Dietary protein/lipid level and protein source effects on growth, tissue composition and lipid metabolism of blackspot seabream (Pagellus bogaraveo)
Abstract
A study was carried out to determine the effects of fish meal (FM) replacement by plant protein (PP) on growth, body composition and lipid metabolism of blackspot seabream fed different protein/lipid levels. Four experimental diets were formulated to contain two protein (P) and lipid (L) levels (60P/6L or 50P/10L), varying in their protein source (100% FM or 50% FM: 50% PP). Dietary inclusion of PP did not affect growth of fish fed 60P/6L, although fish fed 50P/10L exhibited lower final body weight and daily growth index. Fish fed 60P/6L presented the highest protein and the lowest lipid content. FM replacement by PP has decreased muscle n-3 whereas the n-6 fatty acids increased. Glucose-6-phosphate dehydrogenase and fatty acid synthetase (FAS) were depressed in fish fed 50P/10L. FAS was significantly increased with 60P/6L PP which was positively correlated with lipid retention data. Those results suggest the conversion of other nutrient than lipid (protein and/or carbohydrates) into corporal fat. Hepatic lipoprotein lipase activity was lowest in fish fed PP diets. Plasma glucose peaked 1–2 h postfeeding, in all groups and was generally higher with 60P/6L FM. This work shown that besides dietary P/L level, protein source has a strong effect on species lipogenesis and lipid retention. Hence, the 50P/10L FM diet was the most cost-effective for blackspot seabream juveniles.
Introduction
Because of its high commercial value, excellent palatability and scarcity in the fishing grounds, the blackspot seabream (Pagellus bogaraveo) has been recently produced in the Atlantic coast. Considerable progress has been made in terms of prefattening and ongrowing in tanks and cages (Peleteiro et al. 1994, 2000; Olmedo et al. 2000), but growth rates of cultured blackspot seabream are very low, when compared with other farmed Sparidae such as gilthead seabream (Santinha et al. 1996, 1999; Gómez-Requeni et al. 2003, 2004; Izquierdo et al. 2003; Sitjá-Bobadilla et al. 2005) and generally associated with a high lipid deposition (Linares et al. 2000, 2001, Silva et al. 2006; Ozório 2009). However, and as suggested by Peleteiro et al. (2000) the identified problems during the ongrowing phase could be corrected by specific feed formulations and by optimizing the species production systems.
Silva et al. (2006) has recently estimated that the optimal level of dietary crude protein for blackspot seabream juveniles would be above 400 g kg−1 dry matter (DM). Considering the high dietary protein requirements of carnivorous species, fish meal (FM) replacement by alternative plant protein (PP) sources is fundamental for the sustainable development of aquaculture industry. Moreover, FM market availability fluctuations seriously increased the prices of this important protein source (PS) which is the single most important and expensive dietary nutrient.
The large majority of studies confirm the idea that high dietary PP levels (>40% of total protein) depress growth and feed efficiency (Ballestrazzi et al. 1994; Robaina et al. 1995; Mambrini et al. 1999; Burel et al. 2000; Refstie et al. 2000). Nevertheless, almost total FM replacement by PP sources was shown to be feasible, when amino acids (AA)-supplemented diets were used (Kaushik et al. 1995, 2004; Gómez-Requeni et al. 2004; Dias et al. 2005; Sitjá-Bobadilla et al. 2005).
Blackspot seabream excessive fat accumulation in carcass, muscle, liver and particularly around viscera (Linares et al. 2000, 2001; Silva et al. 2006; Ozório 2009) remains an important problem to be solved. Several studies developed in higher vertebrates reported that dietary protein level and source affect lipid deposition, fatty acid bioconversion and alter serum and liver lipids (Lindholm & Eklund 1991; Terasawa et al. 1994; Potter 1995; Aoyama et al. 2000). In fish, dietary PS and AA unbalances are also known to affect fish metabolic pathways (Gómez-Requeni et al. 2003, 2004; Dias et al. 2005; Sitjá-Bobadilla et al. 2005). Dias et al. (2005) have showed that FM dietary replacement by soybean meal affects seabass liver lipogenesis decreasing glucose-6-phosphate dehydrogenase (G6PD), malic enzyme (ME) and fatty acid synthetase (FAS). Moreover, the ingestion of dietary PP sources has a hypocholesterolemic effect on gilthead seabream (Gómez-Requeni et al. 2004; Albalat et al. 2005; Sitjá-Bobadilla et al. 2005), rainbow trout (Kaushik et al. 1995) and European sea bass (Robaina et al. 1995, 1999; Kaushik et al. 2004; Dias et al. 2005).
The aim of this study was to evaluate the effects of partial FM replacement by PP on the growth performance, body composition and nutrient utilization of blackspot seabream (Pagellus bogaraveo) fed different protein/lipid levels, contributing to a general insight into lipid metabolism of this new species. Protein requirement for blackspot seabream maintenance was estimated as 4.3 g kg−1 day−1 (Silva et al. 2006), which is quite superior to values previously reported for rainbow trout (2.6 g kg−1 day−1) (Kaushik & Gomes 1988), European sea bass (2.0–2.8 g kg−1 day−1) (Ballestrazzi et al. 1994) and gilthead seabream (0.86 g kg−1 day−1) (Lupatsch et al. 1998). Thus, and as suggested by Silva et al. (2006) crude protein requirements for maximum weight gain would be above 400 g kg−1 DM. Considering this, dietary protein/lipid level of 50P/10L or 60P/6L was adopted in this study to ensure maximal blackspot seabream growth and to help understanding lipid metabolism. To this end, and for the first time in blackspot seabream, liver lipogenic enzymes activity, glycaemia and plasma lipids were analysed. Furthermore, considering the key role of lipoprotein lipase (LPL) in lipoprotein metabolism, its activity was also determined in muscle, liver and adipose tissue. This work must be considered an attempt to understand some of the mechanisms associated with blackspot seabream growth and nutrient utilization.
Materials and methods
Experimental diets
Four experimental diets were formulated to contain two different crude protein (P)/lipid (L) levels (60P/6L or 50P/10L) either with FM as the main PS or with the replacement of 50% FM by wheat gluten (PP). All ingredients were supplied by Sorgal S.A. (Ovar, Portugal) and were finely grounded, mixed and dry pelleted through a 2.4-mm die at 50 °C (CPM, C-300 model). Ingredients, proximate composition and gross energy of the experimental diets are presented in Table 1 and the dietary fatty acid profiles in Table 2. Additionally, the theoretical composition of some fish indispensable amino acids (IAA, % of protein) is given in Table 1, based on Sorgal S.A. ingredient composition information.
| Dietary treatments | ||||
|---|---|---|---|---|
| 60P/6L | 50P/10L | |||
| FM | PP | FM | PP | |
| Ingredients (g kg−1) | ||||
| Fish meal1 | 832 | 429 | 672 | 357 |
| Wheat gluten2 | – | 342 | – | 298 |
| Wheat3 | 159 | 202 | 267 | 269 |
| Fish oil | – | 18 | 52 | 67 |
| Mineral mix4 | 5 | 5 | 5 | 5 |
| Vitamin mix5 | 2 | 2 | 2 | 2 |
| Choline chloride6 | 1 | 1 | 1 | 1 |
| Lutavin E507 | 0.5 | 0.5 | 0.5 | 0.5 |
| Lutavin C358 | 0.4 | 0.4 | 0.4 | 0.4 |
| Proximate composition | ||||
| Dry matter (DM) (g kg−1) | 919 | 919 | 916 | 916 |
| Protein (g kg−1 DM) | 622 | 640 | 534 | 569 |
| Lipids (g kg−1 DM) | 63 | 61 | 107 | 104 |
| Ash (g kg−1 DM) | 170 | 133 | 144 | 99 |
| Starch (g kg−1 DM) | 99 | 148 | 154 | 187 |
| Energy (MJ kg−1 DM) | 19.4 | 20.8 | 20.1 | 21.6 |
| DP/DE (mg kg−1) | 33.3 | 31.9 | 27.5 | 27.4 |
| Theoretical composition (%P)9 | ||||
| Lysine | 7.86 | 4.94 | 7.72 | 4.98 |
| Methionine + cysteine | 3.91 | 3.88 | 3.90 | 3.89 |
| Tryptophan | 1.13 | 1.05 | 1.13 | 1.05 |
| Threonine | 4.38 | 3.57 | 4.33 | 3.58 |
- 1 Fish meal composition: DM: 913 g kg−1; protein: 715 g kg−1 DM; lipids: 73 g kg−1 DM.
- 2 Wheat gluten composition: DM: 901 g kg−1; protein: 838 g kg−1 DM; lipids: 16 g kg−1 DM.
- 3 Micronized wheat composition: DM: 876 g kg−1; protein: 144 g kg−1 DM; lipids: 24 g kg−1 DM.
- 4 Minerals (g or mg kg−1 diet): Mn (manganese sulphate), 20 mg; I (potassium iodide), 0.6 mg; Cu (copper sulphate), 5 mg; Co (cobalt sulphate), 0.4 mg; Mg (magnesium sulphate), 500 mg; Zn (Bioplex; Alltech), 30 mg; Se (Sel-Plex 2000, Alltech), 0.3 mg; Fe (iron sulphate), 40 mg; Ca (calcium carbonate), 2.15 g; dibasic calcium phosphate, 5 g; KCl, 1 g; NaCl, 0.4 g.
- 5 Vitamins (IU or mg kg−1 diet): vitamin A, 8000 IU; vitamin D3, 2000 IU; vitamin E, 100 mg; vitamin K, 10 mg; vitamin B12, 0.02 mg; vitamin B1, 15 mg; vitamin B2, 25 mg; vitamin B6, 15 mg; folic acid, 10 mg; biotin, 1 mg; vitamin C, 100 mg; betaine, 500 mg; inositol, 300 mg; nicotinic acid, 100 mg; pantothenic acid, 50 mg.
- 6 1000 mg kg−1 diet.
- 7 Vitamin E, 300 mg kg−1 diet.
- 8 Vitamin C, 500 mg kg−1 diet.
- 9 Theoretical composition for some indispensable amino acids (IAA, % of protein), based on Sorgal S.A. ingredient composition information.
| Dietary treatments | ||||
|---|---|---|---|---|
| 60P/6L | 50P/10L | |||
| FM | PP | FM | PP | |
| 10:0 | 15 | 19 | 6 | 15 |
| 14:0 | 85 | 72 | 83 | 74 |
| 15:0 | 7 | 6 | 7 | 7 |
| 16:0 | 252 | 239 | 226 | 228 |
| 17:0 | 6 | 4 | 5 | 5 |
| 18:0 | 40 | 29 | 35 | 31 |
| 20:0 | 2 | 2 | 2 | 2 |
| Saturates | 407 | 372 | 366 | 363 |
| 16:1 | 81 | 64 | 81 | 71 |
| 17:1 | 2 | 2 | 3 | 3 |
| 18:1 | 112 | 122 | 136 | 143 |
| 20:1 | 10 | 16 | 20 | 25 |
| 22:1 | 5 | 12 | 16 | 19 |
| 24:1 | 1 | 1 | 1 | 1 |
| MUFA | 212 | 218 | 258 | 264 |
| 14PUFA | 6 | 6 | 3 | 4 |
| 16:2 n-4 | 6 | 4 | 9 | 5 |
| 16:3 n-4 | 16 | 9 | 10 | 7 |
| 16:4 n-1 | 18 | 12 | 15 | 11 |
| 18:2 n-6 | 45 | 153 | 48 | 106 |
| 18:3 n-6 | 2 | 2 | 2 | 2 |
| 20:2 n-6 | 1 | 1 | 2 | 2 |
| 20:3 n-6 | 1 | 1 | 1 | 1 |
| 20:4 n-6 | 10 | 6 | 8 | 6 |
| 22:2 n-6 | 1 | 0 | 1 | 1 |
| Σn-6 | 59 | 163 | 61 | 117 |
| 18:3 n-3 | 7 | 14 | 10 | 14 |
| 18:4 n-3 | 18 | 17 | 24 | 23 |
| 20:3 n-3 | 0 | 0 | 1 | 1 |
| 20:4 n-3 | 4 | 4 | 8 | 5 |
| 20:5 n-3 | 98 | 70 | 93 | 75 |
| 21:5 n-3 | 3 | 2 | 3 | 3 |
| 22:5 n-3 | 11 | 8 | 10 | 8 |
| 22:6 n-3 | 85 | 63 | 80 | 62 |
| Σn-3 | 226 | 178 | 229 | 190 |
| PUFA | 331 | 373 | 327 | 335 |
| Sat/PUFA | 12 | 10 | 11 | 11 |
| n3/n6 | 38 | 11 | 38 | 16 |
Growth trial
Experiments were directed by trained scientists (following FELASA category C recommendations) and were conducted according to the European Economic Community animal experimentation guidelines Directive of 24 November 1986 (86/609/EEC). Blackspot seabream juveniles (Pagellus bogaraveo) were obtained from a fish farm (Grupo Isidro de la Cal, Valdoviño, Coruña, Spain), and acclimated to the experimental conditions for 3 weeks before the beginning of the trial. The growth trial was conducted at the experimental facilities of CIIMAR, Porto. Homogenous groups of 37 juveniles with an average initial body weight of 37.5 g (Table 4) were randomly distributed among 12 square fibre glass tanks (500 L), in a recirculating water system. Triplicate groups of fish for each treatment were fed by hand to apparent satiety, two times a day (09:30 and 18:00 hours) for 103 days. Each tank was supplied with filtered, heated (19 ± 1 °C) saltwater (33 g L−1), at a flow rate of 2 L min−1. The pH, ammonia, nitrites, nitrates and phosphates in the water were monitored during the entire trial and maintained at levels compatible with marine species. Fish were exposed to natural photoperiod. Every 4 weeks, fish were bulk weighed under moderate anaesthesia (ethylene glycol monophenyl ether at 50 ppm) and data on feed distributed were recorded. Prior to sampling, fish were fasted for 24 h and killed by a sharp blow on the head. At the beginning of the experiment, a pooled sample of nine fish from the initial stock were taken and stored at −20 °C for subsequent whole body composition analyses. The dorsal muscle and the liver from other nine fish were also collected, immediately frozen in liquid nitrogen and stored at −80 °C for the posteriors muscle lipid content and liver lipogenic enzymes analyses. At the end of the growth trial, three fish per tank were sampled and stored at −20 °C for subsequent whole body composition. Muscle, liver and adipose tissues were collected, frozen in liquid nitrogen and stored at −80 °C for subsequent individual analyses of muscle total lipid and fatty acids (nine fish per treatment) and liver lipogenic enzymes activity (nine fish per treatment). Liver, muscle and adipose tissue of nine fish per treatment were also colleted for determining LPL activity.
| Dietary treatments | Two-way anovaP-value | ||||||
|---|---|---|---|---|---|---|---|
| 60P/6L | 50P/10L | ||||||
| FM | PP | FM | PP | P/L | PS | x | |
| Growth | |||||||
| Initial body weight (g) | 37.7 ± 0.1 | 37.6 ± 0.1 | 37.6 ± 0.1 | 37.6 ± 0.1 | 0.385 | 0.995 | 0.540 |
| Final body weight (g) | 90.2 ± 0.5ab | 89.4 ± 2.2ab | 94.2 ± 2.2a | 85.2 ± 4.9b | 0.947 | 0.019 | 0.038 |
| FCR1 | 1.3 ± 0.04 | 1.3 ± 0.04 | 1.5 ± 0.1 | 1.6 ± 0.1 | 0.000 | 0.817 | 0.119 |
| VFI2 | 1.1 ± 0.03 | 1.0 ± 0.02 | 1.2 ± 0.1 | 1.2 ± 0.1 | 0.000 | 0.072 | 0.836 |
| PER3 | 1.2 ± 0.04 | 1.3 ± 0.04 | 1.3 ± 0.1 | 1.1 ± 0.1 | 0.278 | 0.112 | 0.0434 |
| DGI5 | 1.1 ± 0.01ab | 1.1 ± 0.04ab | 1.2 ± 0.03a | 1.0 ± 0.1b | 0.940 | 0.020 | 0.037 |
| Digestible intake (g or kJ kg−1 ABW6 day−1) | |||||||
| Dry matter | 8.1 ± 0.3 | 7.5 ± 0.2 | 10.6 ± 0.7 | 9.1 ± 0.4 | 0.000 | 0.003 | 0.141 |
| Protein | 6.3 ± 0.2 | 6.2 ± 0.1 | 6.4 ± 0.4 | 6.4 ± 0.3 | 0.241 | 0.772 | 0.793 |
| Energy | 188.0 ± 6.0 | 193.4 ± 3.9 | 233.4 ± 14.7 | 233.9 ± 10.7 | 0.000 | 0.615 | 0.673 |
| Starch | 1.0 ± 0.03 | 1.5 ± 0.03 | 1.9 ± 0.1 | 2.2 ± 0.1 | 0.000 | 0.000 | 0.064 |
- Values are mean ± standard deviation (n = 3).
- Mean values within a row with unlike superscript letters showed a significant interaction between the two tested factors (P/L versus PS) (P < 0.05).
- 1 Feed conversion ratio = dry feed intake/weight gain.
- 2 Voluntary feed intake = 100 × crude feed intake/average body weight/day.
- 3 Protein efficiency ratio = weight gain/crude protein intake.
- 4 Without significant differences after post hoc analysis.
- 5 Daily growth index = 100 × [(final body weight)1/3 − (initial body weight)1/3]/days.
- 6 Average body weight = (final body weight + initial body weight)/2.
Blood collection was carried out, after a slight anaesthesia, in <3 min, at each sampling point, to avoid plasmatic metabolites response induced by handling. Samples were taken from the caudal vein, with syringes and collecting tubes containing 15–20 μL of sodium fluoride and potassium oxalate (4% each), from three fish per tank, at nine different times after the last meal (30 min, 1; 2; 4; 6; 9; 12; 16; 24 h). Fish sampled at the first five times (30 min, 1; 2; 4; 6 h) have been identified to assure that the same fish would not be sampled twice. Plasma was obtained after centrifugation (6000 g for 10 min at 4 °C) and stored at −80 °C until further glucose, cholesterol (CHOL), triacylglycerol (TAG) and non-esterified fatty acids (NEFA) analysis.
Digestibility trial
The apparent digestibility coefficients (ADC) of the dietary components of the four diets were assessed after incorporation of 0.1% of yttrium oxide, as inert marker, to a grounded portion of each diet. The mixtures were then dry pelleted through a 2.4-mm die at 50 °C (CPM, C-300 model). Four homogenous groups of 10 fish (mean body weight of 90 g) were randomly stocked into four digestibility tanks (50 L), specially constructed according to the Guelph system protocol (Cho et al. 1982) and adapted to the new conditions for 15 days. The experimental fish were subjected to a natural photoperiod, water temperature was maintained at 19 ± 1 °C and salinity at 33 g L−1.
The four diets were randomly assigned by tank, and tested in two following 15-day periods that were hence considered the experimental unit (n 2, replicates). The first 2 days of each 15-day period were used for acclimation to the feed and no faeces were collected. This time period was deemed sufficient for the fish to achieve complete evacuation of previous meals. Fish were fed once daily until apparent satiety and faeces were collected every morning over a 4-week period. After collection, faeces were centrifuged pooled and frozen at −20 °C. The ADC were calculated according to Maynard et al. (1969).
Feed, body composition and faeces analyses
Whole fish from each tank were ground, pooled and fresh moisture content was determined. Fish and faeces were subsequently freeze-dried before further analysis. Feed, whole body samples and faeces were analysed for DM (105 °C for 24 h), ash by combustion in a muffle furnace (550 °C for 12 h), crude protein (Micro-Kjeldahl; N × 6.25) after acid digestion, lipid content by petroleum ether extraction (at Soxhlet 40–60 °C), gross energy in an adiabatic bomb calorimeter (IKA, Werke C2000) and starch according to Thivend et al. (1972). Yttrium oxide concentrations were determined in both diets and faeces samples by atomic absorption spectrophotometry (SpectrAA 220FS; Varian, Les Ulis, France), after a chemical digestion with HNO3 (Reis et al. 2008).
Plasma metabolites assays
Plasma glucose, CHOL, TAG and NEFAs were determined using enzymatic commercial kits: no. 61269; no. 61218; no. 61236 from Bio-Mérieux, Marcy-L’Etoile, France and NEFA-C from Wako, Neuss, Germany respectively.
Total lipids and fatty acids analyses
Total lipids were extracted and measured gravimetrically according to Folch et al. (1957) using dichloromethane instead of chloroform. Fatty acid methyl esters were prepared by acid-catalysed transmethylation of total lipids using boron trifluoride methanol according to Santha & Ackman (1990) and were analysed in a Varian 3800 gas chromatograph (Varian). The chromatograph was equipped with a DB Wax fused silica capillary column (30 m × 0.25 mm internal diameter, film thickness: 0.25 μm; J & W Scientific, Folsom, CA, USA). Helium was used as carrier gas (1 mL min−1) and the thermal gradient was 100–180 °C at 8 °C min−1, 180–220 °C at 4 °C min−1 and a constant temperature of 220 °C during 20 min. Injection was made in a split mode (ratio 1 : 40) with 1 μL injected. Injector and flame ionization detector temperatures were 260 and 250 °C respectively. Fatty acid methyl esters were identified by comparison with known standards mixtures (Sigma189-19, St Louis, MO, USA) and quantified using the star computer package (Varian).
Lipogenic enzymes and lipoprotein lipase assay
Liver samples were homogenized in three volumes of ice-cold buffer (0.02 mol L−1 Tris–HCl, 0.25 mol L−1 sucrose, 2 mmol L−1 EDTA, 0.1 mol L−1 NaF, 0.5 mmol phenylmethyl sulphonyl fluoride, 0.01 mol L−1β-mercaptoethanol, pH 7.4) and centrifuged at 30 000 g, at 4 °C for 20 min. Selected lipogenic enzyme activities were assayed on supernatant: G6PD (EC 1.1.1.49) according to Bautista et al. (1988) and FAS (EC 2.3.1.38) according to the methodology of Chang et al. (1967) and modified by Chakrabarty & Leveille (1969). LPL (EC 3.1.1.34) was determined in muscle, liver and adipose tissue following the procedure described by Bengtsson-Olivecrona & Olivecrona (1992). Enzyme activity units IU, defined as μmoles of substrate converted to product, per min, at assay temperature, were expressed per mg of hepatic soluble protein (specific activity) or per gram of tissue. Soluble protein content of tissues was determined on supernatant by the method of Bradford (1976). The unknown protein content of the samples was determined using a standard curve with well-known protein (bovine serum albumin; Sigma) concentrations (0–100 mg protein mL−1).
Statistical analysis
Statistical analyses followed methods outlined by Zar (1996). All data were tested for normality and homogeneity of variances by Kolmogorov–Smirnov and Bartlett tests, and then submitted to a two-way anova with protein/lipid level and PS as main effects, using the statistics 6.0 package (StatSoft, Inc., Tulsa, OK, USA). When these tests showed significance (P < 0.05), individual mean values were compared using Tukey test. Tank average values for feed intake, growth, body composition, nutrient accretion and faeces analysis were used as experimental units for statistical analyses.
Results
Data on ADC of the main dietary nutrients and energy are reported in Table 3. High protein (>95%) and starch (>95%) digestibilities were observed in all dietary treatments. The ADC of dietary protein and energy were not significantly influenced by the dietary treatments. However, starch ADC were significantly affected by different dietary P/L levels and PS. Starch digestibilities decreased with increasing dietary starch levels and were lowest in fish fed 50P/10L PP diet which corresponds to the diet having the highest starch level.
| ADC % | Dietary treatments | Two-way anovaP-value | |||||
|---|---|---|---|---|---|---|---|
| 60P/6L | 50P/10L | ||||||
| FM | PP | FM | PP | P/L | PS | x | |
| Dry matter | 77.2 ± 5.1 | 75.2 ± 6.0 | 85.3 ± 0.3 | 78.1 ± 4.3 | 0.170 | 0.240 | 0.501 |
| Protein | 95.9 ± 0.1 | 96.9 ± 0.5 | 97.0 ± 0.1 | 96.2 ± 0.8 | 0.623 | 0.802 | 0.0491 |
| Energy | 92.3 ± 0.9 | 93.7 ± 1.0 | 94.0 ± 0.7 | 92.5 ± 1.1 | 0.802 | 0.302 | 0.319 |
| Starch | 99.6 ± 0.1 | 98.2 ± 0.6 | 98.3 ± 0.5 | 95.6 ± 0.6 | 0.005 | 0.004 | 0.116 |
- Values are mean ± standard deviation (n = 2).
- Absence of superscript letters indicates no significant interaction between the two factors (P/L versus PS) (P > 0.05).
- 1 Without significant differences after post hoc analysis.
At the end of the growth trial, all fish more than doubled their initial body weight, displaying significant differences among dietary treatments (Table 4). The P/L level had no effect on growth, but significant differences were found in the digestible DM, energy and starch intakes. In general, fish fed 50P/10L diet presented both significantly higher feed conversion rates (FCR), voluntary feed intake and digestible nutrient intake compared with those fed 60P/6L diet. The dietary inclusion of PP significantly reduced final body weight (FBW) and daily growth index (DGI), when low protein diets were used (50P/10L). In addition, the partial substitution of FM by PP decreased digestible DM and lipid intake, while increased starch digestible intake.
The PS did not influence whole body composition. Fish fed the high protein/low lipid level (60P/6L) diets presented the highest protein (FM: 175; PP: 171 g kg−1) and the lowest lipid content (FM: 117; PP: 139 g kg−1) (Table 5). Different P/L levels or PS did not affect either liver or muscle lipid content when expressed as % DM (Table 5). However, when expressed as % of wet weight, liver lipid content was highest in fish fed PP diets. Hepatosomatic index was not affected by dietary treatments while different dietary P/L ratios have slightly (P = 0.046) affected viscerosomatic index, with the highest value found in fish fed 50P/10L FM diet.
| Dietary treatments | Two-way anovaP-value | ||||||
|---|---|---|---|---|---|---|---|
| 60P/6L | 50P/10L | ||||||
| FM | PP | FM | PP | P/L | PS | x | |
| Final body composition1 | |||||||
| Moisture (g kg−1) | 659 ± 13 | 642 ± 9 | 646 ± 9 | 641 ± 10 | 0.308 | 0.116 | 0.369 |
| Protein (g kg−1) | 175 ± 5 | 171 ± 5 | 160 ± 4 | 165 ± 2 | 0.003 | 0.744 | 0.096 |
| Lipid (g kg−1) | 117 ± 16 | 139 ± 1 | 147 ± 8 | 150 ± 10 | 0.010 | 0.071 | 0.147 |
| Energy (MJ kg−1) | 85 ± 5 | 94 ± 3 | 93 ± 3 | 95 ± 5 | 0.098 | 0.060 | 0.230 |
| Liver total lipids | |||||||
| g kg−1 WW | 122 ± 21 | 157 ± 49 | 141 ± 29 | 222 ± 38 | 0.095 | 0.031 | 0.330 |
| g kg−1 DW | 348 ± 43 | 400 ± 131 | 383 ± 57 | 515 ± 48 | 0.176 | 0.108 | 0.452 |
| Muscle total lipids | |||||||
| g kg−1 WW | 34 ± 5 | 38 ± 8 | 38 ± 14 | 39 ± 8 | 0.335 | 0.387 | 0.740 |
| g kg−1 DW | 136 ± 18 | 149 ± 25 | 150 ± 48 | 156 ± 27 | 0.389 | 0.415 | 0.717 |
| HSI (%)2 | 1.2 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.2 | 0.105 | 0.335 | 0.960 |
| VSI (%)3 | 5.0 ± 1.0 | 5.3 ± 0.9 | 5.7 ± 1.0 | 5.3 ± 1.1 | 0.046 | 0.950 | 0.096 |
| Gain [(mg or g)/kg ABW4/day] | |||||||
| Nitrogen (mg) | 238.6 ± 10.0 | 228.7 ± 9.9 | 215.2 ± 3.4 | 205.7 ± 169 | 0.007 | 0.170 | 0.980 |
| Lipids (g) | 0.9 ± 0.5 | 1.1 ± 0.01 | 1.3 ± 0.1 | 1.2 ± 2 | 0.010 | 0.226 | 0.053 |
| Retention (%) of intake | |||||||
| Protein | 22.9 ± 0.9 | 22.5 ± 1.4 | 20.4 ± 1.4 | 19.3 ± 1.4 | 0.005 | 0.035 | 0.645 |
| Lipid | 127.3 ± 29.5b | 188.0 ± 6.4a | 98.0 ± 5.9b | 100.8 ± 15.0b | 0.000 | 0.012 | 0.019 |
| Energy | 32.0 ± 2.8 | 36.9 ± 2.3 | 31.9 ± 0.7 | 29.5 ± 3.9 | 0.039 | 0.449 | 0.0475 |
- Values are mean ± standard deviation (n = 3).
- Mean values within a row with unlike superscript letters showed a significant interaction between the two factors (P/L versus PS) (P < 0.05).
- 1 Initial body composition was: moisture 660 g kg−1; protein 158 g kg−1; lipid 133 g kg−1 and energy 90 MJ kg−1.
- 2 Hepatosomatic index = 100 × liver weight/body weight.
- 3 Viscerosomatic index = 100 × weight of viscera/body weight.
- 4 Average body weight = (final body weight + initial body weight)/2.
- 5 Without significant differences after post hoc analysis.
Nitrogen gain of fish fed the 60P/6L diet was significantly higher (229–239 mg kg−1 ABW day−1) than that of fish fed the 50P/10L diet (206–215 mg kg−1 ABW day−1), while an opposite trend was observed in lipid gain. Protein and lipid retentions were significantly affected by both P/L level and PS. Fish fed 60P/6L diets displayed the highest protein and lipid retention. Furthermore, in fish fed 60P/6L diets, FM replacement by PP significantly increased lipid retention (127–188%).
Muscle fatty acid composition is shown in Table 6. Compared to the initial profile, we observed in all groups an increase in saturated FA and a decrease in n-3 PUFA. Muscle FA composition reflected in general terms that of the diets. Total saturated fatty acids ranged from 37% to 39% of total fatty acids and were not affected by the different dietary P/L levels or PS. Stearic acid (18:0) percentage was systematically higher in muscle than in the diet. Moreover, a significant effect of the dietary P/L level was observed, with fish fed 60P/6L diet displaying the highest 18:0 values. The monounsaturated fatty acids (MUFA) in flesh lipids were mainly represented by 18:1 (oleic acid). Muscle MUFA levels were higher than those supplied by the diets with fish fed the 60P/6L with PP diets attaining the highest proportion. Flesh percentages of linoleic acid (18:2n-6) were higher in fish fed diets with 50% substitution of FM by wheat gluten due to the high levels of this fatty acid in those diets. The proportion of muscle n-3 PUFA was affected by different P/L levels and PS. Fish fed 50P/10L diets presented the highest values mainly due to an increased accumulation of eicosapentaenoic (20:5n-3; EPA) and docosahexaenoic (22:6n-3; DHA) fatty acids compared to fish fed diets 60P/6L. Total n-3 PUFA ranged from 16.5% to 18.9% in fish fed FM diets and from 13.5% to 15.9% in those fed PP diets, for 60P/6L and 50P/10L levels respectively.
| Initial | Dietary treatments | Two-way anovaP-value | ||||||
|---|---|---|---|---|---|---|---|---|
| 60P/6L | 50P/10L | |||||||
| FM | PP | FM | PP | P/L | PS | x | ||
| 10:0 | 5 | 12 ± 2 | 9 ± 2 | 11 ± 5 | 11 ± 4 | 0658 | 0287 | 0162 |
| 14:0 | 45 | 52 ± 5 | 50 ± 4 | 51 ± 8 | 55 ± 7 | 0327 | 0626 | 0127 |
| 16:0 | 193 | 247 ± 8 | 237 ± 11 | 235 ± 17 | 237 ± 7 | 0117 | 0364 | 0125 |
| 17:0 | 4 | 4 ± 0.2b | 3 ± 0.2c | 4 ± 0.2a | 4 ± 0.2a | 0000 | 0000 | 0002 |
| 18:0 | 58 | 66 ± 3 | 65 ± 3 | 62 ± 3 | 62 ± 4 | 0002 | 0777 | 0653 |
| 20:0 | 2 | 2 ± 0.1 | 2 ± 0.2 | 2 ± 0.2 | 2 ± 0.1 | 0017 | 0121 | 0441 |
| Saturates | 312 | 392 ± 10 | 374 ± 13 | 374 ± 24 | 380 ± 9 | 0242 | 0307 | 00281 |
| 16:1 | 55 | 59 ± 4 | 56 ± 3 | 59 ± 5 | 61 ± 5 | 0037 | 0657 | 0088 |
| 17:1 | 2 | 3 ± 0.4 | 3 ± 1 | 4 ± 1 | 4 ± 4 | 0000 | 0954 | 0749 |
| 18:1 | 198 | 222 ± 18 | 247 ± 16 | 208 ± 18 | 209 ± 23 | 0000 | 0045 | 0071 |
| 20:1 | 19 | 15 ± 1c | 16 ± 13bc | 19 ± 2a | 18 ± 1ab | 0000 | 0865 | 0031 |
| 22:1 | 15 | 5 ± 1c | 7 ± 2bc | 11 ± 4a | 8 ± 1ab | 0000 | 0469 | 0023 |
| MUFA | 290 | 307 ± 21 | 331 ± 18 | 304 ± 21 | 303 ± 24 | 0038 | 0116 | 0075 |
| 12PUFA | 1 | 3 ± 2 | 2 ± 1 | 2 ± 1 | 2 ± 1 | 0844 | 0378 | 0057 |
| 14PUFA | 2 | 3 ± 0.4ab | 2 ± 0.3b | 3 ± 1ab | 3 ± 1a | 0004 | 0664 | 0016 |
| 16:2 n-4 | 3 | 3 ± 1 | 3 ± 1 | 3 ± 0.3 | 3 ± 0.2 | 0008 | 0023 | 0459 |
| 16:3 n-4 | 4 | 5 ± 0.4a | 3 ± 0.6c | 4 ± 1b | 4 ± 1bc | 0437 | 0000 | 0003 |
| 16:4 n-1 | 6 | 5 ± 1a | 4 ± 0.4b | 5 ± 1ab | 5 ± 1a | 0359 | 0012 | 0005 |
| 18:2 n-6 | 88 | 60 ± 6 | 87 ± 4 | 58 ± 2 | 86 ± 6 | 0189 | 0000 | 0797 |
| 18:3 n-6 | 2 | 2 ± 0.1 | 2 ± 0.1 | 2 ± 0.3 | 2 ± 0.1 | 0590 | 0000 | 0857 |
| 20:2 n-6 | 3 | 2 ± 0.2 | 3 ± 0.3 | 2 ± 0.2 | 4 ± 1 | 0462 | 0000 | 0367 |
| 20:3 n-6 | 1 | 2 ± 0b | 3 ± 1a | 2 ± 0.2b | 2 ± 0.2b | 0000 | 0000 | 0001 |
| 20:4 n-6 | 7 | 6 ± 1 | 5 ± 1 | 6 ± 1 | 5 ± 1 | 0040 | 0000 | 0329 |
| Σn-6 | 101 | 73 ± 6 | 100 ± 5 | 70 ± 2 | 98 ± 6 | 0220 | 0000 | 0895 |
| 18:3 n-3 | 11 | 7 ± 1 | 9 ± 0.4 | 8 ± 0.3 | 10 ± 1 | 0000 | 0000 | 0398 |
| 18:4 n-3 | 10 | 8 ± 1 | 7 ± 0.4 | 10 ± 1 | 10 ± 1 | 0000 | 0575 | 0125 |
| 20:4 n-3 | 7 | 5 ± 0.4 | 5 ± 0.4 | 7 ± 0.4 | 6 ± 1 | 0000 | 0062 | 0491 |
| 20:5 n-3 | 70 | 54 ± 5 | 44 ± 4 | 59 ± 6 | 51 ± 4 | 0000 | 0000 | 0446 |
| 21:5 n-3 | 3 | 2 ± 0.2 | 2 ± 0.2 | 2 ± 0.4 | 2 ± 0.1 | 0001 | 0000 | 0630 |
| 22:5 n-3 | 24 | 17 ± 2 | 14 ± 2 | 19 ± 4 | 15 ± 2 | 0124 | 0000 | 0555 |
| 22:6 n-3 | 102 | 71 ± 11 | 55 ± 10 | 84 ± 19 | 62 ± 8 | 0023 | 0000 | 0544 |
| Σn-3 | 229 | 165 ± 17 | 135 ± 16 | 189 ± 29 | 159 ± 13 | 0001 | 0000 | 0804 |
| PUFA | 346 | 256 ± 21 | 249 ± 21 | 277 ± 28 | 272 ± 18 | 0007 | 0428 | 0864 |
| Sat/PUFA | 9 | 15 ± 1 | 15 ± 2 | 14 ± 2 | 14 ± 1 | 0011 | 0922 | 0579 |
| n3/n6 | 23 | 23 ± 2 | 13 ± 1 | 27 ± 4 | 16 ± 1 | 0000 | 0000 | 0315 |
- Values are mean ± standard deviation (n = 9).
- Mean values within a row with unlike superscript letters showed a significant interaction between the two factors (P/L versus PS) (P < 005).
- 1 Without significant differences after post hoc analysis.
Fatty acid synthetase and G6PD activities assayed in blackspot seabream liver are reported in Table 7. In general terms, there was a significant interaction between P/L levels and PS for both enzymes activity. FAS activity was significantly higher in fish fed PP diet than in fish fed FM diet, but only at 60P/6L, and was positively correlated with lipid retention data (Pearson correlation = 0.75). Moreover, when PP was used, both enzymes were depressed in fish fed 50P/10L by the increase in dietary lipid level (60P/6L versus 50P/10L). Blackspot seabream LPL activities were within the same range of values in the various tissues when expressed as mIU g−1 tissue (Table 7). In muscle and adipose tissue, no dietary effects were found in LPL activity. However, in adipose tissue, LPL specific activities (mIU g−1 protein) were considerably higher (five- to sevenfold increase) than in muscle or liver. In liver, LPL activity (expressed as mIU g−1 tissue or as specific activity) was significantly affected by PS, being lowest in fish fed PP diets.
| Dietary treatments | Two-way anovaP-value | ||||||
|---|---|---|---|---|---|---|---|
| 60P/6L | 50P/10L | ||||||
| FM | PP | FM | PP | P/L | PS | x | |
| G6PD 1 | |||||||
| Liver | |||||||
| IU g−1 tissue | 13.1 ± 4.3 | 14.6 ± 4.5 | 12.4 ± 2.1 | 10.7 ± 2.6 | 0.063 | 0.949 | 0.179 |
| mIU mg−1 protein | 103.8 ± 32.6ab | 136.7 ± 31.8a | 108.1 ± 28.6ab | 94.6 ± 18.0b | 0.054 | 0.312 | 0.020 |
| FAS 2 | |||||||
| Liver | |||||||
| IU g−1 tissue | 2.6 ± 1.1 | 3.2 ± 0.2 | 2.1 ± 1.2 | 1.8 ± 0.9 | 0.004 | 0.699 | 0.179 |
| mIU mg−1 protein | 20.5 ± 7.8b | 30.0 ± 3.2a | 22.4 ± 8.1ab | 16.5 ± 6.8b | 0.019 | 0.454 | 0.003 |
| LPL | |||||||
| Liver | |||||||
| mIU g−1 tissue | 23.7 ± 8.0 | 20.9 ± 8.6 | 28.6 ± 12.6 | 17.3 ± 3.5 | 0.838 | 0.033 | 0.183 |
| mIU mg−1 protein | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.1 ± 0.01 | 0.236 | 0.032 | 0.246 |
| Muscle | |||||||
| mIU g−1 tissue | 26.6 ± 12.7 | 19.8 ± 8.2 | 16.0 ± 4.9 | 19.4 ± 10.4 | 0.095 | 0.594 | 0.115 |
| mIU mg−1 protein | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.142 | 0.770 | 0.075 |
| Adipose tissue | |||||||
| mIU g−1 tissue | 22.8 ± 8.3 | 20.5 ± 9.5 | 24.4 ± 11.8 | 18.6 ± 8.0 | 0.965 | 0.220 | 0.601 |
| mIU mg−1 protein | 1.4 ± 0.7 | 1.3 ± 0.9 | 1.6 ± 0.9 | 1.4 ± 0.6 | 0.642 | 0.592 | 0.841 |
- Values are mean ± standard deviation (n = 9).
- Mean values within a row with unlike superscript letters showed a significant interaction between the two factors (P/L versus PS) (P < 0.05).
- 1 Initial G6PD activity was: 4.2 IU g−1 tissue or 50.2 mIU mg−1 protein.
- 2 Initial FAS activity was: 0.2 IU g−1 tissue or 2.8 mIU mg−1 protein.
Data on plasma glucose, CHOL, TAG and NEFA levels analysed at several times after the last meal are presented in Table 8. The glucose peak occurred 1–2 h postprandially (Fig. 1) in all dietary treatments. Nevertheless, plasma glucose levels were generally higher in fish fed the 60P/6L FM diet that showed significant higher values 2 h after the last meal (158.5 mg dL−1 plasma) compared to other treatments. In all cases, basal glucose levels were restored between 9 and 16 h after last meal. PS significantly affected plasma total CHOL levels at several sampling times: 0.5; 1; 2; 9 and 12 h. Plasma TAG was only significantly affected by PS 24 h after the last meal, with the highest value found in fish fed the PP diets. Plasma cholesterolemia and NEFA levels were generally higher in fish fed FM-based diets and lower in those fed PP diets. In addition, the different P/L levels have only slightly affected CHOL levels or TAG (P ≥ 0.044).
| Time | 60P/6L | 50P/10L | Two-way anovaP-value | |||||
|---|---|---|---|---|---|---|---|---|
| FM | PP | FM | PP | P/L | PS | x | ||
| 0.5 | Gluc | 76.8 ± 14.2‡ | 74.7 ± 19.8 | 66.3 ± 16.1 | 72.1 ± 25.5‡ | 0.325 | 0.778 | 0.552 |
| Chol | 2.3 ± 0.4†‡ | 1.7 ± 0.5 | 2.2 ± 0.5†‡ | 1.9 ± 0.5†‡ | 0.845 | 0.013 | 0.296 | |
| TAG | 3.3 ± 1.5†‡ | 2.4 ± 0.9 | 2.5 ± 1.1 | 3.4 ± 1.5 | 0.850 | 0.997 | 0.048 | |
| NEFA | 0.13 ± 0.05§¶ | 0.11 ± 0.03‡§ | 0.16 ± 0.06†‡§? | 0.14 ± 0.04‡§? | 0.056 | 0.191 | 0.794 | |
| 1 | Gluc | 161.2 ± 62.4† | 108.0 ± 22.2 | 115.7 ± 41.2 | 103.1 ± 20.3†‡ | 0.078 | 0.024 | 0.152 |
| Chol | 2.7 ± 0.4† | 1.8 ± 0.4 | 2.6 ± 0.7† | 2.0 ± 0.4†‡ | 0.943 | 0.000 | 0.336 | |
| TAG | 3.3 ± 0.7†‡ | 2.8 ± 1.5 | 3.4 ± 1.7 | 4.0 ± 1.4 | 0.186 | 0.881 | 0.242 | |
| NEFA | 0.13 ± 0.04a§?¶ | 0.07 ± 0.02b§ | 0.13 ± 0.04a‡§? | 0.13 ± 0.03a§? | 0.010 | 0.008 | 0.026 | |
| 2 | Gluc | 158.5 ± 42.9a† | 107.3 ± 43.0b | 100.9 ± 31.5b | 117.5 ± 31.2ab† | 0.068 | 0.178 | 0.011 |
| Chol | 2.9 ± 0.9† | 1.7 ± 0.2 | 2.5 ± 0.4† | 2.0 ± 0.6†‡ | 0.687 | 0.000 | 0.092 | |
| TAG | 3.7 ± 1.8† | 2.2 ± 1.1 | 3.5 ± 1.2 | 3.6 ± 1.1 | 0.269 | 0.145 | 0.095 | |
| NEFA | 0.09 ± 0.03¶ | 0.07 ± 0.02§ | 0.12 ± 0.06§ | 0.11 ± 0.03? | 0.024 | 0.269 | 0.723 | |
| 4 | Gluc | 135.0 ± 42.4†‡ | 91.4 ± 13.0 | 102.5 ± 50.1 | 91.6 ± 28.8†‡ | 0.291 | 0.083 | 0.284 |
| Chol | 2.0 ± 0.6†‡ | 1.7 ± 0.4 | 2.2 ± 0.6†‡ | 1.8 ± 0.5†‡ | 0.468 | 0.106 | 0.788 | |
| TAG | 3.6 ± 1.5†‡ | 2.8 ± 1.4 | 2.8 ± 0.8 | 3.3 ± 2.1 | 0.845 | 0.790 | 0.324 | |
| NEFA | 0.12 ± 0.03§¶ | 0.10 ± 0.03§ | 0.15 ± 0.04†‡§? | 0.12 ± 0.1§? | 0.150 | 0.180 | 0.870 | |
| 6 | Gluc | 117.9 ± 48.9†‡ | 102.9 ± 49.2 | 11.6 ± 55.9 | 91.0 ± 28.8†‡ | 0.640 | 0.363 | 0.888 |
| Chol | 2.3 ± 0.3†‡ | 2.0 ± 0.4 | 1.9 ± 0.6†‡ | 2.0 ± 0.4†‡ | 0.381 | 0.764 | 0.184 | |
| TAG | 4.2 ± 1.7ab†‡ | 2.5 ± 0.8b | 2.4 ± 1.5b | 5.6 ± 2.4a | 0.372 | 0.264 | 0.002 | |
| NEFA | 0.15 ± 0.05‡§?¶ | 0.09 ± 0.02§ | 0.14 ± 0.06§? | 0.12 ± 0.01§? | 0.554 | 0.015 | 0.101 | |
| 9 | Gluc | 84.3 ± 35.5†‡ | 91.9 ± 44.2 | 96.9 ± 43.6 | 73.3 ± 31.0‡ | 0.849 | 0.613 | 0.325 |
| Chol | 2.3 ± 0.5†‡ | 1.5 ± 0.3 | 2.3 ± 0.5†‡ | 2.0 ± 0.4†‡ | 0.143 | 0.006 | 0.287 | |
| TAG | 2.1 ± 0.6†‡ | 2.9 ± 1.0 | 3.5 ± 1.0 | 3.3 ± 2.3 | 0.175 | 0.583 | 0.461 | |
| NEFA | 0.24 ± 0.08†‡ | 0.13 ± 0.02‡§ | 0.21 ± 0.08†‡? | 0.18 ± 0.1†‡§? | 0.663 | 0.012 | 0.108 | |
| 12 | Gluc | 69.6 ± 20.1‡ | 89.7 ± 51.9 | 89.7 ± 32.9 | 71.3 ± 27.2†‡ | 0.970 | 0.943 | 0.238 |
| Chol | 2.6 ± 0.5†‡ | 1.6 ± 0.6 | 2.7 ± 0.5† | 2.4 ± 0.5† | 0.045 | 0.009 | 0.132 | |
| TAG | 2.2 ± 0.5†‡ | 2.5 ± 1.4 | 3.8 ± 1.1 | 3.6 ± 2.5 | 0.044 | 0.972 | 0.746 | |
| NEFA | 0.26 ± 0.05† | 0.22 ± 0.06† | 0.23 ± 0.06†‡ | 0.22 ± 0.1† | 0.513 | 0.345 | 0.526 | |
| 16 | Gluc | 72.0 ± 27.0‡ | 63.2 ± 14.3 | 80.1 ± 39.4 | 66.1 ± 19.8‡ | 0.530 | 0.236 | 0.781 |
| Chol | 2.6 ± 0.4a† | 1.6 ± 0.3b | 1.6 ± 0.5b‡ | 2.4 ± 0.5a† | 0.441 | 0.545 | 0.000 | |
| TAG | 2.2 ± 1.3†‡ | 2.3 ± 0.8 | 2.4 ± 1.0 | 3.5 ± 1.0 | 0.046 | 0.083 | 0.138 | |
| NEFA | 0.21 ± 0.06†? | 0.23 ± 0.07† | 0.19 ± 0.05†‡§? | 0.19 ± 0.01†‡§ | 0.133 | 0.547 | 0.731 | |
| 24 | Gluc | 83.5 ± 40.2‡ | 75.3 ± 23.5 | 89.7 ± 23.9 | 75.4 ± 26.3‡ | 0.752 | 0.269 | 0.760 |
| Chol | 1.6 ± 0.6‡ | 1.5 ± 0.4 | 1.7 ± 0.4‡ | 1.3 ± 0.4‡ | 0.589 | 0.199 | 0.502 | |
| TAG | 1.6 ± 0.6‡ | 2.1 ± 0.9 | 1.8 ± 0.4 | 2.7 ± 1.2 | 0.177 | 0.033 | 0.434 | |
| NEFA | 0.19 ± 0.05†‡§? | 0.17 ± 0.05†‡ | 0.24 ± 0.06† | 0.21 ± 0.05†‡ | 0.015 | 0.162 | 0.673 | |
| P-valueOne-way anova– time | Gluc | 0.000 | 0.036 | 0.125 | 0.002 | |||
| Chol | 0.000 | 0.484 | 0.001 | 0.001 | ||||
| TAG | 0.022 | 0.828 | 0.030 | 0.077 | ||||
| NEFA | 0.000 | 0.000 | 0.000 | 0.000 | ||||
- Values are mean ± standard deviation (n = 9).
- a,b Mean values within a row with unlike superscript letters showed an interaction between the two factors (P/L versus PS) (P < 0.05).
- †,‡,§,?,¶ Mean values within a row unlike superscript symbols indicates significant differences between time within treatments (P < 0.05).
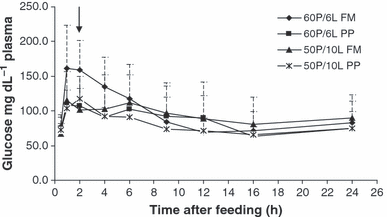
Plasma glucose levels (mg dL−1) measured at different times up to 24 h after the last meal (n 9). Glucose peak is signalled by an arrow.
Discussion
The ADC observed for all nutrients were generally high and in accordance with ADC for carnivore species (NRC 1993). ADC values were not affected by dietary treatments, except for starch which decreased (from 99.6% to 95.6%) with increasing starch dietary levels (from 100 to 190 g kg−1 DM). Similar results were previously reported in others species (Bergot & Breque 1983; Enes et al. 2006) and for blackspot seabream (Silva et al. 2006). Starch digestibilities observed in this study were in general accordance with those described for seabream (Venou et al. 2003; Enes et al. 2008) or sea bass (Peres & Oliva-Teles 2002; Enes et al. 2006) when fed treated starches. It must be taken into consideration that the main dietary starch source utilized in this experiment was micronized treated wheat, known to increase generally starch digestibility (NRC 1993), and thus high starch digestibilities were predictable.
Previous studies with blackspot seabream have registered low growth rates (Peleteiro et al. 1994; Olmedo et al. 2000) when compared with other Sparidae such as gilthead seabream (Santinha et al. 1996, 1999; Izquierdo et al. 2003; Gómez-Requeni et al. 2004; Sitjá-Bobadilla et al. 2005). In previous studies, improvements of both FCR (4.2–1.6) and DGI (0.7–1.4) were reached with increasing dietary protein levels up to 400 g kg−1 DM, but no further increases were obtained with higher protein levels (Silva et al. 2006).
Data on DGI (1.0–1.2), obtained in this work were higher that the ones reported by Olmedo et al. (2000) but slightly lower than those found by Silva et al. (2006). Differences in initial body weight, rearing temperature and genetic origin of the fish can explain those differences. In addition, the present results have evidenced the possibility of replacing 10% protein (60–50%) by an increase of 4% in dietary lipid (6–10%) without affecting growth performance. Nevertheless, compared with 60P/6L, FCR increased in fish fed 50P/10L probably due to a significantly higher fed intake but lower nutrient retention.
The 50% replacement of FM by wheat gluten in diets for blackspot seabream seems to be possible without any adverse effect on growth, only when high protein levels are used. Indeed, DGI and FBW were significantly reduced in fish fed the low dietary protein levels (50%) containing vegetable sources. The feasibility of FM replacement by PP sources has been shown to be highly variable among species. Several studies have shown that FM can be replaced at least up to 60–75% without significantly effects on growth of gilthead seabream (Gómez-Requeni et al. 2004; Sitjá-Bobadilla et al. 2005) or sea bass (Kaushik et al. 2004; Dias et al. 2005). Furthermore, almost total replacement of FM by a mixture of PP sources has also been shown to be possible in rainbow trout (Kaushik et al. 1995) or more recently in European sea bass (Kaushik et al. 2004). In the large majority of these studies, vegetable diets were supplemented with IAA such as lysine and methionine which allowed PP to almost replace FM without significant effects on growth. Data on quantitative IAA requirements of blackspot seabream are not available, but it has been suggested that differences between species would be minor (Mambrini & Kaushik 1995). In addition, the dietary theoretical percentages are within the recommendations for most cultivated species (Kaushik 1998). Considering such, impaired growth registered in fish fed 50P/10L PP was probably due to a lower protein retention and lower nitrogen gain (although without statistical meaning) rather than IAA deficiencies. Low palatability and complex synergistic interactions among antinutritional factors, attributed to PP sources (NRC 1993), could also be responsible for the reduced growth verified with 50/10 PP diet. Nevertheless, the determination of blackspot seabream IAA requirements would eventually allow higher PP inclusion, even at low protein levels, through balancing by addition of crystalline AA.
In this experiment, different P/L ratios (60P/6L or 50P/10L) have significantly affected both protein and lipid whole body content, but no PS effect was found. Blackspot seabream juveniles fed 60P/6L diets presented the highest protein (171–175 g kg−1 WW) and the lowest lipid contents (117–139 g kg−1 WW) comparing to those fed 50P/10L. In sea bass, the use of soy protein as the main dietary PS has significantly reduced fat content (Dias et al. 2005), whereas a significant fat increase was observed when increasing levels of FM were replaced by a mixture of PP sources (Kaushik et al. 2004), indicating a clear PS effect on body fat contents.
Protein and lipid retentions were significantly influenced by both P/L level and PS. Even at low dietary lipid levels (6–10% DM), lipid retentions were generally extremely high (98–188%), attaining maximal values in fish fed 60P/6L diets. Those results suggest the conversion of other nutrient than lipid (protein and/or carbohydrates) into corporal fat. Nevertheless, considering the excessive fat accumulation (>18%) previously described for this species (Linares et al. 2000, 2001; Silva et al. 2006; Ozório 2009) positive improvements were reached with this experiment, and body fat content is well within the values found for other species such as gilthead seabream (Santinha et al. 1996, 1999) or European seabass (Dias et al. 1998, 2005; Kaushik et al. 2004). Liver and muscle lipid content of blackspot seabream were also lower than those previously reported for this species (Ozório 2009), displaying values similar to those described for gilthead seabream (Santinha et al. 1999; Izquierdo et al. 2003) or sea bass (Izquierdo et al. 2003). Dietary treatments have affected neither liver nor muscle lipid content when expressed by DM. However, when expressed by wet weight, the liver lipid content showed a slight increase in fish fed PP diets. Such result does not seem to be related to lipogenesis or LPL activity, but fish individual variability on lipid and/or DM content might explain this result.
Muscle fatty acid composition generally reflects the dietary profile, as showed in previous studies (Bell et al. 2002; Izquierdo et al. 2003; Mourente & Bell 2006), though specific fatty acids were selectively retained. At the end of experimental period, total saturated (37–39%) and monounsaturated (30–33%) fatty acids were the main flesh fatty acid classes irrespective of the dietary treatment. The observed accumulation of stearic acid (18:0) may result from an elevated species lipogenesis capacity as suggested by the high FAS activities displayed by this fish species. An accumulation of the monounsaturated (MUFA) fraction content was also observed in muscle, essentially due to the accumulation of 18:1 and 20:1 fatty acids. Although PS did not affect total saturated, monounsaturated or PUFA lipid fractions, the n-3 and n-6 fractions varied significantly and displayed opposite trends; muscle n-6 content increased, essentially due to an accumulation of linoleic acid (18:2n-6; LA), while a decrease in eicosapentanoic (20:5n-3; EPA) and docosahexaenoic (22:6n-3; DHA) fatty acids significantly contributed to lower muscle n-3 percentages in fish fed PP diets.
Nevertheless, muscle percentages of DHA were similar to those presented in PP diets indicating a selective retention of this fatty acid. On the contrary, EPA proportions were lower in muscle than in diets suggesting that this fatty acid is more efficiently catabolized than DHA (Bell et al. 2002; Stubhaug et al. 2005). The higher assimilation of DHA over EPA implies that DHA might have a higher biological value than EPA during the ongrowing phase. Selective retention of DHA might be a very important characteristic of this species, as the intrinsic structure of this FA is inherently resistant to temperature and pressure changes (Sargent et al. 2002), being particularly advantageous for marine demersal fish species.
Activities found for hepatic lipogenic enzymes in blackspot seabream such as G6PD and FAS were respectively lower and higher than those found in gilthead seabream (Gómez-Requeni et al. 2003) or seabass (Dias et al. 1998, 2005; Richard et al. 2006b), but well within the range of values observed for red seabream (Iritani et al. 1984). Low protein/high lipid diets (50P/10L) significantly decreased G6PD and FAS hepatic activities. In most teleosts, it has been observed an inhibitory effect of dietary lipid level on lipogenesis (Likimani & Wilson 1982; Arnesen et al. 1993; Alvarez et al. 1998; Dias et al. 1998). In higher vertebrates, besides protein level, the dietary protein quality is also known to affect lipogenic enzymes activities (Iritani et al. 1986, 1996; Kayashita et al. 1996; Padmakumarannair et al. 1998). Although the present results showed no significant effects of PS on lipogenic enzymes, fish fed high PP diets showed the highest FAS specific activities due to a significant interaction between the two dietary factors (P/L level and PS). These results are not in completely agreement with those found by Dias et al. (2005), who demonstrated that dietary PP sources affect fat deposition and lipogenic potential in European sea bass, decreasing G6PD and ME activities. The higher dietary FM replacement by different PP sources level (up to 80%) used by Dias et al. (2005) together with differences in PP utilization by fish species are probably in the basis of such differences found on hepatic lipogenesis.
Concerning FAS, results were shown to be dependent on the PP source, decreasing in sea bass fed soy rich diets but increasing with corn-gluten meal-based diets. Dias et al. (2005) suggested that these results could be related to the dietary AA imbalance, particularly a deficiency of the exclusively ketogenic AA such as lysine, which leads to increase catabolism of other AA, leaving thus more carbon chains and glucogenic substrates available for lipogenesis. Assuming that dietary IAA percentages are within the recommendations for most cultivated species (Kaushik 1998), the increased FAS activity verified with 60P/6L PP diet has probably resulted from an inadequate AA ratio to each other. In addition, the increase in hepatic FAS activity corroborates well with the extremely high lipid retention observed in this group of fish (Pearson correlation = 0.75).
Lipoprotein lipase activity or expression has been showed to be markedly higher in adipose tissue than in muscle or liver tissues in rainbow trout and seabream (Lindberg & Olivecrona 1995, 2002; Arantzamendi et al. 2003; Saera-Vila et al. 2005; Richard et al. 2006a). In this work, LPL activities (expressed as mIU g−1 tissue) were similar among the three tissues analysed and no relationship was found between LPL activity and the main lipid depot sites. However, regarding specific activities, a five- to sevenfold higher activity is found in adipose tissue than in muscle or liver tissues which may be due to the differences between these tissues protein content. These similarities between tissues LPL were also found in European seabass (Richard et al. 2006b) suggesting a species-specific LPL control.
Several studies concerning LPL activity or expression, showed a tissue-specific regulation by nutritional conditions in rainbow trout (Lindberg & Olivecrona 2002; Arantzamendi et al. 2003; Albalat et al. 2006; Richard et al. 2006b), red seabream (Liang et al. 2002a,b), gilthead seabream (Arantzamendi et al. 2003; Saera-Vila et al. 2005) and European sea bass (Richard et al. 2006a). The regulatory effect of dietary fatty acids on LPL gene expression has been reported in previous works (Raclot 1997;Liang et al. 2002a,b; Richard et al. 2006a). In red seabream, LPL mRNA levels in liver increased with dietary long-chain n-3 PUFA while high oleic acid had the inverse effect in adipose tissue (Liang et al. 2002b). Similarly, the observed effect of PS on blackspot seabream liver LPL activity could be related to changes in dietary FA composition. Dietary FM replacement by wheat gluten significantly decreased n-3 PUFA contents in diets, which possibly explain the associated reduction in liver LPL activity. This decrease in hepatic LPL activity implies a reduction of liver fatty acid uptake which might be downregulated by the increased fatty acids levels provided by FAS. Moreover, this inverse dietary effect on LPL (downregulated) and FAS (upregulated) activity could explain the final similarity in hepatic lipid contents of fish fed 60P/6L PP diet. In contrast, in gilthead seabream full FM replacements by PP was shown to decrease LPL expression in adipose tissue and enhance it in hepatic tissue, whereas skeletal tissue remained unaffected (Saera-Vila et al. 2005).
The length of hyperglycaemic period is species and condition dependent, depending on species-specific mechanism of glucose homeostasis (Moon 2001). The rates of glucose absorption and time to maximum plasma glucose concentration occurrence are then important to understand plasma glucose regulation and metabolic nutrient relationships. Blackspot seabream juveniles fed the different dietary treatments presented a plasma glucose peak between 1 and 2 h after the last meal. These results are in agreement with those found for common carp and red seabream which showed plasma glucose peaks at 1 and 2 h, respectively, after an oral administration of glucose (Furuichi & Yone 1981). Similar plasma peak times (1–3 h) were observed in gilthead seabream, whereas in several other species the peak was reached 3–6 h after glucose injection (Furuichi & Yone 1981; Hemre et al. 1995; Lin & Shiau 1995; Garcia-Riera & Hemre 1996; Peres et al. 1999; Robaina et al. 1999; Gisbert et al. 2003). In general, the more carnivorous the species, the longer time needed to clear a glucose load (Moon 2001). In our study, the high standard deviations, possibly due to individual genetic variability observed for plasma metabolites, and for glucose in particular, bring some difficulty to results interpretation. The blackspot seabream ability to restore the basal glucose levels was observed between 9 and 16 h after the last meal for all dietary treatments. Robaina et al. (1999) observed higher glucose levels in sea bass fed 30% wheat gluten meal diets compared with those fed FM diets. These differences were explained by the lesser amounts of digestible starch content in these diets. In this work, dietary starch levels ranged from 100 to 190 g kg−1, increasing with vegetal protein incorporation. Curiously, the high plasmatic glucose level was observed in fish fed the low starch dietary level (100 g kg−1). In mammals, the hexokinase IV reaction constitutes an important rate-limiting step in the removal of glucose from circulation by the liver (Hornichter & Brown 1969). Upregulation of this enzyme by high carbohydrate diets has been known for a long time in mammals (Borrebaek et al. 1970) and fish (Borrebaek et al. 1993). Considering this, a carbohydrate upregulation of hexokinase IV activity may be hypothetized to explain the rapidly clearance of glucose from plasma in fish fed high carbohydrate levels. The probably high AA content in 60P/6L FM diets and consequently high gluconeogenic AA content could explain the highest plasma glucose levels in fish fed this diet. Further work on glucose metabolism will be useful to explain the role of dietary nutrients as protein and carbohydrates on glucose metabolism regulation.
High PP diet (60P/6L PP) has lowered from 26% to 41% fish CHOL (at 0.5 and 2 h postprandial time respectively) and NEFA plasma levels. Several studies have previously showed a decrease in lipid deposition and mesenteric fat together with hypocholesterolemia in gilthead seabream (Gómez-Requeni et al. 2004; Albalat et al. 2005; Sitjá-Bobadilla et al. 2005) as well as in other teleostean species (Kaushik et al. 1995, 2004; Regost et al. 1999; Robaina et al. 1999; Dias et al. 2005) when FM is replaced by PP.
In mammals, an inverse correlation between plasma TAG level and LPL activity, increasing the lipid tissue uptake, has been shown (Zampelas et al. 1994; Kayashita et al. 1996). Similarly, in our study an inverse correlation between plasma TAG and liver LPL activity was also observed, particularly in fish fed FM diets.
This work has shown that besides dietary P/L level, PS has a strong effect on blackspot seabream lipogenesis, with excessive PP leading to high hepatic FAS activity and consequently extremely high lipid retention (Pearson correlation = 0.75). In addition, 50% FM replacement seems to be feasible on blackspot seabream, but only when a high dietary protein level is used (60%). Therefore, wheat gluten does not appear to be a good PS to replace FM in diets for blackspot seabream. Moreover, 50P/10L FM based diet was the most cost-effective. Future studies should be conducted to investigate the species AA optimal ratio to each other and their different rates of absorption and catabolism, preventing AA utilization for other purposes than protein synthesis, such as lipogenesis or gluconeogenesis.
Acknowledgements
This work was partially supported by project IDEIA ‘Optidietas’, (Agência de Inovação, Portugal, with the support of the European fund FEDER) and by FCT, Fundação para a Ciência e Tecnologia of Portugal (PhD Grant SFRH/BD/22401/2005). Authors disclose any conflict of interest that could be perceived to bias their work and they have equally contributed to the research and writing of the manuscript. Special thanks to L. Larroquet, C. Vachot, M.J. Borthaire and António Júlio Pinto for technical assistance and Rodrigo Ozório for his critical reading of the manuscript.




