein (N x 6.25) was determined by the Kjeldahl method after acid digestion using an Auto Kjeldahl System (2100-Auto-analyzer; Foss, Hillerød, Denmark). Crude lipid was determined by the ether extraction method using a Soxtec System HT (Soxtec System HT6; Foss). Moisture was determined by oven-drying at 105°C for 24 h. Gross energy was determined using an adiabatic bomb calorimeter. The amino acids of ingredients, diets and faeces were determined using amino acid analyser (Hitachi 8800, Tokyo, Japan) after hydrolysis in 6 N HCl for 22–24 h at 110°C. For sulphur amino acids determination, an oxidative hydrolysis in performic acid for 30 min at 55°C was conducted before hydrolysis by 6 N HCl, and tryptophan was not determined in this study. Free Met was determined directly for distilled water solution. The inert marker (Cr2O3) was determined by inductively coupled plasma atomic emission spectrometry (JY38S; Jobin Yvon, Chilly Mazarin, France). Duplicate analyses were conducted for each sample.
Statistical analysis
All data were subjected to one-way analysis of variance to test the effects of experimental diets using the statistica 6.0 software package (Statsoft, Tulsa, OK, USA). Bartlett's chi-squared test was used for homogeneity test, and there were no differences among variances. Duncan's multiple range test and critical ranges was used to test differences among individual means. Difference was regarded as significant when P was < 0.05.
Results
Apparent digestibility coefficients of PBM
Data on ADCs are presented in Table 4. For the two PBM test diets, nutrient digestibility was generally higher for L. vannamei than for P. monodon, but no significant differences were observed in all the variables except Phe and Arg. However, L. vannamei showed a much higher digestibility of PBM ingredient than that of P. monodon (P < 0.05). For both of shrimp species, dry matter digestibility of PBM was relatively low (47.9% and 63.2% respectively, P < 0.05), but high for ADC of crude protein (77.6% and 84.2%) and gross energy (72.8% and 84.0%). ADC of essential amino acid (EAA) showed similar trends to those of crude protein. Both shrimp species showed relatively high digestibility of Met in PBM (90.5% for L. vannamei and 83.7% for P. monodon).
| P. monodon | L. vannamei | P. monodon | L. vannamei | |
|---|---|---|---|---|
| Test diet | Test diet | Ingredient | Ingredient | |
| Dry matter | 72.2 ± 2.6 | 78.6 ± 0.3 | 47.9 ± 4.8a | 63.2 ± 3.3b |
| Crude protein | 86.3 ± 1.4 | 89.3 ± 0.3 | 77.6 ± 3.0a | 84.7 ± 1.9b |
| Gross energy | 87.7 ± 1.7 | 88.3 ± 0.1 | 74.4 ± 1.7a | 83.4 ± 1.1b |
| Lys | 91.2 ± 1.0 | 93.9 ± 0.3 | 84.2 ± 3.0 | 91.9 ± 1.8 |
| Met | 91.1 ± 1.3 | 91.9 ± 1.0 | 83.7 ± 4.7a | 91.8 ± 2.1b |
| Thr | 85.0 ± 1.2 | 88.3 ± 0.2 | 72.8 ± 2.6a | 86.3 ± 0.9b |
| Cys | 86.6 ± 1.3 | 84.2 ± 0.9 | 86.6 ± 1.9 | 84.1 ± 0.5 |
| Val | 84.4 ± 1.6 | 87.1 ± 0.5 | 62.0 ± 9.9a | 81.0 ± 3.1b |
| Ile | 89.2 ± 0.9 | 91.6 ± 0.1 | 74.0 ± 4.0a | 91.6 ± 0.8b |
| Leu | 89.7 ± 0.9 | 91.9 ± 0.3 | 77.9 ± 3.0a | 89.4 ± 2.0b |
| Phe | 83.2 ± 2.6a | 87.0 ± 0.8b | 69.9 ± 5.1a | 88.5 ± 2.6b |
| His | 90.8 ± 0.9 | 93.3 ± 0.1 | 77.6 ± 3.9a | 89.0 ± 2.6b |
| Arg | 92.7 ± 0.7a | 95.0 ± 0.6b | 75.9 ± 2.4a | 88.7 ± 2.5b |
- All values are presented on a dry matter basis. Values are mean ± SEM, n = 4. Values in the same row with different superscript letters are significantly different (P < 0.05).
Growth performance of P. monodon in experiment 2
Growth performance of P. monodon offered the various test diets over an 8-week period is summarized in Table 5. Survival of all groups was higher than 80%. Final body weight (FBW), weight gain rate and feed conversion ratio were not different among groups (P > 0.05). The PER of PBMA100 was the lowest and fish of PBM75 showed the highest PER among groups (P < 0.05).
| Diets | PBM0 | PBM25 | PBM50 | PBM75 | PBM100 | PBMA100 |
|---|---|---|---|---|---|---|
| IBW (g) | 0.20 | 0.21 | 0.20 | 0.21 | 0.21 | 0.20 |
| FBW (g) | 2.48 ± 0.12 | 2.58 ± 0.06 | 2.71 ± 0.37 | 2.90 ± 0.39 | 2.80 ± 0.61 | 2.64 ± 0.38 |
| WGR (%) | 988 ± 65 | 971 ± 24 | 1100 ± 146 | 1167 ± 139 | 1024 ± 150 | 999 ± 187 |
| FCR | 3.42 ± 0.18 | 3.37 ± 0.05 | 3.13 ± 0.44 | 2.88 ± 0.35 | 3.32 ± 0.58 | 3.59 ± 0.63 |
| PER | 0.65 ± 0.02ab | 0.66 ± 0.01ab | 0.71 ± 0.04ab | 0.77 ± 0.04b | 0.67 ± 0.06ab | 0.62 ± 0.06a |
| Survival (%) | 88.33 ± 1.67 | 85.00 ± 2.89 | 88.33 ± 4.41 | 90.00 ± 2.89 | 83.33 ± 7.26 | 81.67 ± 7.26 |
- Values are mean ± SEM. Values in the same row with different superscript letters are significantly different (P < 0.05).
- IBW, initial body weight; FBW, final body weight.
- WGR (weight gain rate) = 100% × (Wf + Wd−Wi)/Wi, where Wf is the total final body weight, Wd is the total dead body weight, Wi is the total initial body weight.
- FCR (feed conversion ratio) = total dry feed offered (g)/total wet weight gain (g).
- PER = (fish weight gain, g)/(protein intake, g).
- Survival = 100% × the number of survival fish/the number of initial fish.
Whole body and muscle composition of P. monodon
The proximate composition (g kg−1, wet weight basis) of whole body and muscle of P. monodon fed different experimental diets is presented in Table 6. Moisture increased and crude protein and ash of the whole body decreased with the increasing levels of PBM in the diet, and the whole body compositions of PBMA100 group were significantly different from those of shrimp fed PBM0 diet. Besides, crude lipid content of whole body of PBMA100 was the lowest in all groups and significantly lower than that of shrimp fed PBM100 diet (P < 0.05).
| Diets | PBM0 | PBM25 | PBM50 | PBM75 | PBM100 | PBMA100 | |
|---|---|---|---|---|---|---|---|
| Whole body | Moisture | 760.4 ± 15.4a | 783.2 ± 9.2ab | 786.3 ± 1.3ab | 791.6 ± 13.6ab | 779.3 ± 8.0ab | 812.5 ± 6.0b |
| Crude protein | 172.0 ± 1.0b | 157.1 ± 5.4ab | 154.1 ± 0. 5ab | 151.7 ± 1.0ab | 160.4 ± 8.2ab | 137.0 ± 4.3a | |
| Crude lipid | 5.4 ± 0.6ab | 5.7 ± 0.4ab | 4.6 ± 0.2ab | 5.3 ± 1.6ab | 6.8 ± 1.1b | 3.3 ± 0.3a | |
| Ash | 41.7 ± 2.4c | 37.3 ± 1.5abc | 38.7 ± 0.3bc | 37.8 ± 1.6abc | 35.8 ± 1.2ab | 33.0 ± 1.0a | |
| Muscle | Moisture | 786.8 ± 2.5b | 774.4 ± 2.0a | 780.7 ± 1.7ab | 782.4 ± 4.2ab | 778.4 ± 3.7ab | 785.6 ± 4.3b |
| Crude protein | 194.9 ± 2.6ab | 203.8 ± 2.1b | 198.9 ± 1.3ab | 198.2 ± 3.4ab | 202.0 ± 3.2ab | 192.7 ± 3.4a | |
| Crude lipid | 4.9 ± 0.2 | 4.9 ± 0.2 | 4.9 ± 0.5 | 4.7 ± 0.3 | 5.0 ± 0.5 | 4.6 ± 0.2 |
- Values in the same row with different superscript letters were significantly different (P < 0.05).
The muscle of shrimp fed diet PBM25 showed remarkably lower moisture and higher crude protein content than that of PBMA 100 group. There were no differences in crude lipid content of the muscle among groups (P > 0.05).
Crystallized Met leaching experiment
The diet PBMA100 was placed in the same tank without shrimp to determine the losses of crystallized DL-Met and the result is shown in Table 7. Additional crystallized DL-Met in PBMA100 diet was rapidly decreased with the longer leaching time in water. The content of crystallized DL-Met in PBMA100 decreased 58.01%, 79.39%, 90.84% and 97.33% after 30, 60, 90 and 120 min leaching respectively.
| Time (min) | Free Met(g kg−1)in diet |
|---|---|
| 1.31 ± 0.07 | |
| 30 | 0.55 ± 0.05 |
| 60 | 0.27 ± 0.03 |
| 90 | 0.12 ± 0.02 |
| 120 | 0.035 ± 0.003 |
Discussion
The determination of nutrient digestibility is the first step in evaluating the potential of an ingredient to utilize in animal feed (Allan, Rowland, Mifsud, Glendenning, Stone & Ford 2000). Digestibility reflects the percentage of a feedstuff absorbed from an animal's intestinal tract (Lin, Guo, Yang, Zheng & Li 2004). ADC of dry matter provides a measure of the total quantity of an ingredient that is digested and absorbed. Because all components of a feedstuff are not digested equally, apparent dry matter digestibility coefficients can provide a better estimate of the quantity of indigestible material presented in a feed ingredient than digestibility coefficients for individual nutrients (Brunson, Romaire & Reigh 1997). The present study showed that ADC values of dry matter of PBM for P. monodon and L. vannamei were 47.9% and 63.3% respectively. Similar results for L. vannamei (Akiyama, Coelho, Lawrence & Robinson 1989; Yang, Zhou, Zhou, Tan, Chi & Dong 2009) and P. setiferus L (Brunson et al. 1997) had been reported. Similar low results of ADC of dry matter have been reported in other feed ingredients consumed by shrimp tended to decrease with the fibre and ash content of the ingredients increased (Yang et al. 2009). In addition, high level of ash should be the main reason for low digestibility for the two shrimp species in this trial. In the present study, ADCs of all tested index of P. monodon were significantly lower than those of L. vannamei. The results suggested that the practice diet of P. monodon should be designed with higher nutrients level than that of L. vannamei.
Rapid growth and high density of shrimp culture require the use of the high quality feeds. Using high digestibility feedstuffs is especially important under high-density culture conditions, where accumulations of undigested feed can foul the water, increase the cost of water treatment and increase the chance of shrimp disease and mortality (Lin, Li, Chen, Zheng & Yang 2006). Fishmeal was traditionally used as a major or even the sole protein source in the commercial shrimp diets. However, high price and resource deficiency of FM cannot meet the rapid growth of aquaculture. Compared with most of plant protein sources, PBM holds better amino acids profile, higher phosphorus utilization and better palatability and relatively high production in the world (Samocha et al. 2004). Pet food grade PBM is a source of highly digestible ingredients for several fish species, such as Siberian sturgeon (Acipenser baerii Brandt), cobia (Rachycentron canadum) and hybrid striped bass (Morone chrysops x M. saxatilis) (Zhou, Tan, Mai & Liu 2004; Rawles, Riche, Gaylord, Webb, Freeman & Davis 2006; Liu, Wu, Zhao, Xue, Guo, Zheng & Yu 2009) and a very high inclusion level were reported in the diet of humpback grouper (Cromileptes altivelis) that 740 g kg−1 pet food grade PBM could totally replace 694 g kg−1 Danish FM without negative effects on fish growth (Shapawi, Ng & Mustafa 2007). In view of the high protein requirement of shrimp (35–40% crude protein), the findings of suitable protein replacement source are considered important. Feed costs will be substantially reduced with the inclusion of greater quantities of high-grade PBM in the diets of shrimp without negative effect on growth performance of shrimp. Partial replacement of FM in shrimp feeds has met with different degrees of success (Sudaryono et al. 1999; Paripatananont et al. 2001; Forster et al. 2003). Davis and Arnold (2000) observed that co-extruded soybean PBM and flash dried PBM could be used to reduce the FM content from 300 to 60 g kg−1 in practical diets for juvenile L. vannamei without any adverse effect on growth performance. Yang, Xie, Lei, Zhu and Yang (2004) showed that PBM could replace 285 g kg−1 FM in diets for Macrobrachium nipponense. Cruz-Suárez et al. (2007) found that pet food grade PBM can adequately replace w/w up to 80% of FM (313.68 g kg−1) in commercial diets for L. vannamei. Menasveta and Yu (2002) reported that P. monodon grew significantly faster when 60% of FM was replaced with PBM. In the present study, the growth performance and survival were not significantly influenced when FM was totally replaced by PBM. Based on the above research studies and this trial, PBM could be a good protein source for shrimp.
The studies about balanced EAA in aquatic animal diets have shown varying results. Gaylord and Rawles (2005) found that 100% FM could be replaced by PBM when Lys and Met were balanced in the diet of hybrid striped bass. Other studies also showed that some carnivorous fish species, such as turbot (Pestta maxima), European seabass (Dicentrarchus labrax), rainbow trout (Oncorhynchus mykiss) and Siberian sturgeon can utilize CAAs efficiently (Fournier, Huelvan & Desbruyeres 2004; Peres & Oliva-Teles 2006; Nang Thu, Parkouda, Saeger, Larondelle & Rollin 2007; Zhu, Gong, Wang, Wu, Xue, Niu, Guo & Yu 2011). However, common carp, Cyrinus carpio apparently does not respond well to amino acid supplementation without adjustment of diet pH (Cowey 1994). Teshima, Kanazawa and Koshio (1992) reported that supplementation of P. japonicus diets with crystalline Met did not improve performance in terms of weight gain or feed efficiency. It seems that amino acid supplementation is more useful for fish species with stomach than those without stomach and shrimp. In the present study, although PBM100 diet showed 18% lower than the control diet (Table 2), the similar growth performance of all groups indicated that no Met deficiency was observed for PBM100 diet. The shrimp is a kind of bottom-feeder and eats slowly. Water stability of Met is important for shrimp that feed on the bottom of ponds and respond relatively slowly to the presence of feed. Similar fast leaching rate had been reported by Alam, Teshima, Koshio and Ishikawa (2004). Water stability of DL-Met in PBMA100 was poor and DL-Met had lost almost 60% in 30 min, and lost 97.33% after 120 min of dipping in water. PER of PBMA100 shrimp were the lowest in all groups. Crystallized DL-Met in diet seemed to negatively affect the shrimp protein retention, which was mainly because of the significantly reduced whole body protein content in shrimp of PBMA100 group. Whole body protein content of crustaceans determined by Kjeldahl method might be affected by chitin content of shrimp shell during moulting periods.
The present investigation reveals that PBM can totally replace FM in diet of P. monodon without additional CAA supplement. The cost-effectiveness of substituting PBM for FM varies depending on the local cost of the ingredients (Yu 2007). In the conditions of the present study, 100% replacement of FM would lead to more than 30% cost reduction in shrimp feed.




