Effect of plant protein concentrates on nutrition physiology of Litopenaeus vannamei (Boone, 1883) juveniles
Abstract
This study was designed to test the effect of soy protein (SPC), wheat gluten (WHG) and potato protein (PPC), in vitro and in vivo digestibility of protein and energy in the juveniles Litopeneaus vannamei. A completely random design was used with nine 400-L tanks (with three repetitions by treatment). Ten respirometric chambers (500 mL) were used for energy distribution. In vitro digestibility for SPC (8.8%) was higher than for PPC (5.8%) and for WHG (4.3%, P < 0.05). Diets’ degree of hydrolysis ranged between 0.75% and 1.2%, with lowest value in potato protein concentrate diet (0.75 ± 0.09%, P < 0.05). No significant differences were obtained in apparent digestibility coefficient (ADC) for protein (63.4–74.1%). ADC for amino acids ranged between 80% and 90%. Daily growth coefficient ranged from 0.86% to 1.1% day−1, being the best in soybean protein concentrate diet (SPCd) (P < 0.05). Significant differences on heat increment were observed (P < 0.05); highest value was in wheat gluten diet (1.0 ± 0.1 kJ shrimp day−1) that coincided with a peak of trypsin specific activity (16.5 ± 3.7 mU mg protein−1). Highest retained energy for growth was observed in shrimp fed SPCd (0.7 ± 0.03 kJ day−1, P < 0.05). Muscle collagen content presented a minimum of bands with SPCd, whereas shrimp post-mortem collagenase activity was not affected by any of the three diets (P > 0.05).
Introduction
Currently, a reduction in fishmeal content in feeds is the major challenge in nutrition of farmed marine species (Naylor, Hardy, Bureau, Chiu, Elliott, Farrell, Forster, Delbert, Gatlin, Goldburg, Hua & Nichols 2009). As long as palatability and digestibility remain at a high level, the use of plant protein sources in compounded feed for shrimp is still viable (Lawrence, Castille, Sturmer & Akiyama 1987; Davis, Arnold & McCallum 2002). At the beginning, ingredients selection for shrimp feed was of prime, high interest for nutritionists due to the absence of defined dietary requirements as in Penaeus merguiensis (Aquacop, 1987) or Litopenaeus vannamei (Tacon & Akiyama 1997), and recently with Farfantepenaeus duorarum (Gaxiola, Cuzon & Guillaume 2009).
Several works on the use of ingredients such as wheat gluten (WHG), squid meal (Cruz-Suárez et al. 1992), meat and bone meal and fishmeal (Tapia-Salazar 2004), as well as pea meal (Cruz-Suárez & Nolasco 2006) or lupin (Smith, Tabrett, Glencross, Irvin & Barclay 2007a; Smith et al. 2007b), encouraged fish meal substitution in shrimp feeds. Protein concentrates look like a promising way to replace fishmeal. WHG was initially chosen as a source of protein for aquafeeds due to its physical properties and its excellent level of digestibility or its nutritive value (Cuzon & Gehin 1998). In fact, native WHG was found to be highly energy content digestible when compared with other ingredients and tested on Litopenaeus setiferus (Brunson, Romaire & Reigh 1997) or L. vannamei (Roquette 1990; Lemos, Lawrence & Siccardi 2008). Such quality favoured its inclusion in shrimp feed despite a deficit in lysine. Moreover, the selection of such concentrates such as soybean, lupin and sunflower limits the presence of complex carbohydrates (cbh) (Guillaume 1999). Soybean meal (SBM) was identified as a protein in numerous studies (Aquacop 1976; Tacon 1987; Smith, Tabrett & Moore 1998; Davis, Miller & Phelps 2007; Suarez, Gaxiola, Mendoza, Cadavid, Garcia, Alanis, Suarez, Faillace & Cuzon 2009) with an amino acid (aa) profile similar to that of shrimp muscle (Shigeno 1975).
However, SBM quality can vary in function of heating time during preparation (Cuzon & Williams 1997; Cruz-Suárez et al., 2010). Digestibility will depend on the presence of an anti-trypsic factor (Cheng & Hardy 2003). Such factor could be found in SBM and in concentrate even though, in theory, concentrates would contain lower amount (Tacon 1987) as evidenced by its apparent digestibility coefficient for protein (ADCprotein) for L. vannamei juveniles (Suarez et al. 2009). To a certain extent, a similar situation was observed in potato meal processed to get tubermine® (Roquette, Lestrem, France). By and large, concentrates remain an option to replace fishmeal in L. vannamei diets; quality protein sources used in place of regular meals sustained weight gain for whiteleg shrimp (Davis & Arnold 2000; Suarez et al. 2009). Concentrates replaced fishmeal better than did regular meals and, when mixed with low fishmeal content, they should ensure feed consumption and texture (Smith et al. 1998; Suarez et al. 2009). Nevertheless, texture might be affected as a result of a reduction in collagen content or collagenase activity. In turn, muscle texture, as observed earlier with blue shrimp (Aquacop, 1987), could change according to feed composition as shown with whiteleg shrimp fed a mixture of protein sources (Ezquerra Brauer, Salazar, Alvarado & Rouzaud-Sandez 2003).
The present work was designed to compare practical diets including three plant concentrates: soy protein (SPC), WHG and potato protein (PPC) incorporated at 30% of the diet. The parameters taken into account were weight gain, survival rate, enzymatic activity, in vitro and in vivo digestibility and energy distribution. In terms of shrimp quality, muscle texture was examined by collagen and collagenase post-mortem activity and reported to feed composition.
Materials and methods
Litopenaeus vannamei juveniles were obtained from a multiple spawn in the laboratory and were reared until postlarvae. They were also transported to outdoor tanks for growth until they reached 5 g average weight.
Experimental design and diets formulation
In vivo digestibility was set with a complete randomized design including three treatments and three replicate treatments. Diets included 30% protein-rich plant source and celite as a marker (Table 1). WHG with 80% crude protein (CP), 6% lipid, 10% starch, soy concentrate with 62% CP, 2% lipid, 15% cbh, PPC with 72% CP, 4% lipid, 15% cbh. Elaboration of practical diets with one major plant protein source and adjustments with other ingredients did not have an impact on the overall composition. A basis of 30% inclusion of such ingredient represents between 52% and 60% of diet protein content. Such composition was set not only from proximate analysis but also from aa profile. To calculate ADC for each ingredient, the level of fishmeal was regulated to maintain palatability, as well as to get collets readily ingested. Enough fatty acids (20:5n-3 and 22:6n-3) were added by fish oil. Carbohydrates were chosen due to their digestibility (Cousin et al., 1996). Stability of finished cold extruded feed was ensured adding carboxylmethylcellulose. A marker was included for digestibility trial. The regimes were isoproteic, isoenergetic (GE) and after the digestibility study, ADCenergy maintained isoenergy (DE) necessary for equal feed intake as an initial step for energy partitioning. Furthermore, aa profile gave the first limiting factor based on essential amino acid (EAA) while referring to aa composition of abdominal muscle. Each diet presented a different limiting factor (Table 2). It was then possible to calculate chemical score. Protein digestibility gave access to a corrected amino acid score varying in feeds between 0.5 (WHG) and 1 (SPC).
| SPCd | WHGd | PPCd | |
|---|---|---|---|
| Menhaden meala | 190 | 140 | 140 |
| Soybean paste | 150 | 150 | 150 |
| Wheat flourb | 85 | 115 | 130 |
| Wheat starchb | 155 | 165 | 165 |
| Soy protein concentrate | 300 | ||
| Wheat glutenb | 300 | ||
| Potato protein concentrate | 300 | ||
| Cod liver oil | 50 | 60 | 45 |
| Soy lecithin | 20 | 20 | 20 |
| Cholesterol | 5 | 5 | 5 |
| Vitamin and mineral premixc | 20 | 20 | 20 |
| Stay-Cc | 0.3 | 0.3 | 0.3 |
| CMC | 10 | 10 | 10 |
| Celite | 15 | 15 | 15 |
| Fishmeal | Soybean paste | Soy protein concentrate | Wheat flour | Wheat gluten | Potato meal | Potato protein concentrate | |
|---|---|---|---|---|---|---|---|
| Crude protein (Nx 6.25) | 652 | 460 | 700 | 110 | 800 | 95 | 800 |
| Humidity% | 79 | 120 | 100 | 130 | 120 | 120 | 100 |
| Crude fat% | 66 | 5 | 30 | 20 | 12 | 5 | 30 |
| Ash% | 180 | 70 | 70 | 15 | 5 | 45 | 7 |
| Fibre% | 11 | 55 | 45 | 25 | 13 | 30 | 7 |
| NFE | 110 | 290 | 78 | 700 | 50 | 705 | 56 |
| kJ g−1 | 35.1 | 35 | 15 | 37.9 | |||
| ARG | 41 | 34 | 73.4 | 4.3 | 48 | 2.9 | 44 |
| HIS | 19 | 11.2 | 24.1 | 2.5 | 22 | 1.2 | 19.2 |
| LYS | 51 | 27.6 | 56 | 2.5 | 28 | 3.4 | 64 |
| ILE | 28 | 21.4 | 46 | 4.7 | 36 | 2.9 | 47.2 |
| VAL | 32 | 22.7 | 43.8 | 5 | 45 | 4.3 | 56.8 |
| LEU | 46 | 31.2 | 63 | 8.7 | 65 | 4.8 | 86.4 |
| THR | 29 | 16.2 | 33.4 | 3.3 | 28 | 2.8 | 48 |
| MET | 21 | 59 | 9 | 1.8 | 13.5 | 1 | 19.2 |
| PHE | 26 | 20 | 43 | 6 | 44 | 3 | 55.2 |
| TRP | 6 | 61 | 9 | 1.2 | 11 | 0.7 | 10.4 |
- a Menhaden meal from Chile; soyabean paste.
- b Gluten y Almidones de México, S.A de CV. Soy protein concentrate Profine; potato protein concentrate from Roquette frères, France.
- c Stay C + vitamin mix and minerals premix from DSM Products.
| EAA | Chemical score (%) | ADCEAA (%) | Corrected digestibility (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SPCd | WGHd | PPCd | SPCd | WGHd | PPCd | SPCd | WGHd | PPCd | |
| ARG | 98 | 55 | 72a | 94 | 94 | 94 | 100 | 58.5 | 77 |
| HIS | 97 | 75 | 85 | 94 | 92 | 94 | 100 | 81.5 | 90 |
| LYS | 94 | 47a | 100 | 93 | 93 | 93 | 100 | 50.5 | 100 |
| LEU | 97 | 89 | 100 | 92 | 92 | 92 | 100 | 96.7 | 100 |
| ILE | 100 | 79 | 100 | 91 | 90 | 91 | 100 | 87.8 | 100 |
| VAL | 91 | 76 | 100 | 90 | 89 | 90 | 100 | 85.4 | 100 |
| THR | 81a | 60 | 100 | 91 | 89 | 91 | 89 | 67.4 | 100 |
| MET | 83 | 73 | 100 | 91 | 92 | 91 | 91.2 | 79.3 | 100 |
| ALA | 100 | 100 | 100 | 89 | 86 | 89 | 100 | 100 | 100 |
| TRP | 95 | 81 | 100 | 92 | 90 | 92 | 100 | 90.0 | 100 |
- a First liming amino acid; corrected digestibility = (chemical score of amino acid)/100)/(ADCaa).
Diets’ chemical analyses were carried out according to the following methods: dry matter (AOAC 934.01); ash (AOAC 942.05); lipids (AOAC 920.39); protein (AOAC 984.13); crude fibre (AOAC 962.09) and nitrogen-free extract NFE (Table 3). Amino acid composition of diets was obtained using an auto-analyser technique after acid hydrolysis (AOAC 982; tryptophan, AOAC 988.15) and it was compared with aa composition of juvenile L. vannamei muscle (Lee & Lawrence 1985).
| SPCd | WHGd | PPCd | |
|---|---|---|---|
| Moisture | 5.4 | 6.1 | 5.4 |
| Crude protein (%) | 42 | 44 | 44 |
| Lipid (%) | 10.1 | 11.8 | 10.7 |
| NFE | 32.4 | 29.4 | 30.1 |
| Crude fibre | 3.0 | 2.1 | 2.9 |
| Ash% DM | 7.7 | 6.6 | 6.8 |
| GE (kJ g−1) | 16 | 16 | 16 |
| DE (kJ g−1) | 11 | 11 | 12 |
| LYSa | 3.0 | 2.3 | 3.3 |
| ARG | 2.6 | 2.0 | 2.3 |
| HIS | 1.1 | 1.1 | 1.1 |
| LEU | 3.2 | 3.1 | 3.8 |
| ILE | 1.6 | 1.4 | 1.8 |
| VAL | 1.8 | 1.7 | 2.2 |
| THR | 1.8 | 1.5 | 2.1 |
| MET | 0.7 | 0.8 | 0.9 |
| PHE | 2.0 | 2.0 | 2.2 |
| TRP | 0.4 | 0.3 | 0.4 |
| PRO | 2.1 | 3.9 | 2.0 |
| Chem. score | ARG | ARG | ARG |
| PD-CAAS | 0.52 | 0.40 | 0.41 |
- NFE, nitrogen-free extract; DM, dry mater.
- a aa in g% product.
In vitro digestibility analysis for ingredients and diets was conducted in a pH-Stat with a measure of initial rate of hydrolysis (Nieto-López, Cruz-Suárez, Risque & Ezquerra 2005). Litopenaeus vannamei fed commercial diet (30% protein) in ponds were dissected rapidly and hepatopancreas (HP) were extracted and frozen in liquid nitrogen, stored at −40°C. HP was individually homogenized in distilled water using an ultra-grinder. Homogenate in pH 8 was centrifuged at 13 370 g for 30 min at 4°C and stored at −20°C.
The degree of hydrolysis (DH) was measured in triplicate for both ingredients and diets. Samples were sieved through a 250-μm mesh and homogenized at 13 370 g in 5 mL of distilled water. They were placed in a recipient for hydrolysis and pH adjusted at 8 with NaOH 0.1 M. The reaction started with the addition of 1 mL enzyme homogenate for a proteolysis activity of 6 U mg−1.
The volume of NaOH 0.1 N was measured for 5 min at 28°C to keep pH value at pH 8. The DH for protein was determined using the following formula (Dimes and Haard, 1994): DH% = [(B × Nb × 1.5/M × (S/100)/8] × 100 where: B = mL NaOH 0.1 N used to control pH; Nb = NaOH normality with a 1.5 calibration factor at pH 8 and 28°C; M = g of mixture; S = protein concentration in the mixture (%); 8 = total content of peptides bonds (meqv g−1) for trypsin or casein (Alder-Nissen 1986).
As a further modification, the degree of self-hydrolysis was determined by incubating samples in the same test conditions, without adding the enzyme homogenate to estimate DH corrected by the addition of extract: DH corr. = [(B−B′) × Nb × 1.5/M × (S/100)]/8 × 100 where B′ for mL NaOH 0.1 N was used to keep pH 8.0 for 5 min at 28°C. The absolute DH value was calculated and attributed to casein; casein was chosen as a pure protein source and then reported for each diet in percentage. Total protein concentration was measured in a 5-μL sample diluted at 1/10 and put into a microplate mixed with 125 μL reagent, incubated for 5 min at a room ambient temperature and read at Abs595nm (Bradford, 1976).
A re-circulating semi-open seawater system including nine 400-L tanks was used to collect faeces over a span of 42 days. In each tank, there were 50 juveniles with an initial mean weight of 5.3 g (Table 4). Seawater parameters were 28 ± 1°C (mercury thermometer), 35.0 ± 0.5 ppt (refractometer; Vitalsine SR-6, Apopka, FL, USA) and 6.5 ± 1.0 mg O2 mL−1 (oxymeter; HACH mod 40, Loveland, CO, USA). Water exchange was set at 10% using sand-filtered seawater on cartridges of 20 and 5 μm and passed through UV.
| SPCd | WHGd | PPCd | |
|---|---|---|---|
| DH | 1.00 ± 0.34a | 1.20 ± 0.15a | 0.75 ± 0.09b |
| H (%) | 32.6 ± 11.1a | 39.6 ± 5.0a | 23.7 ± 2.8b |
- Different letters in superscript indicate significant differences (P < 0.05).
At the end of the experiment, all shrimp were counted to estimate survival; the difference between initial and final number of shrimp per tank gave survival rate. Shrimp were weighed using an OHAUS balance at 0.01 g. Daily growth coefficient (DGC; mg day−1) of juveniles L. vannamei was calculated based on live weight gain using the formula: DGC = final wt1/3−initial wt1/3/days × 100.
In vivo digestibility
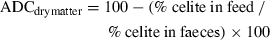
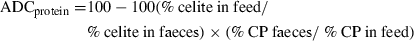
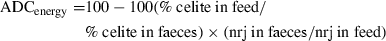
Formulae were set on a protein basis similar for the three diets; at 30% inclusion; each plant protein source brought about 20% CP; therefore, it contributed approximately 40% to the overall dietary protein content; then, based on digestibility with its property of additivity, a difference in ADCprotein could be observed on each plant protein source.
Energy budget was measured after 42 days and oxygen consumption monitored individually on juvenile for each treatment using nine 500 mL chambers (Acrident SA, Mexico D.F, México). A flow through respirometer in a closed system (Rosas, Martinez, Gaxiola, Diaz, Brito & Soto 1998) helped to monitor oxygen tension in water using an YSI 50B oxygen electrode (Apopka, FL, USA) at the entrance and exit of the chambers.
Shrimp were previously acclimated in the chamber for 14 h. Oxygen consumption was first determined at 08:00 on shrimp fasted for 24 h. Early juveniles were then fed collets in each treatment and oxygen consumption was measured every hour from 09:00 until 14:00.
Respiration (HeE + HiE) was measured in mgO2 h−1 shrimp−1, where HeE was the oxygen consumption of unfed animal (maintenance) and HiE was the peak corresponding to the heat increment of feeding. These values were converted to energy by the coefficient 14.3 J mg−1 O2 (Lucas 1996). We performed calculation of HeE (J day−1 shrimp−1) daytime on unfed shrimp as well as HiE (J day−1 shrimp−1) considering an area below the peak after feeding and also considering the number of rations per day (n = 3) .
Similarly, excretion was estimated as routine nitrogen excretion using the formula UE + ZE = 0.08 × (RE + HeE + Hie + HxE) (Bureau, Azevedo, Tapia-Salazar & Cuzon 2000). These values were converted to energy by the coefficient 20.5 J mg−1 N–NH3 in J day−1 shrimp−1. The initial and final energy content of shrimp from each treatment was measured in an adiabatic calorimeter bomb (PARR, Moline, IL, USA) previously calibrated with benzoic acid.
Both individual wet and dry weights were measured using an analytical OHAUS balance with 0.001 g accuracy. Shrimp were washed with distilled water and oven-dried at 60°C for 24 h. The same procedure was used to obtain final dry weight of animals in each treatment. Data on individual dry weight gain in mg−1 day−1 were transformed into energy with corresponding units to express RE in J day−1 shrimp−1.
DE derived from indirect calorimetry study for several hours was calculated as: DE = RE + (HeE + HiE) + (UE + ZE) + EEx.
RE energy channelled into growth was calculated from daily growth rate transformed into energy values using kJ carcass content; other parameters concerned were ‘HeE + HiE’ energy lost in respiration, ‘UE + ZE’ energy lost in excretion and total cost of an ecdysis given for 1.4 kJ (Read & Caulton 1980).
Digestive enzymatic specific activity
The HP soluble protein concentration was measured by using a Bio-Rad-500-0006 kit and by reading on spectrophotometer at 595 nm (Bradford, 1976). Activities were expressed as a specific activity in mU mg−1 protein. HP from intermolt shrimp of each treatment were dissected, frozen in liquid nitrogen, stored at −40°C until analysis. HP was individually homogenized in 500 mL of distilled water, using a tissue homogenizer and centrifuged at 16 170 g for 20 min at 4°C. Supernatant was removed for further analysis.
The trypsin activity determination followed Geiger and Fritz (1988) and Geiger (1988) for chymotrypsin. For trypsin activity, a 100-mM BAPNA (N-benzoyl-l-arginine-p-nitro-anilide; Sigma B7632, Estado de México, México) was used as a substrate in TRIS buffer 0.1 M, pH 8 at 40°C. The hydrolysis rate was measured, as an absorbance increment, using a spectrophotometer (Spectronic model 21D, Madison, WI, USA) at 405 nm for 2 min, with an extinction coefficient the ε405 = 1.02 L mol−1 cm−1 formula (Geiger 1988). A unit was defined as the corresponding 1 mM of p-nitroanilide released in 1 min. Chymotrypsin activity was measured with the same method, but another substrate succinil-alanin-2-proline-phenil-p-nitro-anilide (SAPPNA) and an extinction coefficient of є405 = 1.02 L mmol−1 mm−1 (Geiger & Fritz 1988).
Post-mortem shrimp analysis
The entire extraction process to evaluate collagen from the muscle of L. vannamei was carried out at 4°C. The extraction of collagen (Sivakumar & Chandrakasan 1998) supported slight modifications (Torres et al., 2008). Fresh shrimp tail muscle was homogenized and mixed with 6 M urea containing 0.5 M sodium acetate (pH 6.8). Then, it was sequentially extracted with a neutral buffer (0.05 M Tris/1 M NaCl, pH 7.2) for soluble collagen, 0.5 M acetic acid for acid-soluble collagen. Pepsin (10 mg g−1 tissue in acetic acid, CH3COOH 0.5 M) served as pepsin-soluble collagen. The collagenase activity was measured on post-mortem abdominal shrimp muscle using bovine collagen type I as a substrate.
Three pools of abdominal muscle per treatment were placed in tubes. For each sample, a blank was prepared using TRIS buffer 1.25 mL + 1 M NaCl + extract. A second tube was prepared using TES buffer N-tris (hydroxymethyl) methyl-2-amino ethane sulphonic acid 1.25 mL + NaCl 1 M + bovine collagen 6 mg and, finally, a third tube was prepared with a TES buffer (1.25 mL + 6 mg collagen + 25 μL) extract sample incubated for 5 h at 37°C, together with a blank sample containing only a water sample centrifuged at 14 000 g for 5 min at 4°C. Moreover, 1.5% ninhydrin was added to 0.5 mL of supernatant incubated at 100°C for15 min and 2.5 mL of ethanol. Absorbance was read at 600 nm. Collagenase activity was reported as mg protein min−1 using TES buffer from Sigma.
Statistical analysis
Percentages were previously transformed to arcsine to choose the statistical test (Zar 1999). Kruskal–Wallis and multiple range tests helped to determine significant differences for in vitro data on ingredients. ADC values, weight gain and survival rate were previously transformed to arcsine for a nested anova followed by Tukey multiple range tests. Energy parameters followed a one-way anova and Tukey test to detect differences between shrimp fed the three diets. A linear regression on diets was applied between DH (%) and ADCprotein. All statistical tests were run at a probability of 5%.
Results
Diets’ composition was similar for protein content in a range of 42–44% CP. Amino acid composition was slightly better with SPC based on basic aas (Table 3). Chemical score differed among the three diets with THR, LYS, ARG for soybean protein concentrate diet (SPCd), wheat gluten diet (WHGd), and potato protein concentrate diet (PPCd) respectively. ADCaa fell in the same range except for ALA below 90%. Corrected digestibility reflected the chemical score with THR (89%), LYS (51%) and ARG (77%, Table 2).
The degree of hydrolysis values for ingredients and diets analysed by the pH-stat method are summarized in Table 2. Soybean paste (SBM) used as a reference showed a DH value (19.4 ± 3.8%) superior to DH values observed in plant protein concentrates and wheat meal (P < 0.05). DH for diets showed significant differences (P < 0.05); SPCd and WHGd displayed the highest DH, whereas the lowest value was found in PPCd (Table 5). ADCs for dry matter, protein and energy were not different (P > 0.05). The linear regression y = 90.18−0.67 (x) applied between DH (%) and ADCprotein for diets was significant (R = −0.99, Fig. 1) indicating an inverse relationship between in vitro and in vivo digestibility for the three treatments concerned. Feeds and faeces aa composition gave a range of ADCaa from 89% to 94% for SPCd and PPCd and from 86% to 94% for WGHd. ADCaa was corrected using the chemical score (Table 3), and values for WGHd were below 100% for almost all EAA.

| Ingredients | DH total | %H |
|---|---|---|
| Menhaden meal | 14.7 ± 7.6ab | 46.1 ± 23.8 |
| CPSP70a | 11.4 ± 0.2ab | 35.6 ± 0.6 |
| Soybean meal | 19.4 ± 3.8a | 60.9 ± 11.9 |
| Soy protein concentrate | 8.8 ± 2.3b | 27.5 ± 7.2 |
| Wheat meal | 7.8 ± 1.3b | 24.6 ± 4.1 |
| Wheat gluten | 4.3 ± 1.3b | 13.7 ± 4.1 |
| Potato protein concentrate | 5.8 ± 0.7b | 18.2 ± 2.2 |
- a Sopropêche, France.
Digestive enzymes were measured at the end of the 42 days experiment. Trypsin activity peaked at 16.5 ± 3.7 mU mg of protein−1 (P < 0.05) in shrimp fed WGHd, whereas no significant differences were observed for chymotrypsin activity (Table 6).
| Diet | Soluble protein (mg mL−1) | Trypsin | Chymotrypsin |
|---|---|---|---|
| SPCd | 3.3 ± 1.1a | 7.8 ± 2.5b | 30.8 ± 7.6a |
| WHGd | 1.9 ± 0.6b | 16.5 ± 3.7a | 25.2 ± 7.3a |
| PPCd | 2.1 ± 0.6b | 10.1 ± 5.4b | 31.3 ± 4.7a |
- a Different letters in superscript indicate significant differences (P < 0.05).
Survival rates were similar between treatments with a mean value of 97% (P > 0.05). Weight gain (Table 7) showed shrimp fed SPCd with a high final live weight (10.9 ± 0.2 g) expressed in DGC=1.1 ± 0.06% day−1 (P < 0.05) (Table 8). Energy partitioning indicated a significant difference between treatments (Table 7). Heat increment (HiE) differed between diets and WGHd produced a high value (1 ± 0.1 kJ shrimp−1 day−1) (Table 9). Maintenance (HeE) presented large variations among the three diets; then, no significant differences could be evidenced (P > 0.05). RE values were different (P < 0.05) and shrimp fed SPCd showed the highest value (0.7 ± 0.03 kJ day−1), whereas those fed PCCd retained 0.4 ± 0.02 kJ day−1 (Table 9).
| ADC dry matter | ADC protein | ADC energy | |
|---|---|---|---|
| SPCd | 70.0 ± 2.4 | 68.5 ± 2.5 | 67.5 ± 2.6 |
| WHGd | 68.3 ± 10.5 | 63.4 ± 12.1 | 71.1 ± 9.6 |
| PPCd | 68.3 ± 2.4 | 74.1 ± 1.9 | 75.1 ± 1.9 |
| Diet | Initial mean weight (g) | Final mean weight (g) | DGC% mg day−1 | g week−1 | Survival (%)a |
|---|---|---|---|---|---|
| SPC | 5.4 ± 0.3 g | 10.9 ± 0.22a | 1.1 ± 0.06a | 0.9 ± 0.02a | 98 ± 3a |
| WHG | 5.4 ± 0.3 g | 9.52 ± 0.18b | 0.86 ± 0.03b | 0.7 ± 0.02b | 97 ± 3a |
| PPC | 5.2 ± 0.15 g | 9.25 ± 0.25b | 0.87 ± 0.02b | 0.7 ± 0.02b | 96 ± 3a |
- a Different superscripts letters within the columns indicate significant differences (P < 0.05).
| Diet | DE | UE+ZE | HeE | HiE | EEXV | RE |
|---|---|---|---|---|---|---|
| SPCd (n = 7) | 2.2 ± 0.1a | 0.1 ± 0.001a | 1.2 ± 0.1a | 0.07 ± 0.01 a | 0.03 ± 0.003a | 0.7 ± 0.03a |
| WHGd (n = 4) | 3.3 ± 0.03b | 0.2 ± 0.02b | 1.4 ± 0.1a | 1 ± 0.1b | 0.03 ± 0.006a | 0.5 ± 0.03b |
| PPCd (n = 4) | 2.2 ± 0.5a | 0.1 ± 0.03a | 0.7 ± 0.4a | 0.7 ± 0.004c | 0.027 ± 0.003a | 0.4 ± 0.02c |
- a DE, digestible energy; HeE, basal metabolism (maintenance) based on value measured at t0; HiE, heat increment of feeding on the basis of one feeding per day; UE + ZE, urinary and gills excretion about 6% DE; RE, recovered energy; Eexv, exuviae energy.
- b Values are means of five replicates. Means within columns with the same letter are not significantly different (P = 0.05).
Electrophoretic pattern of collagen fractions from tail muscle showed fewer bands at 120 kDa for SPCd compared with WGHd and PCCd (Fig. 2). Other bands did not differ in position for all other molecular weight.
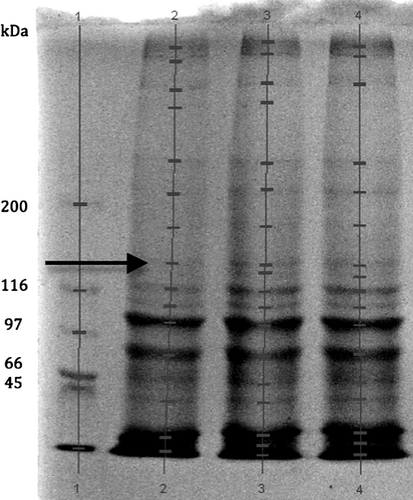
The activity scores of collagenase on abdominal muscle of juvenile L. vannamei fed a SPCd, WHGd and PPCd were 293 ± 20, 326 ± 14, 373 ± 47 U h−1 (μg of leucine liberated in 5 h by using bovine collagen type I as a substrate) for SPCd, WHGd and PPCd respectively (P > 0.05).
Post-mortem collagenase activity based on the hydrolysis in collagen with tyrosine exposed to ninhydrin combined to form a complex, indicating the same activity between treatments (P > 0.05) (Table 10).
| Diet | U h−1b |
|---|---|
| SPC | 293 ± 20a |
| WHG | 326 ± 14a |
| PPC | 373 ± 47a |
- a Superscript letter indicates significant differences (P < 0.05).
- b μg leucine liberated in 5 h using bovine collagen type I as substrate.
Discussion
Considering ingredients one at a time, WHG was available either as a regular form or as native gluten (Viten®; Roquette, Lestrem, France). Data obtained in this study clearly showed that WHG was not a native form, since evidence of a low ADCprotein, of an increase in trypsin specific activity, and of comparatively poor weight gain and muscle texture found to be inferior to other sources. On the other hand, a native gluten proved to produce very high ADC protein, with a GE ~23 kJ, DE ~22 kJ g−1 and ME (21 kJ g−1) applying coefficients 21.3/39.5/17.2 (Cuzon & Guillaume 1997b).
Three ingredients were incorporated at 30% in shrimp diets. CP, DP, GE, DE, ME were measured or calculated to derive enough information for further formulation of all-plant grower feeds. Diets including 30% of each concentrate will represent ~52–60% protein content and all things are equal elsewhere when compared with the incidence of each source at the level of a compounded feed called SPCd, WHGd, and PPCd. The results obtained in the present study demonstrate a good capacity for the digestion, absorption and assimilation of the SPC compared with WHG or PPC for juvenile L. vannamei.
In vivo protein and energy digestibility of diets reflected primarily the values for ingredients included at 30% because all the three diets had an equal proportion of other protein sources. Besides, the additivity of digestibility values was also considered. Altogether, in vivo values were obtained from a true physiological process with a possibility of getting ADCprotein as well as dry matter, carbohydrates and energy. In contrast, in vitro determination on ingredients provided only the protein value. In vitro digestibility values for diets were consistent with previous results on the same species (Ezquerra, Garcia-Carreño & Carrillo 1998). Although all diets contained approximately the same CP amount, DH values were different and PPCd showed a low digestibility value. Protein hydrolysis of the whole diet due to HP proteases should be efficient in both cases and such differences could be attributed to the digestibility of protein sources and the way they were processed. The correlation between ADCprotein and DH% with diets offered an answer because (i) the ADC value combined the addition of basal part, which was similar to each diet, and ingredient to be tested; (ii) a relationship between in vitro (DH) and in vivo (ADCprotein) digestion seemed to exist at a minimum value of 75% ADCprotein (Lemos & Nunes 2008; Lemos et al. 2008). One would get the following for ADCnutrients: 100, 92, 90 for protein, lipid, cbh, respectively, and DH: 40, 33, 24 in WHG, SPC, PPC respectively. In theory, a high DH for WHG should correspond to a high ADCprotein, but it was not reflected because in vivo proteases would poorly hydrolyse a protein with a rearranged quaternary protein structure. A significant difference appeared between SPCd on one side as well as on WHGd and PPCd on the other one, as it was the case for Macrobrachium (Tidwell, Webster, Yancey & D'Abramo 1993) or cobia (Chou, Her, Su, Hwang, Wu & Chen 2004). Shrimp fed SPCd (68. 5 ± 2.5%) gave an ADC value lower than the previously reported one (93.5%). This difference might be attributable to the efficiency of a marker. In this study, celite replaced chromic oxide because of discrepancy between transit of organic material and marker as in Homarus americanus (Leavit 1985; Smith & Tabrett 2004) and Procambarus clarkii (Brown & Bern, 1986). ADCprotein showed a positive trend with only SPCd and PPCd compared with WHGd. It is consistent with the values encountered for ADCprotein in Procambarus clarkii (Brown & Bern 1986; Chen, Leu & Roelams 1992) and L. vannamei (Akiyama, Coelho, Lawrence & Robinson 1989). Soy protein possessed a high digestibility (Siccardi, Lawrence, Gatlin, Fox, Castelle & Perez-Velazquez 2006) coinciding probably with a low level of an anti-trypsic factor. Highly digestible WHG for monogastrics (Blasco, Fondevila & Guada 2005) may vary due to genetic and environmental factors (Díaz Tenorio 2006), or to how it is processed (Roquette 1990). Processing would modify the protein structure of two main units, gliadin and glutenin, that make about 10–14% of the grain with different solubility levels (Cuniberti, Roth & Macritchie 2003). The value of 63% ADCprotein found in this study contrasts with previous results on L. setiferus (Brunson et al., 1997), crayfish (Brown 1995) and L. stylirostris (Roquette 1990) due to a difference in the extraction techniques. WHG analysed in the study came from a local company using a process at 80°C and then contrasted with native gluten with up to 100% ADCprotein (Robaina, Izquierdo, Moyano, Socorro, Vergara, Montero & Fernandez-Palacios 1995) because the quaternary structure of the protein was intact, and then the proteases could hydrolyse protein more easily than heat-processed gluten. SPC remained an efficient protein source, despite a lack of differences in ADC values between treatments, but a high RE. The lack of difference in ADC was explained by an elevated standard error noticeably in determining ADCprotein of WHG.
Apparent digestibility coefficient for amino acids reached a consistent high value; however, it was difficult to separate the results except for ALA. As mentioned above, ADCprotein was not found as expected especially for WHG. It could emphasize an overestimation for ADCaa or a rapid absorption at the intestine level (Deshimaru 1976) with in final a low aa content in faeces as a consequence of a poor hydrolysis. A rapid absorption was described with probably a low saturation of sites and low competition between aa for example for leucine, valine and methionin at the same site of absorption as described for fish.
It was not possible to identify the respective incidence of variability in the measure in spite of the replicates or of the effect of the processing on the ingredient. The coefficients 23/35/15 proposed for protein, lipid and cbh respectively (Cuzon & Guillaume 1997b) allowed calculating DE (203 kJ kg−1) that led to an ADC of about 82% considering GE = 25 kJ g−1, well above what was found in this study. The coefficients emphasized the distinction between native gluten (Viten®; Roquette) and a regular one and explained some differences in the literature for this ingredient (Brown & Kisiel 2003).
Indirect calorimetry was used to extend the comparison and to try to show a difference among the three diets. Feed intake can be estimated on a daily basis, expressed in mg day−1 and then converted to DE kJ g−1 with ADCenergy calculated separately. Five per cent body weight was fitted for the different treatments; energy expenditure during inanition (HeE) and just after feed ingestion (HiE) or the sum (HeE + HiE) allowed examining incidence of feed intake on protein synthesis. Also, a high capacity to use protein as an energy source for growth was previously described in shrimp (Cuzon & Guillaume 1997b).
Basal metabolism (HeE) did not present any significant difference between protein sources tested, signalling a similar physiological status of juvenile L. vannamei. HiE basically involved (i) energy required for deamination and nitrogen excretion (Kleiber 1975); (ii) post-absorptive process in relation to feed intake, in particular protein-rich feed, and protein and fat synthesis from substrates absorbed; (iii) energy expenditure for digestion and absorption process (Emmans 1994) that could vary from 10% to 50% in relation to quality protein. A difference was observed between the tested diets. There is a drastic change in HiE among the three diets (P < 0.05). A similar value was expected as shrimp received the same amount of feed with only one difference consisting of protein quality between diets. SPCd did not enhance HiE contrasting with the high values observed with SBM fed M. japonicus (Koshio, Teshima & Kanazawa 1993). In addition to this, juveniles seemed to utilize SPC better than SBM.
Soybean protein concentrate diet provided an acceptable weight gain as recorded after 45 days on shrimp in clear water indoor tanks that corroborates RE value found with energy partition. Weight gain with SPCd tended to prove that anti-nutritional factors not determined in this study were not detrimental and a gain close to 0.9 g week−1 reflected a kind of reference norm for the species in the environment of Yucatan. Finally, a high variability remained, but a beneficial effect of SPCd was evidenced. A low level of trypsin inhibitor (1.88 mg g−1) and antigens (glycinin and conglycinin with 14 and 8 wells of inhibition respectively at haemaglutinin test) in a regular SBM or its absence in SPCd could contribute to such nutritive value.
Shrimp feed quality was compared in larvae, juvenile, and adult muscle as a reference for such a flesh mussel (composition and freshness) that would match Marsupenaeus japonicus requirement. Weight gain may be optimum when a diet with a basic aa profile close to the whole shrimp composition was obtained. Alternatives to fresh food, such as crab or casein protein, evidenced differences in weight gain with EAA deficiency on arginine (Fox, Lawrence & Li-Chan 1995). However, 40% protein was the basis for elaborating diets for juvenile L. vannamei raised in clear water (Cuzon & Guillaume 1997a). The aa profile of each diet helped to identify a limiting factor (Table 3) on ARG. Limiting LYS level could refrain weight gain, as this aa is much needed in fast-growing animals such as chicken. All results converged to the evidence that feed was as good as its ingredients (Glencross, Booth & Allan 2007). However, any incidence of quality ingredients could have an impact on shrimp quality at harvest. Abdominal muscle texture could change with dietary protein content as well as with growth performances (Rivas-Vega, Goytortua-Bores, Ezquerra-Brauer, Salazar-García, Romaire & Lutz 1989). Previous observations on juveniles L.stylirostris fed on all plant protein during a month could produce a combination of reduced feed intake with poor muscle texture. It is possible that although diets had the same protein content, the quality of the protein could affect digestion and absorption with subsequent incidence on bioavailability that would change the texture of abdominal muscle (Haard-Norman 1992; Ezquerra Brauer et al. 2003). Parameters such as dietary aa (limiting EAA) and ADC part of indirect calorimetry during short-term period of contention helped to discriminate or qualify diets with vegetable protein sources. Such approach proved efficient in trout (Cho & Bureau 1998) and it is still in use, even though there is a trend for dynamic energy biomass approach (Koojiman 2007) and, in the future, it could be possible, with some well-defined parameters, to run such a programme and find a way to optimize feed input and reduce waste.
A particular role of proline (PRO) as a constituent of free aa in the muscle was described on P. esculentus (Smith & Tabrett 2003). For each diet, a range was calculated from 2 to 4 g (Table 3) seemingly similar in normal feeding conditions. Nevertheless, at this stage, no relationship can be set for an improvement in texture of farmed shrimp. Muscle texture decreased in toughness during storage due to proteases hydrolysis action on myofibrillar with a low calpain inhibitor activity (Chen, Guttman, Xiong, Webster & Romaire 2008), elastases, and collagenases contributing to ageing in fish (Delbarre-Ladrat, Cheret, Taylor & Verrez-Bagnis 2006). The later being crucial, as proteolytic enzymes measured in shrimp HP and muscle of several other marine organisms (Lindner, Angel, Weinberg & Granit 1988) were associated with some changes in muscle texture due to a production of trypsin as found in L. vannamei (Torres-Mendoza 2003).
In this case, collagenase activity influenced muscle quality leading to post-mortem alteration. Collagenase activity, shear force and muscle collagen content were positively modified in L. vannamei fed with sardine or squid meal-based diets. Protein source could act on the enthalpy of transition of collagen from shrimp muscle, collagenase activity and texture in shrimp tail meat (Ezquerra Brauer et al. 2003). However, a loss in initial texture seemed independent of proteases effect during finfish freezing process (Ladrat, Verrez-Bagnis, Noel & Fleurence 2003; Shigemura, Ando, Tsukamasa, Makindan & Kawai 2003). In this study, muscle texture did not respond in the range of fishmeal replacement chosen in this experiment. The comparison between muscle collagenase activity in shrimp fed SPCd, WHGd or PPCd did not show any difference. None of the three diets altered muscle composition in spite of 3.9% PRO being slightly higher in WHGd (Table 3). Although chemical score bore on basic aa for two diets and THR for SPC had no apparent difference in relation to texture for the rearing period concerned, collagen extracted from muscle of shrimp fed SPCd had a lower number of bands and revealed five major fractions comprised between 21 and 77 kDa.
Conclusion
The results of this study confirm that of the three plant protein concentrates evaluated, SPC has the best nutrient quality for the L. vannamei feeds as a good source for fishmeal replacement, in spite to their aa deficiency (MET and THR). Digestion and absorption parameters measured through trypsin activity, HiE and ADCprotein indicated that SPCd was more efficient than WHGd and PPCd. It was correlated also with the growth values as index of assimilation. However, a negative impact of SPCd on shrimp muscle texture was observed through collagen numbers of bands at 120 kDa, probably due to an aa deficiency (MET).
Acknowledgements
We thank the following technicians: Adriana Paredes, Gabriela Palomino, Miguel Arevalo, Gabriel Taboada, Manuel Valenzuela, Maribel Badillo and Ariadna Sánchez for their technical support. PAPIIT-UNAM IN 2140602 and CONACyT 49406/24750 helped financing the study. We thank CONACyT for the PhD scholarship to C. Maldonado. Roquette Lestrem France provided potato protein Tubermine® (Roquette) and DSM for the vitamin premix and minerals. Translation and style correction services were offered by Noé Chirino, PhD & Larisa Herrera, MA.




