Effect of temperature on survival, growth and development of Mytilus galloprovincialis larvae
Abstract
Mussel aquaculture is widely prevalent worldwide, but generally relies on natural seed collection, which does not always meet the needs of the producers. Thus, development of mussel hatcheries is of economic interest in some parts of the world, such as Europe; it provides opportunities not only on annual reliability of seed but also on genetic improvements. To broaden knowledge on mussel larval physiology, we carried out temperature treatments (17, 20 and 24 °C) on Mytilus galloprovincialis larvae under laboratory conditions. The trials ended when 30% of the larval population was in the post-larval stage. The temperature coefficient Q10 indicated a strong relationship between temperature and increase in growth from 17 to 20 °C, but not between 20 and 24 °C. Exposure of M. galloprovincialis larvae to 17 °C resulted in poor growth, low survival and a delayed development and was considered to be inadequate for M. galloprovincialis larval culture. Rearing the larvae at 20 or 24 °C produced better growth, higher survival rates and faster metamorphosis as compared with 17 °C. The temperature region within 20 and 24 °C was suggested as adequate for the mussel M. galloprovincialis larval culture, and implications of these results on the development of commercial hatcheries were discussed.
Introduction
Mussel Mytilus galloprovincialis is mainly cultured in China and in the coastal waters from Galicia (NW Spain) to the northern shores of the Mediterranean Sea. Excluding China, global production of this bivalve species in 2008 was 280 015 tons, of which 64.37% (180 261 tons) was produced in Spain (FAO 2010; Spanish Government 2010).
The culture of mussels in Europe depends entirely on wild seed (juvenile) harvesting. However, the seed stocks are variable in space and time (Smaal 2002) and appear to be decreasing due to overexploitation. Thus, in spite of a rapidly increasing market for mussels, low-spat falls may result in a constraint to industry production. This is the case of mussel aquaculture in Andalusia (S Spain), an emerging industry, which is mainly limited by a lack of natural juvenile (Tirado, Macías, Villarías, Gaiteiro, Gómez, Martín, Rueda, Álamo, Manchado & Infante 2005).
Bivalve hatcheries are a reliable solution to dependence on natural seed, provide opportunities for genetic improvement and allow producers to meet the requirements of the consumers year round more easily. Methods for culturing bivalves in a hatchery are well established for oysters and clams (Utting & Spencer 1991; Helm & Bourne 2006). However, the economics of mussel production in most parts of the world are based on the low value of the mussel seed, and hence hatchery production has not been a realistic option (Galley, Batista, Braithwaite, King & Beaumont 2010). Only a few mussel hatcheries exist (e.g. US, Canada, Australia and New Zealand) and they are all outside Europe. Nevertheless, with increasing demand and continued unreliable seed supply, development of mussel hatchery techniques has become of economic interest in Europe also, and it was one of the goals of the European Commision's 6th Framework-funded project Blueseed. This project provided guidelines on how to reduce the fluctuations in annual production and to sell high-quality blue mussels year round (Blueseed 2008). However, to optimize culture methods, more knowledge on mussel larval physiology is still needed (Smaal & Wijsman 2010).
The effect of temperature on some other bivalve larvae is well documented (Ruiz-Azcona, Rodriguez-Sierra & Martin 1996; Cataldo, Boltovskoy, Hermosa & Canzi 2005; Verween, Vincx & Degraer 2007; Rico-Villa, Pouvreau & Robert 2009; Galley et al. 2010). However, few published studies have focused to date on the effect of temperature on M. galloprovincialis larvae. His, Robert and Dinet (1989) carried out tests within a wide temperature region (15, 20, 25 and 30 °C) on M. galloprovincialis larvae; however, only the results corresponding to the first 8 days of culture (not the whole larval period) were reported. Ruiz, Tarifeño, Llanos-Rivera, Padget and Campos (2008) tested the effect of three different temperatures (12, 16 and 20 °C) on M. galloprovincialis larvae coming from broodstock collected in Chile.
In Andalusia, seawater temperature is often between 17 and 25 °C in spring and summer, when most of the wild broodstock is ripe and ready to spawn (Tirado et al. 2005). The aim of the present study was to test the effect under laboratory conditions of three temperatures (17, 20 and 24 °C) on survival, growth and development of M. galloprovincialis larvae coming from Andalusian mussel broodstock. We also focused on the implications that culture temperature may have on a hatchery located in Andalusia.
Materials and methods
Spawning, fertilization and production of veligers
Mytilus galloprovincialis broodstock used for spawning was collected on May 2009 from culture populations growing on La Atunara harbour, S Spain (N 36°10′ W 5°19′, Fig. 1). On day 0, 150 mussels were cleaned of epifauna and induced to spawning by thermal shock (they were kept out of water at 4 °C overnight and then transferred to 1 μm filtered and UV-treated seawater warmed to 26 ± 0.5 °C). The first individual responding to the stimulus was detected 20 min after being introduced in the warm water. Eggs and spermatozoa released were used within 90 min of spawning. Fertilization was accomplished in a glass beaker with gametes coming from 11 males and five females at a ratio of approximately 200 sperm egg−1. Once polar bodies were detected, this suspension was poured into a 200 L tank filled with 1 μm filtered and UV-treated seawater kept at 22 ± 0.5 °C, and provided with slight aeration. The density of eggs in the incubation tank was 25 eggs mL−1. When development to early veliger was complete (day 2), larvae were sieved on to a 40 μm mesh, counted and assessed for the percentage that exhibited normal morphology from a random sample of 300 larvae.
Site of broodstock collection (La Atunara harbor, Spain).
Growth of veligers
Veliger larvae were reared in 2 L glass beakers containing 1 μm filtered and UV-treated seawater at an initial stocking density of 15 larvae mL−1 per beaker. The larval cultures were slightly aerated using sterile glass tubes to provide oxygen and to minimize organic matter deposits, which might encourage bacterial concentrations. The water in the beakers was changed two times a week: larvae were sieved on to a 40 μm mesh, kept submerged while random samples were taken for microscope examination (report to the section ‘Sampling’) and finally replaced (including sampled animals and dead shells) in sterile beakers with new seawater. Day 2–7 larvae were fed a single diet of Isochrysis galbana (clone T-iso) at a concentration of 50 000 cells mL−1. From day 8 up to the end of the experiments, a mixed diet of 1:1 (cell volume) of T-iso and Chaetoceros calcitrans was provided at a concentration of 100 000 cells mL−1. Larvae were fed every other day. Feedings not coinciding with water changes were made by adjusting the microalgal concentration in the beakers.
Temperature treatments (17 ± 0.5, 20 ± 0.5 and 24 ± 0.5 °C) were conducted in triplicate. For each treatment, temperature was maintained by keeping the beakers in different culture chambers.
Sampling
During water changes, random subsets of larvae were taken with a plastic pipette for assessment of larval growth, survival and development by microscopic examination. Larval records did not take larvae with abnormal morphology into account. For each sample, shell anteroposterior length was measured from at least 10 (n=30) random larvae using a Nikon Digital Sight DS-L1 device (Nikon Corporation, Tokyo, Japan) and the software ImageJ for image capture and analysis respectively. Survival rate was estimated by counting the live larvae in a sample of 100 (n=300) randomly selected individuals and by expressing the count as a percentage of the initial larval stocking population. Developmental stage (i.e. veliger, pediveliger, postlarva) was assessed in a sample of 100 (n=300) randomly selected live larvae. Larval rearing was terminated when 30–34% larvae from the entire population was in the post-larval stage. All larval parameters were compared among treatments at the end of the experiment.
Q10 calculation
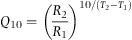
Before Q10 factor calculation, exponential relationship between growth of veliger larvae and time was statistically tested and confirmed to fit to our data. Q10 factor was calculated using the growth rate corresponding to the final average length and time recorded (when 30–34% larvae were in the post-larval stage).
Statistical analysis
Chi-square tests were used to test whether the development stage of the larval population at each sampling point was independent of the rearing temperature. Data on percentage of survival were arcsine transformed before statistical analysis. Normality and homoscedasticity were tested using a Kolmogorov–Smirnov and Cochran tests respectively. One-way anovas were used to test the effect of temperature on growth, survival and developmental stage. When significant differences were detected among means, post hoc Tukey's pairwise multiple comparison test was performed. The SigmaStat® 3.5 software package was used to perform these analyses.
Results
On day 2, the percentage of larvae showing normal morphology was 85.46%. Larvae all grew over time (from 105 ± 4 μm at day 2), with increasing larval size on each sampling day as the temperature was increased (Fig. 2). No significant differences were found in larval shell length between treatments on day 5; however, from day 8 to the end of the experiment, mean larval shell length at 17 °C was significantly smaller than at the other tested temperatures (anova on growth data on day 8: F=5.789; P=0.004; anova on growth data on day 13: F=41.033; P<0.001; anova on growth data on day 19: F=42.507; P<0.001) (Fig. 2). No significant differences in mean shell length were found over the entire culture period between larvae reared at 20 and 24 °C (Fig. 2). For all treatments, growth of veliger larvae fit an exponential model (R2=0.992, P=0.01 for the 17 °C treatment; R2=0.975, P=0.001 for the 20 °C treatment and R2=0.946, P=0.005 for the 24 °C treatment): a first period of slow growth (around 1 μm day−1) was recorded until day 5, followed by a second period of higher growth rates. However, in the case of the 17 °C treatment, growth rate was smaller compared with those at 20 and 24 °C (Table 1).
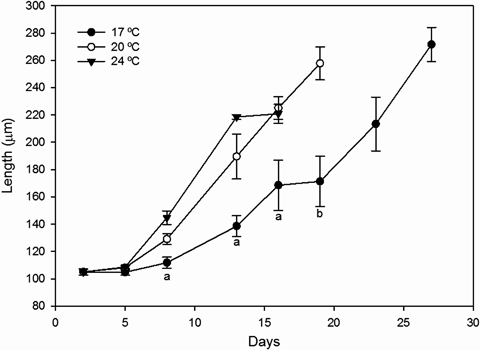
Larval growth (mean ± standard error) of Mytilus galloprovincialis, reared at three temperatures (17, 20 and 24°C). Letters indicate a significant difference: ‘a’ indicates that 17°C is different from 20 and 24°C and ‘b’ indicates that 17°C is different from 20°C.
| Temperature (°C) | Final shell length (μm ± SD) | Growth rate (μm day−1 ± SD) |
|---|---|---|
| 17 | 272 ± 18a | 6.65 ± 0.2a |
| 20 | 258 ± 21a | 9.06 ± 1.2b |
| 24 | 221 ± 12c | 8.71 ± 0.4b |
- Different superscript letters indicate a significant difference (P<0.05; one-way anova).
Growth rate was 6.65 ± 0.2, 9.06 ± 1.2 and 8.71 ± 0.4 μm day−1 for the larvae reared at 17, 20 and 24 °C respectively (Table 1). The increase in growth rate expressed as Q10 between 17 and 20 °C was 2.699, between 17 and 24 °C was 1.569 and between 20 and 24 °C was 0.957.
Survival was similar in all treatments up until day 13 (Table 2). Between day 5 and day 13 all temperature treatments suffered a great loss of survival: on day 13, only 10.8–21% over the initial larval population was alive. Significant differences between treatments on survival were detected on day 16 (anova: F=5.226; P=0.049) and 19 (anova: F=8.291; P=0.045), and both days survival was found to be higher at 20 than at 17 °C.
| Treatment | Age of larvae (days) | ||||||
|---|---|---|---|---|---|---|---|
| 5 | 8 | 13 | 16 | 19 | 23 | 27 | |
| 17°C | 76.0 ± 3.0a | 64.3 ± 12.2a | 10.8 ± 5.5a | 7.1 ± 3.9a | 3.1 ± 2.9a | 0.9 ± 5.2 | 0.5 ± 5.2+ |
| 20°C | 70.3 ± 5.2a | 55.3 ± 6.4a | 21.0 ± 3.7a | 21.3 ± 3.7b | 13.7 ± 2.2b* | ||
| 24°C | 68.7 ± 1.8a | 32.3 ± 7.5a | 16.3 ± 1.2a | 10.7 ± 1.4a,b* | |||
- Data are represented as percentage of live larvae over the initial larval population (on day 2) ± standard error. Different superscript letters indicate a significant difference (P<0.05; one-way anova) in mean survival rate in the same day. Different superscript symbols indicate a significant difference (P<0.05; one-way anova) in final mean survival.
Results of larval development at the three tested rearing temperatures are shown in Fig. 3. Until day 8, all larvae were in the veliger stage. On day 13, all larvae at 17 °C remained in the veliger stage. Conversely, on day 13, larvae at 20 and 24 °C were all in the pediveliger stage. On day 16, only 20% of the total larval population at 17 °C was pediveliger, while postlarvae were already found in treatments at 20 and 24 °C (representing 16% and 34% of the total larval population respectively). On day 19, at 20 °C, postlarvae reached 31% of the total population. On day 27, 10% of the total larval population at 17 °C remained in the veliger stage, while 60% and 30% had developed into pediveliger and post-larval stages respectively. At the end of the experiment, veliger larvae were found only in the 17 °C treatment. Chi-square tests (P<0.001) revealed that the development stage was independent from the rearing temperature until day 8, and was not independent from temperature from day 13 up to the end of the experiments.
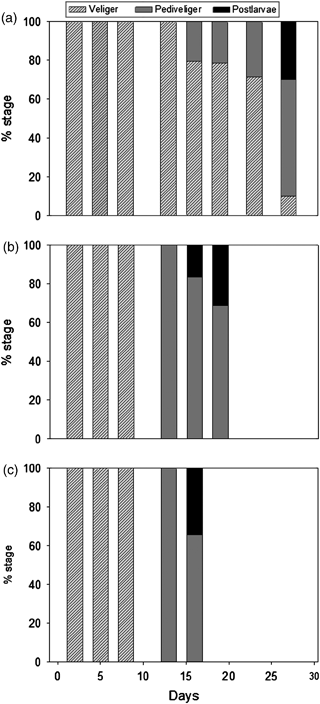
Larval development stages of Mytilus galloprovincialis, reared at three temperatures (A, 17°C; B, 20°C; C, 24°C). Data are represented as percentages of the total larval population.
All the temperature treatments ended with an equivalent post-larval rate (30%, 31.3% and 34% for cultures at 17, 20 and 24 °C, respectively), but the rearing ended at different times (Fig. 2), final shell lengths (Table 1) and survival (Table 2) for each temperature. The treatment at 24 °C ended on day 16 with a mean shell length of 221 ± 12 μm and a survival of 10.7%. The treatment at 20 °C ended on day 19 with a mean shell length of 258 ± 21 μm and a survival of 13.7%. The treatment at 17 °C ended on day 27 with a mean shell length of 272 ± 18 μm and a survival of 0.5%. Statistical analyses revealed that mean shell length was smaller at 24 °C than at 20 and 17 °C (anova: F=6.122; P=0.004) (Table 1), and survival at 17 °C was lower than at 20 and 24 °C (anova: F=19.946; P=0.002) (Table 2).
Discussion
As a general rule, increasing temperatures accelerate the metabolic processes, and feed assimilation is primarily affected due to an enhanced enzyme activity. Accordingly, bivalve larval growth often increases with increasing temperature (Nair & Appukuttan 2003; Cataldo et al. 2005; Liu, Gurney-Smith, Beerens & Pearce 2010) up to a point, after which this positive relationship saturates and even becomes detrimental (Bayne 1965; His et al. 1989; Widdows 1991).
In the current investigation, a rise in culture temperature from 17 to 20 °C accelerated growth rate from 6.65 to 9.06 μm day−1, resulting in a high Q10 value (2.699). Accordingly, Ruiz et al. (2008) also found a significant rise in growth rate with temperature between larval cultures reared at 16 and 20 °C. Our results also suggest that the maximum temperature for the culture of M. galloprovincialis larvae could be close to 24 °C, because no significant differences in growth rates were observed between 20 and 24 °C (and thus corresponding to a low Q10 value, 0.957). These results support those obtained by His et al. (1989), who reared M. galloprovincialis larvae during 8 days at temperatures from 15 to 30 °C and suggested an optimal temperature range from 20 to 25 °C. On the other hand, this optimal range is slightly warmer than this suggested previously for the larvae of the M. galloprovincialis' sister species, and is also important in aquaculture of M. edulis (Hrs-Brenko & Calabrese 1969; Beaumont & Budd 1982; Galley et al. 2010), going from 17 to 21 °C.
As discussed previously, the relationship between temperature and growth rate expressed as Q10 was stronger in the range from 17 to 20 °C (2.699) than from 20 to 24 °C (0.957) and thus was not a constant within the temperature range tested. These Q10 values correspond to short-temperature-range responses and are high and low, respectively, from what would be expected for bivalve larvae. Nevertheless, in our study, Q10 value when using a wider temperature range (17 to 24 °C, Q10=1.569) better adjusts to the literature: Sprung (1984) reported a Q10 value of 1.9 for Mytilus edulis larvae cultured at 12 and 18 °C; Drent (2002) reported a Q10 value of 1.5 for Macoma balthica larvae grown at 10 and 20 °C.
Ruiz et al. (2008) observed that M. galloprovincialis larvae reached metamorphosis 6 days earlier with a rise of 8 °C in the culturing temperature (12 to 20 °C). In our study, increasing temperature also accelerated the development process. Our results show that development of M. galloprovincialis larvae is dependent on the rearing temperature from day 13 (Chi-square test, P<0.001) and clearly delayed at 17 °C. At this temperature, veliger larvae were present throughout the whole experiment, and the post-larval stage appeared 21 days delayed when compared with the 24 °C culture. As mentioned previously, growth rates at 20 and 24 °C were not significantly different in our study. However, we detected that metamorphosis on day 16, counted as the rate of postlarvae in the cultures, was double at 24 than at 20 °C, and the larvae reared at 20 °C reached 30% of postlarvae 3 days delayed (day 19) from 24 °C (day 16).
As it was observed by Widdows (1991), any factor that reduces growth rate and thus extends the period of mussel larval development in the water column, will have a major effect on mortality and the chances of survival to the settlement stage and beyond. In our study, the larval period at 17 °C (27 days) was much longer than at 20 and 24 °C (16 and 19 days respectively), and accordingly accumulated survival when metamorphosis occurred was dramatically lower at 17 °C (0.5%) than at 20 and 24 °C (13.7% and 10.7% respectively).
Size at metamorphosis has been found to be independent of rearing temperature in some bivalve species: for example, for M. balthica larvae (Drent 2002) and for Crassostrea gigas (Rico-Villa et al. 2009). Conversely, our results showed that temperature affected size at metamorphosis, as the larvae reared at 17 and 20 °C reached metamorphosis at a larger average length than those reared at 24 °C. Similar results were described previously for M. edulis larvae reared at different temperatures by Pechenik (1990) and Galley et al. (2010).
Culture of bivalve larvae in hatcheries is often carried out in great volumes and flow through systems; the current investigation was carried out in small volumes and closed systems. However, our results suggest that 17 °C is too low for culturing M. galloprovincialis larvae because they showed a low growth rate (6.65 μm day−1) and an extremely low survival (0.5%). In our study, the growth rates and survival of larvae reared at 20 and 24 °C suggest that this could be the optimum temperature region for M. galloprovincialis larvae. However, larvae at 24 °C were smaller when metamorphosis occurred, and potential implications of this finding on the further juvenile culture are still to be highlighted.
In terms of energy optimization in M. galloprovincialis commercial hatcheries, needs for cooling or warming the water for rearing larvae can be minimized or can even disappear as soon as seawater temperature in the hatchery would be situated within the region suggested as optimal in this study (20–24 °C). For example, in Andalusia (Spain), the costs for cooling or warming the seawater would be minimal during a great part of the year, as seawater temperature in spring and summer goes from 17 to 25 °C in this Spanish region (Junta de Andalucía 2006).
Acknowledgments
This work was funded by the Subprograma de Formación de Personal Investigador en Agroalimentación en los Centros de Investigación INIA-CCAA (Government of Spain) and by the projects 0251_ECOAQUA_5_E and Viabilidad del cultivo de mejillón (Mytilus galloprovincialis) a partir de semilla producida en criadero. We are grateful to Professor Fernando Fernández Palacín for his valuable advice on statistical analysis.




