Molecular identification and tissue distribution of peroxisome proliferators activated receptor gamma transcript in cultured Thunnus orientalis
Abstract
Pacific bluefin tuna (PBT) peroxisome proliferator-activated receptor γ (PPARγ) gene was characterized to know the expression of its transcript contribution to the development of PBT, because PPARγ is the key molecule for adipose cell differentiation. Resulting cDNA and deduced amino acid sequence had high similarities to other teleosts that consisted of A to F domains. Phylogenetic tree analysis indicated a close relationship PBT PPARγ to cobia and flatfishes ones among the teleosts with the similarity of characteristic insertion sequences at amino terminal region of E/F domains. PPARγ transcripts quantification profile in the tissues of a 13-month-old PBT indicated the correlation between its quantity with the muscle types of different lipid content. The transcripts were also detected in the head kidney, digestive organs and red muscle with higher level inferred the PPARγ contribution to the multiple physiological processes. PPARγ transcripts were quantified for PBT juveniles fed artificial diets to investigate the effect of phospholipid supplementation on the growth, survival and PPARγ expression, to verify the efficacy of phospholipid source. No obvious difference in growth performance, survival and gene expression of PBT juveniles was identified, this may be due to DHA/EPA ratio analogy between diets and indicating that phospholipid sources in the juvenile diet might be adaptable.
Introduction
The aquaculture industry continually strives to improve fish flesh quality, with lipid accumulation and metabolism being given the prime importance (Einen & Skrede 1998; Rora, Kvale, Morkore, Rorvik, Steien & Thomassen 1998; Todorčević, Vegusdal, Gjøen, Sundvold, Torstensen, Kjær & Ruyter 2008). This is especially significant in the case of the tuna species whose lipid content is the key factor in grading the flesh quality (Miyake, De La Serna, Natale, Farrugia, Katavic, Miyabe & Ticina 2003; Nakamura, Ando, Seoka, Kawasaki, Sawada, Miyashita, Okada, Kumai & Tsukamasa 2006). During the early development of fish, lipids and their constituent fatty acids, play essential roles in maintaining optimum growth, survival, feed efficiency, health, neural and visual development, and the response to various stressors in addition to being their main energy source (Dhert, Lavens & Sorgeloos 1990; Ashraf, Bengston & Simpson 1993; Kanazawa 1997). Among the lipids and their constituents, phospholipids, highly unsaturated fatty acids and their precursors have attracted particular attention due to their large contribution in the aforementioned biological processes (Coutteau, Geurden, Camara, Bergot & Sorgeloos 1997; Weirich & Reigh 2001; Cahu, Infante & Takeuchi 2003). Appropriate uptake and accumulation of lipids improve growth and survival of fish in their early developmental stages, with lipid storage being the source of energy and physiological substances. Lipids are much more important in highly active fish species which experience rapid growth, due to massive energy consumption and an increased metabolism (Dickson 1994; Miyashita, Sawada, Okada, Murata & Kumai 2001; Blank, Morrissette, Farwell, Price, Schallert & Block 2007).
Tunas are prime examples of fish with such characters; this has resulted in much attention being spent on understanding their lifestyle and energy metabolism (Korsmeyer & Dewar 2001). The tuna aquaculture industry has grown drastically in recent years, with the increasing demand for higher catches endangering the survival of the wild stocks. Tuna aquaculture has an essential role in the attempt to reduce fishing pressure on the wild stock, and in decreasing our dependency on natural resources; though unfortunately the survival rate is poor in artificially hatched Pacific bluefin tuna (PBT) (Miyashita, Sawada, Hattori, Nakatsukasa, Okarda, Murata & Kumai 2000; Sawada, Okada, Miyashita, Murata & Kumai 2005; Seoka, Kurata & Kumai 2007). In order to promote better growth, development and survival of cultured PBT it is necessary to identify a number of essential biochemical compounds and molecular biological processes. Recent studies have found that salmon roe phospholipid supplementation has a beneficial effect on growth and survival of PBT juveniles (Seoka, Kurata, Tamagawa, Biswas, Biswas, Yong, Kim, Ji, Takii & Kumai 2008). Although salmon roe improved growth and survival of PBT juveniles, the exact physiological mechanism is unknown. An added disadvantage is the high cost of salmon roe phospholipid which makes it impractical for use in aquaculture.
Peroxisome proliferator-activated receptor γ (PPARγ), which forms a heterodimer with a retinoid × receptor, is a ligand-activated transcriptional factor that belongs to the nuclear hormone receptor superfamily (Issemann, Prince, Tugwood & Green 1993; Tontonoz, Hu, Graves, Budavari & Spiegelman 1994). PPARγ plays an important role in adipocyte differentiation (adipogenesis) and lipid accumulation (Chawla, Schwarz, Dimaculangen & Lazar 1994; Tontonoz, Hu, Graves et al. 1994; Tontonoz, Hu & Spiegelman 1994; Hu, Tontonoz & Spiegelman 1995; Rosen, Sarraf, Troy, Bradwin, Moore, Milstorne, Spiegelman & Mortensen 1999; Schadinger, Bucher, Schreiber & Farmer 2005). A variety of substances have been suggested to be natural ligands of PPARγ, for example, fatty acids and eicosanoids (Yu, Bayona, Kallen, Harding, Ravera, Mcmahon, Brown & Lazar 1995; Kliewer, Sundseth, Jones, Brown, Wisely, Koble, Devchand, Wahli, Willson, Lenhard & Lehmann 1997; Desvergne & Wahli 1999), components of oxidized low-density lipoproteins (Nagy, Tontonoz, Alvarez, Chen & Evans 1998), oxidized alkyl phospholipids including lysophosphatidic acid (Mcintyre, Pontsler, Silva, St Hilaire, Xu, Hinshaw, Zimmerman, Hama, Aoki, Arai & Prestwich 2003) and nitrolinoleic acid (Schopfer, Lin, Baker, Cui, Garcia-barrio, Zhang, Chen, Chen & Freeman 2005). Numerous studies focusing on PPARγ have been conducted to identify its structure, functions, exogenous and endogenous ligands, tissue expression under different conditions; contributing to the comparative physiology, medical, livestock and aquaculture sciences. Recent publications have identified various kinds of marine fish PPARs that possess a role in regulating adipogenic genes (Andersen, Eijsink & Thomassen 2000; Vegusdal, Sundvold, Gjøen & Ruyter 2003; Leaver, Boukouvala, Antonopoulou, Diez, Favre-krey, Ezaz, Bautista, Tocher & Krey 2005; Oku & Umino 2008; Tsai, Chen, Tseng & Chang 2008; Cho, Kong, Nam, Kim, Noh, Lee, Kim & Cheong 2009).
Identifying PBT PPARγ and its tissue distribution may be useful to understand the regulation of adipocyte growth or differentiation and nutritional condition, which could be a solution for the improvement in low survival rate of the juvenile. However, no information for PBT PPARγ is available until now. In this study, we identified PBT PPARγ gene and investigated PPARγ expression profiling in various tissues of a 13-month-old fish. Additionally, the effect of phospholipid supplementation was examined on the growth, survival and PPARγ expression in the liver and muscle tissue of juvenile fish. The diet test was also conducted aiming to verify the efficacy of low-cost bonito oil polar lipid fraction as the dietary phospholipid source.
Materials and methods
Cloning of PBT PPARγ cDNA
RNA purification and reverse transcription were conducted as per the manufacturer's protocol. Total RNA was obtained from the liver of a 2-year-old male PBT (20.4 kg body weight) artificially hatched and raised in Kinki University. The liver was immediately frozen in liquid nitrogen after dissection, and thereafter kept in the freezer at −80 °C for 1 week until RNA extraction. One hundred and ten milligrams of liver was homogenized with 3 mL of Isogen (Nippongene, Tokyo, Japan), then 60 μg of total RNA was obtained; 30 μg of total RNA was DNaseI (TaKaRa Bio, Otsu, Japan) treated. cDNAs were synthesized in 20 μL volume containing 5 μg of total RNA, 50 pmol of random 9-mer primer (Toyobo, Tokyo, Japan), 0.5 mM of each dNTPs, 20 U of RNase inhibitor and 200 U of Prime Script Reverse Transcriptase (TaKaRa Bio). Total RNAs were quantified by UV spectrometer. Reverse transcript was used as a template for the PCR-based cloning of PPARγ. The following synthetic oligonucleotides were used for the amplification of PPARγ cDNA. Degenerated primers, DpF-1 and DpR-1 (Table 1) were designed depending on the cDNA alignment of teleosts PPARγ sequences. The PCR condition was as follows: 25 μL reaction volume containing 7.5 pmol of DpF-1, DpR-1 primers, 0.1 mM of dNTPs, 0.125 U of Ex Taq DNA polymerase (TaKaRa Bio) and 0.2 μL of reverse transcripts. The PCR product was confirmed by agarose gel electrophoresis, single band corresponding to 806 bp was observed. Qiaquick Gel Extraction (Qiagen, Duesseldorf, Germany) was used for gel extraction of 806 bp band, then cloned into pCR2.1 T-A cloning vector (Invitrogen, Carlsberg, CA, USA), then sequenced using BigDye terminator ver. 3.1, M13 forward and M13 reverse primer. Sequence data were analysed by FASMAC (Atsugi, Japan).
| 5′–3′ | |
|---|---|
| For molecular cloning | |
| DpF-1 | CATGYCTGYGAGGGCTGYAAGGG |
| DpR-1 | GGYTTCCTSAGRCTCTTGAGRAA |
| GSP-1 | AGTGACAGAATTCGCCAAGAGC |
| GSPanti-1 | CCTTGGCCTTGGTGAGGGGGAA |
| GSP-2 | TAATCATCATGATGTCGCC |
| GSPanti-2 | TCTCCGCCTGGGGCATTCGG |
| 3 sites Adaptor primer | CTGATCTAGAGGTACCGGATCC |
| For real-time PCR | |
| PPARγ rtF | ACCTGACCAACATGGACTAC |
| PPARγ rtR | GAGAAAACAGGACTGTCAGC |
| Tunaβ-act rtF | ACCCACACAGTGCCCATCTA |
| Tunaβ-act rtR | TCACGCACGATTTCCCTCT |
- R=A/G, S=C/G and Y=C/T; PBT, Pacific bluefin tuna.
RACE (rapid amplification of cDNA end)
3′ RACE was performed using 3′-Full RACE core set (TaKaRa Bio), following the manufacturer's protocol. A 20 μL reaction solution containing 10 mM Tris-HCl pH 8.3, 50 mM KCl, 1.0 μg of liver total RNA, was reverse transcribed using Oligo dT-3sites adaptor primer and AMV reverse transcriptase XL (Life Sciences, Petersburg, FL, USA). Reverse transcript was amplified by PCR in 25 μL reaction volume containing 0.125 U of Ex Taq DNA polymerase, 7.5 pmol of sense strand gene-specific primer GSP-1 and 7.5 pmol of Oligo dT-3sites Adaptor primer. Thermal condition was 94 °C for 2 min, 30 cycles of 94 °C for 20 s, 55 °C for 30 s, 72 °C for 1 min, 72 °C for 2 min, and then kept at 4 °C. Several weak bands were observed after electrophoresis, then nested PCR was performed under the following conditions, basically same as first PCR. One microlitre of first PCR product was diluted by addition of 99 μL TE buffer, then 1.0 μL of diluted solution was used as second PCR template. Sense strand gene-specific primer; GSP-2 and 3 sites Adaptor primer (Table 1) were used for second PCR. After the second PCR, clear major band, which is approximately worth to 700 bp was confirmed by 0.8% agarose gel electrophoresis. The band was gel extracted and cloned into pCR2.1 TA cloning vector, then independent five clones were sequenced as same as above.
5'RACE
Before performing 5′ RACE, 3.2 μg of poly-A RNA was purified from 200 μg of liver total RNA using Oligotex-dT30 mRNA purification kit (TaKaRa Bio). For reverse transcription, 1.0 μL of 5′ CDS primer and 120 ng of poly-A RNA were filled up to 3.75 μL with DEPEC-treated water, and their mixture was incubated at 72 °C for 3 min, followed by the incubation at 42 °C for 2 min on the thermal cycler. Later procedures were performed as per the manufacturer's protocol. 5′ RACE was performed using PPARγ-specific antisense primers: GSPanti-1, GSPanti-2 and Universal Primer A Mix (UPM), which corresponds to SMARTer oligonucleotide partially. 5′ RACE product was confirmed by 0.8% agarose gel electrophoresis. A single band of approximately 700 bp was observed when the primers of GSPanti-2 and UPM were used together.
Phylogenetic analysis
Vertebrate PPARγs amino acid sequences were obtained from DNA Data Bank of Japan (DDBJ) for the phylogenetic analysis. The species and those accession numbers are as follows; Ctenopharyngodon idella (EU847421), Danio rerio (DQ839547), Dicentrarchus labrax (AY590303), Homo sapiens (X90563), Lateolabrax japonicus (DQ345545), Mus musculus (BC021798), Onchorhynchus keta (AB210272), Oryzias latipes (AB469414), Pagrus major (AB298549), Platichthys flesus (CAB51396), Pleuronectes platessa (AJ539469), Rachycentron canadum (DQ321817), Salmo salar (AJ416951), Sparus aurata (AY590304), Takifugu rubripes (AB275885), Tetraodon nigroviridis (CAAE01014979), Thunnus orientalis (AB574331 PBT PPARγ cDNA sequence was obtained by this study then submitted to DDBJ), Xenopus laevis (AJ310087). Human Rev-Erb Alpha (X72631) was used as an outgroup. Amino acid alignment and phylogenetic tree was generated by ClustalW (1.83) program using neighbor-joining method [DDBJ, http://clustalw.ddbj.nig.ac.jp (Saitou & Nei 1987)]. Inferred tree was confirmed using TreeDyn software obtained from Institute National de Recherche en Informatique et en Automatique (INRIA, France), and NJPlot software from Pôle Bio-Informatique Lyonnais (PBIL, France).
Quantitative real-time PCR
For the quantitative real-time RT-PCR, RNA was treated with RNase-free DNase I (TaKaRa Bio) and used as a template for real-time PCR using a Real-Time PCR 7300 system (Applied Biosystems, Carlsbad, CA, USA). cDNA was synthesized using random 9-mer oligonucleotides and ReverTra Ace (Toyobo). In diet-tested fish, 50 mg of liver and 100 mg of dorsal ventral ordinary muscles were used. Both absolute PPARγ and β-actin gene transcripts were measured and PPARγ transcripts were normalized by that of β-actins. Absolute quantities were calculated by using plasmid containing PPARγ and β-actin fragment as a standard. In the expression profiling of 13-month-old fish, 50–100 mg of organs and muscles were subjected to RNA isolation and quantitative PCR. A 13-month-old of 2.8 kg PBT, which was artificially hatched and raised in Kinki University, was immediately dissected on the boat after capture and tissues were frozen using liquid nitrogen. Each sample measurement was repeated four times from one reverse transcript. Quantitative PCR was performed using 20 μL solution containing 10 μL of SYBER premix Ex Taq II (TaKaRa Bio), 8.0 pmol of forward and reverse primers and 10 ng total RNA derived reverse transcript. Quantification of PCR products were measured by SYBER Green intercalation. For absolute quantification, plasmids containing PCR target region were cloned into pCR2.1 vector then amplified by liquid LB medium culture and purified plasmid were quantified UV spectroscopy. 105 to 109 fold diluted plasmid dilutions were used to approximate absolute quantity.
The following synthetic oligonucleotides were used to detect reverse transcripts: PPARγrtF (corresponding to 232–251 bp of PPARγ cDNA NCBI accession no. AB574331) and PPARγrtR (corresponding to 331–350) were used for PPARγ gene generated a 180 bp product Tuna β-act rtF and Tuna β-act rtR (Adachi, Kato, Yamamoto, Ishimaru, Kobayashi, Osamu Murata & Kumai 2008) were for β-actin gene (Table 1) generated a 170 bp product. Real-time PCR quantifications were performed four repeated measurements.
Experimental diet test and fish rearing
Ingredients and proximate composition of the experimental diets are provided in Table 2. Results of lipid class and fatty acid composition analysis of each diet are provided in Table 3a. Enzyme-treated fishmeal was the main protein source of the diets. Lipid content of all diets (D1–4) were equally adjusted to 12.5% in the total diet weight. The following lipid sources were used in each diet: D1; skipjack tuna neutral lipid fraction, D2; skipjack tuna polar lipid fraction, D3; 7.5% skipjack tuna polar and 5% soybean polar lipid fraction, D4; soybean polar lipid fraction (Table 2). Fatty acid composition of total fatty acid of the experimental diets was shown in Table 3b. Fatty acids of experimental diets were analysed by Ueda Oils and Fats MFG (Kobe, Japan). A total of 150 individuals were reared in each 500 L volume tank and fed for 7 days, the diet test was conducted in triplicate. Five fish were randomly collected for the expression analysis from each tank at the commencement of experiment, first, fourth, and seventh day of the diet test. Diet-tested PBT juveniles were fed every 2 h from 06:00 to 22:00 hours, and the PBT juveniles subjected to the measurement were caught at 9 am, 3 h after the daily first feeding. Sampled juveniles were immediately frozen in the liquid nitrogen and stored at −80 °C in a freezer until the expression analysis was conducted.
| Ingredients (%) | Experimental diets | |||
|---|---|---|---|---|
| D1 | D2 | D3 | D4 | |
| Enzyme-treated fish meala | 45.0 | 45.0 | 45.0 | 45.0 |
| Casein | 20.0 | 20.0 | 20.0 | 20.0 |
| Corn gluten | 10.0 | 10.0 | 10.0 | 10.0 |
| Bonite neutral lipid | 12.5 | |||
| Bonite polar lipid | 12.5 | 5.0 | ||
| Soybean polar lipid | 7.5 | 12.5 | ||
| Halver's vitamin mixturesb | 7.0 | 7.0 | 7.0 | 7.0 |
| Halver's mineral mixturesb | 5.0 | 5.0 | 5.0 | 5.0 |
| Taurine | 0.5 | 0.5 | 0.5 | 0.5 |
| Feeding stimulantsc | 0.7 | 0.7 | 0.7 | 0.7 |
- a Enzyme-treated brown fishmeal (Bio-CP®, Nagase Bio-Chemical Sales, Japan).
- b Halver's mineral and vitamin mixtures (Halver 1957).
- c l-glutamic acid, 0.013%; l-histidine monohydrochloride monohydrate, 0.357%; inosine 5′-monophosphate disodium salt, 0.308% (Ji, Takaoka, Seoka, Kohbara, Hosokawa, Shimeno, Jeong, Lee & Takii 2007).
| % Lipid | D1 | D2 | D3 | D4 |
|---|---|---|---|---|
| TL (% of wet weight) | 22.49 | 20.81 | 21.15 | 23.08 |
| PL (% of total weight) | 13.38 | 41.63 | 55.30 | 61.88 |
| NL (% of total weight) | 86.62 | 58.37 | 44.70 | 38.13 |
| PL class (% of PL content) | ||||
| PE | 6.78 | 3.85 | 7.73 | 11.68 |
| PC | 41.51 | 39.01 | 53.16 | 61.96 |
| SPM+LPC | 9.85 | 26.23 | 13.83 | 3.29 |
| PS+PI | ND | ND | ND | ND |
| Others | 41.86 | 30.91 | 25.28 | 23.07 |
| NL class (% of NL content) | ||||
| S | 1.60 | 3.03 | 2.82 | 2.52 |
| TAG | 92.00 | 82.94 | 85.93 | 86.01 |
| SE | 0.84 | 3.25 | 2.27 | 2.96 |
| Others | 5.56 | 10.78 | 8.98 | 8.50 |
- TL, total lipid; PL, polar lipid fraction; NL, neutral lipid fraction; PE, phosphatidylethanolamine; PC, phosphatidylcholine; SPM, sphingomyelin; LPC, lysophosphatidylcholine; PS, phosphatidylserine; PI, phosphatidylinositol; S, sterol; TAG, triacylglycerol; SE, sterol ester.
| TL % | D1 | D2 | D3 | D4 |
|---|---|---|---|---|
| C14:0 | 3.64 | 4.02 | 3.04 | 1.83 |
| C15:0 | 0.87 | 1.10 | 0.75 | 0.34 |
| C16:0 | 17.84 | 25.16 | 20.11 | 14.50 |
| C16:1 | 4.19 | 2.75 | 2.07 | 1.27 |
| C17:0 | 0.88 | 1.28 | 0.82 | 0.33 |
| C17:1 | 0.26 | 0.30 | 0.24 | 0.15 |
| C18:0 | 4.65 | 8.18 | 6.05 | 3.71 |
| C18:1n-9 | 14.39 | 14.87 | 13.67 | 12.82 |
| C18:1n-7 | 2.16 | 2.22 | 2.03 | 1.84 |
| C18:1 total | 16.55 | 17.09 | 15.70 | 14.65 |
| C18:2n-6 | 3.65 | 4.38 | 18.21 | 35.86 |
| C18:3n-3 | 0.83 | 0.70 | 2.12 | 3.91 |
| C20:1 | 4.46 | 5.89 | 5.05 | 4.09 |
| C20:4n-6 | 1.27 | 0.93 | 0.73 | 0.38 |
| C20:5n-3 (EPA) | 5.39 | 3.49 | 3.37 | 2.75 |
| C22:1 | 2.24 | 2.58 | 2.21 | 1.93 |
| C22:5n-6 | 1.67 | 1.80 | 1.57 | 1.14 |
| C22:6n-3 (DHA) | 22.76 | 12.65 | 12.12 | 9.39 |
| Others | 8.86 | 7.70 | 5.85 | 3.74 |
| DHA/EPA | 4.22 | 3.62 | 3.60 | 3.41 |
- EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
Statistics
PPARγ transcript quantity in juvenile liver, muscles (Fig. 4), final total length, body weight, condition factor and survival rate in the diet test (Table 4) were expressed as mean ± standard deviation (SD) and were analysed by two-way analysis of variance (anova) with Bonferroni's correction. Statistical analyses were performed by using the Statistical Package for the Social Science program for Windows (version 16.0J).
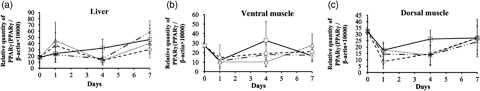
Relative gene expression pattern of PPARγ/β-actin in diet-tested juvenile (a) liver and (b) dorsal and (c) ventral ordinary white muscles in PBT. Line with dots (X); skipjack neutral lipid fraction was used as lipid source of artificial diet (diet 1; D1); solid line (□): skipjack polar lipid (D2); small dotted line (Δ): skipjack polar and soybean polar (D3); spaced line (○): soybean polar lipid (D4) respectively. Quantifications were performed by quantitative RT-PCR. 23 dph juveniles were fed four different diets for 1 week. Five independent fish were measured, measurements were repeated four times (n=5 × 4). Vertical lines indicate the standard errors. Values are statistically not different (P>0.05). PPARγ, peroxisome proliferator-activated receptor γ.
| Parameters | ||||
|---|---|---|---|---|
| Total length (mm) | Body weight (g) | Condition factor | Survival rate (%) | |
| Initial | 29.8 ± 4.1 | 0.3 ± 0.1 | 15.7 ± 2.5 | |
| Final | ||||
| D1 | 45.0 ± 4.3 | 1.0 ± 0.3 | 14.4 ± 2.4 | 63.6 ± 10.4 |
| D2 | 45.3 ± 5.5 | 1.0 ± 0.4 | 14.4 ± 4.1 | 66.0 ± 1.3 |
| D3 | 47.2 ± 5.3 | 1.1 ± 0.4 | 13.6 ± 1.5 | 74.9 ± 2.7 |
| D4 | 48.1 ± 5.0 | 1.2 ± 0.4 | 14.8 ± 2.0 | 74.7 ± 1.8 |
- TL, BW, CF; n=30, survival rate; n=3. Values in final are statistically not different (P>0.05).
- TL, total length; BW, body weight; CF, condition factor.
Results
cDNA cloning of PPARγ gene and phylogenetic analysis
Fresh liver of a 2-year-old PBT was subjected to total RNA extraction for reverse transcript based PCR screening. We identified 806 bp conserved sequence of PBT PPARγ cDNA using degenerated primers DpF-1 and DpR-1 which were designed on 806 bp regions to perform 5′ and 3′ RACE reactions. RACE reactions were successfully performed using these primers, and the full-length cDNA sequence of PBT PPARγ was characterized. PPARγ cDNA consisted of 1660 bp of that ORF of 534 amino acids.
Deduced amino acid sequence of the PBT PPARγ shares a close similarity to a number of other teleosts; for example cobia, Rachycentron canadum is the closest with 92% amino acid identities in whole PRF (Tsai et al. 2008), plaice, Pleuronectes platessa, 90% (Leaver et al. 2005), red sea bream, Pagrus major, 88% (Oku & Umino 2008), tiger puffer, Takifugu rubripes, 81% (Maglich, Caravella, Lambert, Willson, Moore & Ramamurthy 2003). In addition, amino acid identities of the PBT PPARγ C and D domains to other vertebrates was 100% in R. canadum, P. platessa and P. major, 99% in gilthead sea bream, S. aurata and T. rubripes, and 78% in Atlantic salmon, Salmo salar respectively (Fig. 1). Therefore, the obtained cDNA sequence was concluded to be the PBT PPARγ gene. A/B domain and amino terminal region of E/F domain were diverged between species. Other regions, carboxyl terminal of E/F domain, C (containing conserved two Cys-4 type zinc fingers) and D domains were highly conserved among species, these function as a sequence specific DNA-binding region of PPAR response elements (PPREs), suggesting that PBT PPARγ may also activate the transcription of adipogenic genes carrying PPREs.
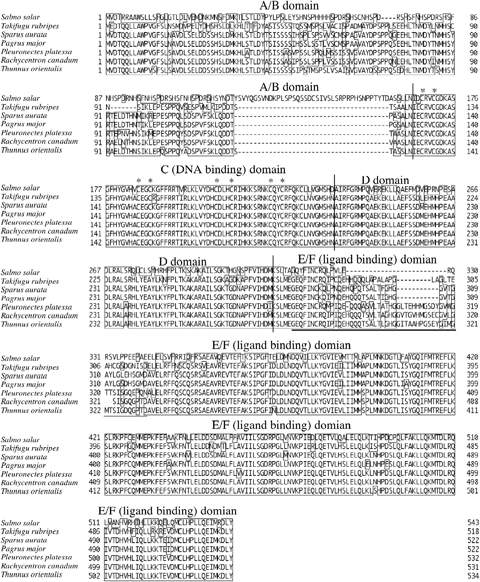
Deduced amino acid sequences and multiple alignments of teleost peroxisome proliferator-activated receptor γ (PPARγs) are provided. Specific names are provided on the left of sequences. Enclosed residues were identical between sequences, gaps (−) were introduced to maximize identities. The DNA-binding domain contains two zinc-binding modules with the eight conserved cysteine residues involved in Zn2+ coordination that are shown by asterisks. Underline indicates thermal mobile loop of the protein (Andersen et al. 2000).
The phylogenetic tree generated for PBT and 17 other vertebrates clustered the PPARγ sequences into several groups, all of which had the significant statistical indicators of bootstrap value higher than 500 (Fig. 2). Salmonids, Atlantic and Chum salmon, O. keta, were distinguishable from other vertebrates in the tree, because their amino acid sequences were highly diverged in the A/B domain compared with others, with a long insertion at A/B domain in particular (Andersen et al. 2000). PBT PPARγ was similar to that of cobia, flatfishes (P. flesus, and P. platessa), followed by the sparids (P. major and S. aurata) and sea basses (L. japonicus and D. labrax).
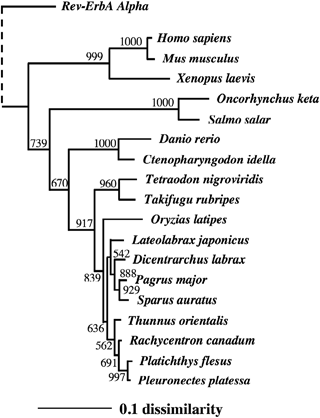
PPARγs) phylogenetic tree constructed using neighbour-joining method based on deduced amino acid sequences of vertebrates (Saitou & Nei 1987). Human Rev-ErvA alpha was used as an outgroup. Bootstrap value of 1000 repeated calculation was shown at branches. Scale bar shows 10% sequence dissimilarity. Accession numbers of sequence subjected to the tree are described in ‘Materials and methods’.
Tissue distribution of PPARγ transcripts in a 13-month-old PBT
This study determined the tissue expression profile of PPARγ transcripts in a 13-month-old fish, to identify the characteristic expression of PPARγ in PBT. There are two ways to indicate the transcript quantity. One is to indicate it by relative quantity of the gene interest versus quantity of internal standard gene; another is to show absolute quantity of the transcripts versus fixed quantity of total or poly-A RNA. At first, we measured transcripts of PPARγ/β-actin relative quantity, however the quantity of β-actin/fixed quantity of total RNA differed between tissues. Therefore, we indicated PPARγ quantity by absolute quantity versus 10 ng of total RNA-derived reverse transcripts (Fig. 3). The highest number of PPARγ transcripts was observed for the head kidney followed by the pyloric caeca and intestine. PPARγ transcripts were detected in large numbers in lipid-rich muscles (Roy, Miyake, Ando, Kawasaki & Tsukamasa 2010) with the exception of the inner red muscle. We examined the PPARγ expression in the following three types of white muscle: O-toro is the disto-ventral white ordinary muscle containing the highest fat content; and Chu-toro is in the central portion of the ventral muscle with a lower lipid content when compared to O-toro; Akami is the proximal white ordinary muscle with the lowest fat content (Roy et al. 2010). High levels of PPARγ transcripts were detected in O-toro then Chu-toro and Akami muscle. Extraocular muscle, which is present behind the eyes, and masticatory muscle, which closes the mouth, contained high lipid levels.
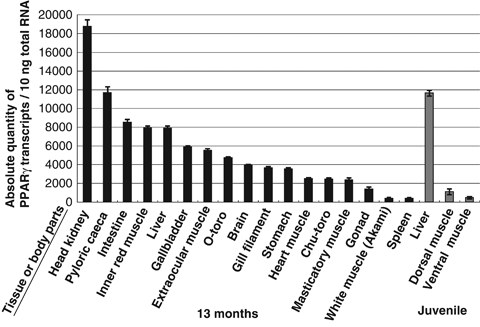
Peroxisome proliferator-activated receptor γ (PPARγ) gene quantification profile of various tissues and muscle parts in cultured Thunnus orientalis. Absolute quantities (left of the figure) of PPARγ are shown. Left: quantification of 13-month-old fish are shown in black; right: 30 dph juveniles are shown in grey. Fatty white ordinary muscle was designated as O-toro, moderately fatty white ordinary muscle (Chu-toro); fatless white ordinary muscle (Akami). Vertical line indicates standard errors. Measurements were repeated four times (13-month-old fish; n=1 × 4, 30 dph juveniles; n=5 × 4). Vertical lines indicate the standard errors. Quantification was performed by quantitative RT-PCR. Tissues and muscle parts measured are shown at the bottom in the figure.
Diet test of juvenile PBT
Experimental fish experienced a relatively high growth rate almost tripling the body weight (1.0 g, 30 dph, n=30) compared with the initial (0.3 ± 0.1 g, 23 dph, n=30) body weight (Table 4). The final body weight and condition factor [BW/SL3; body weight (BW), standard length (SL)] was higher in fish fed D4 diet than others (Table 4). Survival rates between diet groups, which varied from 63.6% to 74.7%. However, statistical differences were not confirmed in BW, TL, SL and CF (Table 4). To know the character of the diets, lipid class compositions (Table 3a) and fatty acid compositions (Table 3b) were analysed. Phosphatidylethanolamine (PE) and phosphatidylcholine (PC) were higher in diet D3 and D4 than other diets (Table 3a), C18:2n-6 and C18:3n-3 were higher in D3 and D4 (Table 3b), which showed better growth performances (Table 4). To know whether diet nutrition affects the PPARγ transcript quantity, we determined relative quantities of PPARγ transcripts during the diet test in the juvenile liver, dorsal and ventral muscle (Fig. 4). There was no significant statistical difference in PPARγ transcripts between diets.
Discussion
Amino acid sequence of the PBT PPARγ had the highest similarity to that of cobia and flatfishes (1, 2), which was referred from the 11 residue insertions at amino terminal region of E/F domain of these splices differing from other teleosts (Leaver et al. 2005; Tsai et al. 2008, Fig. 1). This region is reported as the thermally mobile loop of direct ligand binding (Nolte, Wisely, Westin, Cobbs, Lambert, Kurokawa, Rosenfeld, Willson, Glass & Milburn 1998; Andersen et al. 2000), and is highly variable among species. Phylogenetic analyses of PPARγ conducted in separate studies supports our current categorization in fish (Leaver et al. 2005; Oku & Umino 2008; Tsai et al. 2008), and it also shown that cobia and flatfishes are close to PBT. As the current information of PPARγ sequences in marine fish is very limited, it is difficult to infer the phylogenetic implication among these species from the point of PPARγ gene molecular evolution. Further studies of scombrid PPARγs amino acid sequence may enable them to identify species close to PBT as shown in the tree of mitochondria sequences (Setiamarga, Miya, Yamanoue, Mabuchi, Satoh, Inoue & Nishida 2008).
Among the tissues in fish, the highest number of PPARγ transcripts was detected in the adipose tissues in red sea bream (Oku & Umino 2008), cobia (Tsai et al. 2008), gilthead sea bream and plaice (Leaver et al. 2005), and moderately in Atlantic salmon (Andersen et al. 2000). Interestingly, PBT do not possess visceral fat deposits except in the periphery of both sex gonads, instead, they have a lot of intramuscular and/or inner-muscular fat deposits (Fujimoto, Yamamoto, Sudo, Haga, Kurata, Okada, Miyashita, Sawada & Kumai 2008). Lipid-rich muscles of a 13-month-old PBT, such as extraocular muscle, masticatory muscle, fatty white ordinary muscle (O-toro), and moderately fatty white ordinary muscle (Chu-toro) showed a large amount of PPARγ transcripts than lean white ordinary muscle (Akami) and juvenile white ordinary muscles (Fig. 3). Recent biochemical analysis on the lipid content of muscles in cultured PBT has elucidated that the white ordinary muscles of O-toro, Chu-toro, and Akami contained approximately 40%, 15% to 25%, and 10% of lipid respectively (Roy et al. 2010). The results of molecular biological analysis in this study together with the aforementioned results in histology and biochemistry suggest that PPARγ transcript quantities are positively correlated with the lipid accumulation in the 13-month-old PBT ordinary muscles. In contrast, where PBT red muscle is concerned there is a discrepancy in PPARγ transcript quantity and lipid content. Pacific bluefin tuna (30 kg body weight) red muscle had approximately 10% lipid content, and was not higher than the white ordinary muscles as reported by Roy et al (2010). However, during the current study the red muscle of a 13-month-old individual (2.4 kg body weight) had the highest quantity of PPARγ transcripts of all muscle groups (Fig. 3). A possible explanation for this is that PBT red muscle functions not only as a mechanical muscle but also an active energy production organ.
This is supported by the fact that fish red muscle is known to metabolize lipids like other internal organs such as liver (George & Stevens 1978; Murata & Shiraishi 1979), with the lipid distribution of the PBT inner red muscle reported to be similar to that of liver (Katada, Zama & Igarashi 1960). Tuna red muscle contains increased numbers of mitochondria (George & Stevens 1978) which are the centre of β-oxidation (lipid metabolism for energy production) as well as the peroxisomes. In addition, the mitochondrial enzyme for citrate synthetase activity was higher than that of bill fish or slower moving fish species (Dickson 1995). Such biochemical traits of tuna red muscle give tuna their characteristic continuous and aerobic swimming (Dickson 1996). In addition, lipids are the major energy source in the red muscle (Nag 1972), and tuna red muscle has the largest ratio in relation to the total body mass compared with other fish (Graham, Koehrn & Dickson 1983). Another explanation may be that tuna red muscle contains many blood vessels (Carey & Teal 1966). In humans and rats, PPARγ transcripts were largely detected in vascular smooth muscle cells (Law, Goetze, Xi, Jackson, Kawano, Linda Demer, Fishbein, Meehan & Hsueh 2000). High detection of the transcript in the PBT red muscles may indicate the presence of many blood vessels in the red muscles.
Larger amounts of PPARγ transcripts were detected in the following digestive organs; intestine, pyloric caeca and liver of the PBT coinciding with information from other fish (Leaver et al. 2005; Tsai et al. 2008). The function of PPARγ in internal organs might be to assist energy production, which is used in the secretion of digestive enzymes and absorption of nutrients. Along with adipocyte differentiation (Tontonoz, Hu & Spiegelman 1994; Hu et al. 1995), PPARγ is involved in multiple biological processes (Lehrke & Lazar 2005) including macrophage differentiation (Nagy et al. 1998; Tontonoz, Laszlo Nagy, Alvarez, Thomazy & Evans 1998) and tumour suppression (Grommes, Landreth & Heneka 2004). In PBT, the largest quantity of PPARγ transcripts were detected in the head kidney (Fig. 3). Because of the thymus, kidney and spleen has been regarded as major immune organs in fish, with head kidney having a functional specialization for haematopoiesis and lymphopoiesis. The melanomacrophage centre is a cytological structure of aggregated macrophages present in the head kidney (Lin, Lin & Yang 2005), indicating that the PBT head kidney may also possess a melanomacrophage centrr containing large quantities of PPARγ transcripts (Nagy et al. 1998; Tontonoz et al. 1998). In PBT (Fig. 3), olive flounder (Cho et al. 2009), cobia (Tsai et al. 2008), gilthead sea bream and plaice (Leaver et al. 2005), PPARγ transcripts were detected in low levels in the spleen. In a preliminary study of the mRNA detection of immune-related gene rag-1 (recombination activating gene 1), a marker gene of an earlier lymphopoiesis was detected in the thymus, head and trunk kidneys of the malabar grouper, Epinephelus malabaricus, but not in the spleen (Lin et al. 2005), indicating that the involvement of the spleen in the immune system might be limited. As PBT PPARγ transcripts were widely detected, it suggests that the gene products may have a contribution in multiple biological processes of fish.
In the PPARγ transcripts quantification following the diet test, there were no significant differences between diet-tested fish as shown in growth and survival data. This may not only be a similarity of dietary fatty acid composition (Tables 3a and 3b) but also a capture timing of the fish subjected to quantification. As juvenile PBT possess strong appetites, the fish examined may possibly be under the induced condition of PPARγ expression by feeding after the evening fasting period. In gilthead sea bream (S. aurata), PPARγ transcripts of the liver increased by feeding immediately after fasting (Leaver et al. 2005), it indicated a change of PPARγ gene expression in S. aurata due to nutritional status. Diet-tested PBT juveniles were fed every 2 h from 6 am to 10 pm, and the PBT juveniles subjected to the measurement were caught at 9 am, 3 h after the daily first feeding. It is necessary to monitor PPARγ transcript alterations daily in order to estimate the optical time for quantification in a day.
Beneficial effects of dietary phospholipids have been reported in many fish larvae and juveniles (Kanazawa, Teshima & Sakamoto 1985; Coutteau et al. 1997; Cahu et al. 2003; Macqueen Leifson, Homme, Lie, Myklebust & Strøm 2003; Gisbert, Villeneuve, Zambonino Infante, Quazuguel & Cahu 2005); however quantitative and qualitative requirements of phospholipids during early development have not yet been determined (Tocher, Bendiksen, Campbell & Gordon Bell 2008). For larval and juvenile scombrids, there is little information on the phospholipid requirements apart from the beneficial effects of salmon roe phospholipid supplementation on growth and survival of PBT larvae and juveniles along with the accumulation of phospholipids, DHA and EPA in their bodies (Seoka et al. 2008). The diet test in this study on the effect of dietary phospholipid supplementation resulted in no significant improvement in growth and survival, as well as the quantity of PPARγ transcripts in the liver and muscles of PBT juveniles. There are two possible reasons of this result. The first may be the low DHA and EPA content in the polar lipid fraction of the bonito oil used in this study. The DHA and EPA contents in the polar lipid fraction of bonito oil used during this study (DHA; 5.82–20.65%, EPA; 1.30–4.52% of total fatty acids, Table 3b) were much lower than that of salmon roe (DHA; 26.3%, EPA; 13.7% of total fatty acids, Seoka et al. 2008). The polar lipid n-3 HUFA and/or DHA have been reported to be essential fatty acids for many fish, especially for the growth, survival, development and lipid accumulation during early developmental stages (Kanazawa 1997; MacQueen Leifson et al. 2003; Gisbert et al. 2005). The shortage of these n-3 HUFA and/or DHA might cause the similarity in growth and survival of juveniles fed diets with different content level of phospholipids in this study. In addition, some specific phospholipids improve the growth and survival of PBT larvae and juveniles (Seoka et al. 2007) such the growth promoting effect of PC and/or phosphatidylinositol in fish (Coutteau et al. 1997). Regarding other essential fatty acids in carnivorous fish, the content of arachidonic acid (C20:4n-6), the precursor of eicosanoids (Miyazaki & Ntambi 2008), in the test diets used in this study was slightly lower than that in the salmon roe phospholipid supplementation. This also might cause the similarity in performance of test diets in this study. Further study is needed to identify the phospholipid and fatty acid requirements of the PBT juveniles.
The polar lipid fraction of bonito oil used in this study is the by-product from the fish oil production for human, domestic animals and cultured fish use, and is much less expensive than the roe oil. Such low-cost alternative materials for fish feed are fundamental not only for sustainable aquaculture industry development but also for animal resource husbandry sectors. In addition, in the case of this diet test, there was no difference between lipid sources in growth, survival and PPARγ gene transcript promotion, thus indicating that soybean lipid may be for a possible lipid source in the PBT juvenile diet. It is statistically not different however, better growth performance of diet D3 and D4 (Table 4) contained much PE and PC (Table 3a), and C18:2n-6 and C18:3n-3 (Table 3b) than D1 and D2. The possibility of beneficial effect of PE and PC in polar lipid had suggested (Coutteau et al. 1997; Seoka et al. 2008), and coincide with the previous report in PBT juvenile (Seoka et al. 2008), we also found better performance diet contained much PE and PC (Table 3a). Recently, beneficial effect of soybean PC had reported in Caspian brown trout (Kenari, Sotoudeh & Rezaei 2011). Further studies of PE and PC are needed to understand their effect in PBT juvenile diets. While the C18:2n-6 and C18:3n-3 is concerned, they were enriched using soybean polar lipid fraction to the diets D3 and D4, because they are typical plant fatty acid and beneficial effect is unclear. Complete replacement of fish polar lipid to plant may prove difficult. However, if partial replacement is possible it may help reduce consumption of fish resources in aquaculture. This study has highlighted the need to identify the polar lipids method of action in improving fish growth and survival, thereby improving overall aquaculture production.
In conclusion, this study found that the quantity of PPARγ transcripts is correlated with fat deposition in the PBT white ordinary muscles. In addition, this study provided some information about PPARγ gene expression in PBT internal organs. Further studies are needed to investigate lipid accumulation and metabolism during development of the PBT. Emphasis must be given on the expression analysis of genes related to lipid accumulation and metabolism combined with histological and biochemical investigation of tuna lipid metabolism and its industrial application.
Acknowledgments
We would like to thank Dr Y. Tsukamasa and Dr B. C. Roy (Department of Agriculture Kinki University) for helpful discussion with lipid content of PBT muscles. We are grateful to Dr M. Andrews for critical reading of the manuscript and valuable comment. We thank the FLKU staff for the assistance in the PBT rearing experiments. This study was supported by the Global COE programme of the Ministry of Education, Culture, Sports, Science and Technology of Japan.




