Girding for migratory cues: roles of the Akt substrate Girdin in cancer progression and angiogenesis
Abstract
Cell migration is a fundamental aspect of a multitude of physiological and pathological processes, including embryonic development, inflammation, angiogenesis, and cancer progression. A variety of proteins are essential for cell migration, but context-specific signaling pathways and promigratory proteins must now be identified for our understanding of cancer biology to continue to advance. In this review, we focus on the emerging roles of Girdin (also designated KIAA1212, APE, GIV, and HkRP1), a novel component of the phosphatidylinositol 3-kinase (PI3-K)/Akt signaling pathway that is a core-signaling transduction pathway in cancer progression. Girdin is expressed in some types of cancer cells and immature endothelial cells, and is therefore at the crossroads of multiple intracellular processes, including reorganization of the actin cytoskeleton, endocytosis, and modulation of Akt activity, which ultimately lead to cancer invasion and angiogenesis. It also acts as a nonreceptor guanine nucleotide exchange factor (GEF) for Gαi proteins. A significant observation is that Girdin, although vital for cancer progression and postnatal vascular remodelling, is dispensable for cell migratory events during embryonic development. These findings suggest that Girdin and its interacting proteins are potential pharmaceutical targets for cancer therapies and pathological anigiogenesis, including tumor angiogenesis.
(Cancer Sci 2010; 101: 836–842)
The development and progression of cancer are multistep processes characterized by permanent alterations in the genome of a single line of cells.(1) Among these processes, acquisition of the ability to migrate and invade, pushing aside and degrading the surrounding extracellular matrix and tissues, is one of the master switches that contribute to cellular transformation and, ultimately, to highly invasive and usually fatal cancers. While advances in cell biology and integrative genomic and proteomic analyses have greatly improved our appreciation of possible precipitating factors underlying the invasion of cancer cells, there is still much more to explore in the identification of cancer-specific proteins that govern the migration/invasion of cancer cells. It is not unusual to find that proteins that govern the migration/invasion of cancer cells are also critical for migratory events during embryonic development and for maintenance of fundamental physiological cellular processes. Therefore, consideration of the context and timing of physiological function, both in vitro and in vivo, is vital when studying proteins of interest in cancer development and progression.
Intracellular signaling pathways elaborately regulate various aspects of cellular functions, such as proliferation, differentiation, and apoptosis, etc., the dysregulation of which leads to the initiation and progression of cancers.(2–4) Especially, proteins encoded by oncogenes or tumor-suppressor genes act as accelerators and brakes of cell proliferation respectively. Mutations in these genes lead to abnormal regulation of intracellular signaling pathways and the loss of proliferation control by which the cells become malignant. The signaling pathway mediated by phosphatidylinositol-3-OH kinase (PI3-K) and the serine/threonine kinase Akt (also termed Protein Kinase B, PKB) is no exception to the progression of cancers;(5–12) it has been established as a critical signaling node within all cells of higher eukaryotes and as one of the most important protein kinases with versatile functions at the core of human physiology and a wide spectrum of human cancers.(13) Akt has a wide range of its substrates; the activation of Akt and these substrates regulates many important cellular functions, such as cell survival, growth, proliferation, metabolism, migration and invasion.(14) In this review, we focus on the roles of Girdin (girders of actin filaments), which was identified as a novel Akt substrate that is capable of binding to actin cytoskeleton.(15) Subsequent studies identified that Girdin and its phosphorylation are important for cell migration of cancer cells, immature endothelial cells, and neural progenitors, which regulates the invasion of cancer cells,(16) postnatal angiogenesis,(17) and neurogenesis,(18) respectively. It should be noted that the function of Girdin and its phosphorylation largely depends on contexts and tissue types. Here we summarize our current understanding of Girdin function with a particular emphasis on its roles in cancer progression and the cell biology that leads to cell migration.
Primary structure and expression of Girdin
Girdin was independently discovered in 2005 by four groups as a novel protein that interacts with Akt,(15,19) the trimeric G proteins Gαi/s,(20) and dynamin,(21) a large GTPase necessary for endocytosis.(22) Our laboratory isolated Girdin using Akt1 as bait in a yeast two-hybrid screen of a human fetal brain cDNA library, followed by 5′-rapid amplification of cDNA ends (5′-RACE).(15) Human and mouse Girdin genes (CCDC88A and ccdc88a, respectively) encode large proteins of 1870 (1871 in an isoform of human Girdin) and 1845 amino acids, with a predicted molecular mass of 220–250 kilodaltons (kDa). The structure of Girdin shows hydrophilic properties and a tendency to assume an α-helical coiled-coil conformation that spans more than two-thirds of the protein in its middle domain (the coiled-coil domain) (Fig. 1). The N-terminal domain (253 amino acids) shows significant homology to the microtubule-binding domain identified in the Hook family of proteins, which functions to link organelles to microtubules.(21) It is thought that the N-terminal domain and coiled-coil domain contribute to the oligomerization of Girdin. The C-terminal domain (495 amino acids) contains the Akt- and actin-binding sites and is also assumed to contribute to the interaction of Girdin with the plasma membrane (Fig. 1A). Upon stimulation with various types of growth factors, Akt phosphorylates Girdin at position Ser-1416 (1417 in an isoform of human Girdin) in the C-terminal domain, which plays an important role in remodeling of the actin cytoskeleton during cell migration.(15) It is of special interest that no other AGC (cAMP-dependent, cGMP-dependent and protein kinase C) kinases interact with the C-terminal domain of Girdin, indicating that Girdin is exclusively involved in the Akt signaling pathway.
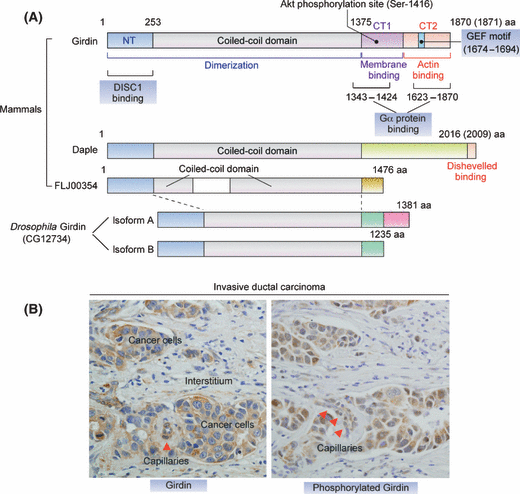
Primary structure of Girdin and its expression in breast cancer. (A) Proposed domain structures of Girdin and its family of proteins. Girdin can be divided into three domains, an N-terminal domain (NT) that shows high homology with the microtubule-binding domain of the Hook family of proteins, a central coiled-coil domain, and a C-terminal domain that binds to actin filaments and the Gα protein family. The N-terminal and coiled-coil domains are thought to contribute to Girdin oligomerization. The Gα proteins-binding site within the CT2 domain possesses GEF activity that specifically activates Gαi proteins. The primary structures of Daple and FLJ00354, members of the Girdin protein family, and Drosophila Girdin (dGirdin) are also shown. Among the members, the greatest sequence variation is located within the C-terminal domain, which specifies the binding partners. dGirdin gene has two alternative sites of translation termination, which gives rise to two isoforms of the protein (dGirdin-PA and dGirdin-PB). (B) Expression and phophorylation of Girdin in breast cancer. Shown are immunohistochemistry data of Girdin and its phosphorylated form on sections from invasive ductal carcinoma tissue. Note that Girdin is also moderately expressed and phosphorylated in microvessels (arrowheads) in the carcinoma tissues.
Although Northern and Western blotting analyses have revealed that Girdin is expressed in virtually all tissues and in various immortalized and tumor-derived cell lines, it has not been completely understood which cell types express Girdin in physiological contexts as well as in disease states. This review focuses on the expression of Girdin in human cancer, immature endothelial cells, and certain neuronal subsets.
During mouse embryonic development, Girdin expression is temporally and spatially restricted:(21)in situ hybridization experiments detect expression at embryonic day (E) 10.5 in the branchial arches, nasal processes, limbs, somites and dorsal root ganglia. At E11.5–E12.5, its expression persists at these sites and was also found in the fore-, mid-, and hindbrain, and the interdigital mesenchyme of the limbs as well as in the developing eye.
An extensive bioinformatical analysis revealed Girdin paralogues, Daple (Dishevelled-associating protein with a high frequency of leucine residues) and FLJ00354, in the human, rat, and mouse genomes (termed the Girdin family of proteins) (Fig. 1A). Daple was identified as a novel binding protein of Dishevelled, a modulator of the Wnt/β-catenin signaling pathway, but without any functional similarity to other well-defined proteins.(23) Recently, a Girdin-like protein was identified in Drosophila melanogaster (termed dGirdin), whose N-terminal and coiled-coil domains show high homology to human Girdin, indicating that the Girdin protein family arose from gene duplication events from a single Girdin-like protein early in evolution (Fig. 1A).(24) Similar to the function of Girdin in mammals, dGirdin is important for the regulation of actin organization and appropriate cell size control downstream of the Akt signaling pathway in Drosophila.
Expression of Girdin in human cancers
Considering that aberrant Akt signaling is heavily involved in the initiation and progression of human cancers, it is likely that the Akt/Girdin pathway also contributes to the malignant behavior of cancer cells. Several case studies have found that Girdin is expressed in a wide range of cancer cell lines and cancer tissues. One report stated that Girdin is expressed exclusively in colorectal carcinoma cells with high metastatic potential (such as HCT116 and DLD1 cells) and is virtually undetectable in those with poor metastatic potential (such as HT29p and LS174T cells), implying the involvement of Girdin in cell motility.(25) A comparison of the expression of Girdin between invasive ductal breast carcinoma and normal mammary tissues also showed that Girdin is highly and moderately expressed in ∼10% to 35% of breast carcinoma tissues, which is in contrast to normal tissues where it was not expressed in the normal epithelium of milk ducts but was weekly expressed in myoepithelial cells (Fig. 1B).(16) In addition, an investigation of 180 human cancer specimens, including carcinomas of the digestive tract, uterine cervix, lung and thyroid, detected high expression of Girdin in 10% to 50% of these carcinomas.(16) Aberrant expression of Girdin is not limited to epithelium-derived malignant tumors; in concert with Girdin expression in immature endothelial cells (see below), it is also expressed in endothelium-derived tumors such as capillary hemangiomas and angiosarcomas(17) (A.E. and M.T., unpublished data). A recent study showed that Girdin is highly phosphorylated by Akt2 in malignant glioma cells.(26) These expression profiles of Girdin in cancer cell lines and tissues indicate that Girdin has important roles in cancer development and progression, although we should know much more information about which processes of transformation, growth, invasion, and metastasis require Girdin’s function. A legitimate question is how the expression of Girdin is regulated in these cancer cells. At present, neither DNA amplification of Girdin gene nor loss of heterozygosity (LOH) of markers spanning Girdin on 2p16.1 has been reported.
Regulation of cancer cell motility by Girdin downstream of Akt
Many cancer cells possess the ability to invade and metastasize to adjacent and distant tissues, which is the leading cause of cancer death worldwide. Therefore, investigating the motility mechanism used by cancer cells not only increases our understanding of cancer progression but also provides new diagnostic approaches and targets for treatment.
We know that, among intracellular organelles, the cytoskeleton is the force-generating apparatus that moves cells from one place to another. Among the three types of cytoskeletal proteins (actin filaments, microtubules, and intermediate filaments), actin filaments regulate many aspects of dynamic cell motility.(27) The process of cell motility is very complex. The cells first polarize and extend protrusions (lamellipodia and filopodia) in the direction of migration. These protrusions are stabilized by adhering to the extracellular matrix (ECM) via transmembrane receptors such as integrins to form focal adhesions. Contraction of the actin stress fiber network then generates sufficient tension to drag the cells forward over focal adhesions. These adhesions disassemble at the rear of the cells, allowing them to retract and be dragged in the direction of migration.(28) A multitude of actin-binding proteins and Rho family of small GTPases, which are controlled by a variety of intracellular signaling such as the PI3-K/Akt pathway, play central roles in regulating actin reorganization.(29)
As a primary target of PI3-K, Akt has important roles in promoting cell survival, proliferation, and growth (Fig. 2A).(30) Abnormal expression of Akt has been reported in several malignant tumors. For example, Akt2 is highly expressed in breast, ovarian and pancreatic cancers,(31–33) and clinical studies have revealed that increased expression of Akt is associated with poor prognosis in cancer patients.(30,34,35) Moreover, a growing body of evidence indicates the PI3-K/Akt signaling pathway also regulates the migration of cancer cell types through several mechanisms, and one of which is the phosphorylation of Girdin at the leading edge of migrating cells.(16)
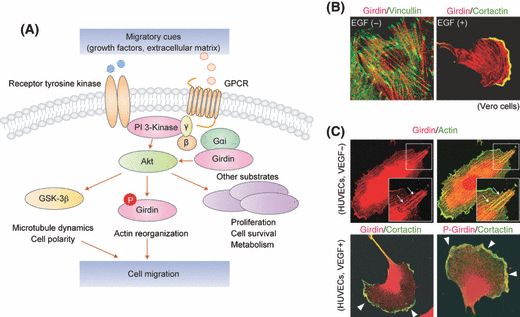
Role of Girdin and its subcellular localization in migrating cancer cells. (A) The roles of Girdin in the migration of cancer cells. The binding of various growth factors, extracellular matrix components, and other migratory cues to cell-surface receptors triggers an activation of Akt through PI3-K. Activated Akt subsequently phosphorylates several substrates (GSK-3β, Girdin, and other substrates) to regulate actin remodeling and microtubule dynamics, which ultimately leads to cell migration and polarization. Note that Girdin also binds to heterotrimeric G proteins to reciprocally regulate the activity of Akt downstream of GPCR. In collaboration with trimeric G proteins, Girdin activates Akt, which in turn phosphorylates Girdin to regulate actin-based cell motility. For detailed mechanism and speculated function of the Girdin/Gαi complex, see Fig. 3. (B and C) Subcellular localization of Girdin and its phosphorylated form in quiescent and growth factor-stimulated cells. In the left panels in (B), in quiescent Vero fibroblasts, Girdin (red) colocalized with stress fibers that span the cytoplasm and terminate in focal adhesions labeled with vincullin (green), whereas, in EGF-stimulated cells, Girdin accumulates at the leading edge and co-localizes with cortactin (green), a leading edge marker. Shown in (C) are immunofluorescent data of quiescent and VEGF-stimulated HUVECs. Girdin and its phosphorylated form (red) was detected on actin stress fibers, diffusely in the cytoplasm, and at the leading edge of migrating HUVECs (arrowheads). Figures (B) and (C) are reproduced from refs.(15,17) with permission from Elsevier and Nature Publishing Group, respectively.
The role of the Akt/Girdin pathway in cell motility was initially investigated in immortalized fibroblasts and the breast cancer cell line MDA-MB-231.(15,16) In quiescent cells without added growth factors, Girdin primarily localizes along actin stress fibers and is seen diffusely throughout the cytoplasm, often concentrated around the Golgi apparatus and the nucleus,(20) whereas, in migrating cells, it preferentially localizes to the actin meshwork structure of the lamellipodia at the leading edge (Fig. 2B,C).(15–17) In response to migratory cues such as growth factors, the phosphorylation of Girdin by Akt occurs at the leading edge, which is required for directional cell migration that, in the case of cancer cells, ultimately leads to invasion and metastasis (Fig. 2A). The importance of Girdin in cell motility is not confined to epithelium-derived tumors, as it is expressed in glioma cells arising in the central nervous system as well. In glioma cells, Akt2 is vital for the phosohorylation of Girdin and cofilin, a LIM-Kinase 1 substrate that is essential for the regulation of actin polymerization and depolymerization, for the regulation of cell migration and invasion.(26)
The mode of action underlying the regulation of cell migration by Girdin has been uncovered. Girdin associates with the plasma membrane through its CT1 domain, formerly half of the CT domain (15) (Fig. 1). In vitro binding assays have shown that the purified recombinant CT1 domain of Girdin specifically binds to membrane-bound phosphoinositides PI(4)P and PI(3)P, which reside in the plasma membrane and Golgi apparatus. This protein-lipid interaction is mediated in vitro by a positively charged sequence of 19 amino acid residues located near the Akt phosphorylation site (serine-1416), and is attenuated by Akt phosphorylation.(15) Although this interaction has not been confirmed in vivo, one speculation is that Girdin interaction with the plasma membrane and their dissociation by Akt phosphorylation are tightly regulated and fine-tuned only at the leading edge of migrating cells, which gives rise to the remodeling of the membrane skeleton that propels the cells forward.
Reciprocal regulation of Akt and Girdin signaling
The function of Girdin in intracellular signaling cascades is not a case of simple regulation downstream of Akt. It has also been shown that Girdin overexpression markedly enhances the phosphorylation of threonine-308 and serine-473 in Akt in the absence of growth factor stimulation (Fig. 2A), leading to subsequent phosphorylation of downstream substrates of Akt such as GSK-3β and the Forkhead Transcription Factor FKHR.(19) The mechanism behind the inducement of Akt phosphorylation by Girdin, a cytoskeletal protein, has not been discovered, but the biological significance of Girdin-mediated Akt activation has been examined in several ways using cultured cells. One study has shown that in discordance with the fact that Akt promotes cellular proliferation and inhibits apoptosis in many cell types, the overexpression of both Akt and Girdin inhibits cellular proliferation and cytokinesis, but induces apoptosis.(19) Furthermore, as mentioned in the next section, the Girdin-mediated Akt activation is positively regulated by Gαi, a Gα subunit of heterotrimeric G proteins (Fig. 2A). The guanine nucleotide exchange (GEF) motif located in the C-terminal domain of Girdin activates and sequesters Gαi (Fig. 1A), thereby enhancing Akt signaling through the Gβγ-PI3-K pathway.(36) Taken together, the synergistic relationship between Akt and Girdin may have biological relevance in various cellular processes, an example being the migration and differentiation of neural progenitors in postnatal and adult hippocampi as described below.
Girdin and Gαi: a new route from GPCR to Akt and cell migration
Another designation for Girdin is GIV (Gα-interacting vesicle-associated protein), which is derived from the interaction of Girdin with members of the Gα subfamily of heterotrimeric G proteins.(20) Heterotrimeric G proteins, which include α, β, and γ subunits, act as intracellular transducers to mediate signals from G protein-coupled receptors (GPCR) to effectors such as adenylyl cyclases and phospholipase C.(37) In the past, hundreds of different GPCRs that respond to a wide variety of agonists have been identified with great hopes that they will prove to be useful drug targets for pharmacological intervention. The binding of agonists to their specific GPCRs causes a change in the conformation of the receptors and, consequently, the interaction with the heterotrimeric G proteins, which in turn releases bound GDP and binds GTP. Gα is converted to the “on” state, leading to the dissociation of Gα from the Gβγ subunit. Both of the receptor-activated GTP-bound Gα and the Gβγ subunit then stimulate several downstream effectors,(38) which induces cell migration(39) and tumorigenesis.(40)
It has become evident that, among the Gα subfamily, Gαi1 - 3 are preferred targets of Girdin. There is compelling evidence suggesting the importance of signaling through Gαi for cell motility. For example, the activation of Gαi is responsible for increased leukocytes chemotaxis after stimulation with platelet-activating factor.(41) An important unanswered question was how the Gαi-mediated signaling pathway regulates cell motility. A research group led by Marilyn G. Farquhar tackled this issue and found that Gαi interacts with two independent domains of Girdin. One is located in the region of amino acids 1343–1424, which spans from the coiled-coil domain to the C-terminal domain of Girdin. The second region lies in the latter part of the C-terminal domain (amino acids 1678–1694) (Fig. 1A).(20,36) It was also disclosed that the latter Gαi-binding domain of Girdin specifically interacts with a GDP-bound form of Gαi and has GEF activity toward Gαi (Fig. 1A). The Girdin C-terminal domain is required for activation and sequestration of Gαi and release of the Gβγ subunit for subsequent PI3-K activation, leading to Akt phosphorylation and the migration of cancer cells (2, 3). Thus, Girdin may serve as a mediator of the GPCR-Akt signaling pathway.
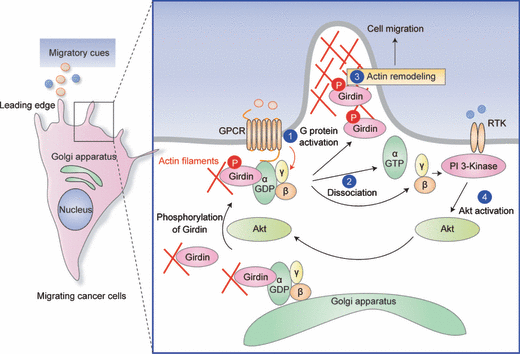
Proposed mechanism of Girdin-mediated Akt activation and cancer cell migration. In this model constructed by Ghosh et al.,(25) Girdin exhibits GEF activity towards Gαi and preferentially binds GDP-bound (inactive) Gαi at the plasma membrane and/or the Golgi apparatus. Stimulation with migratory cues such as growth factors and chemokines activates Akt, leading to the phosphorylation of Girdin, which is facilitated by its interaction with Gαi (1). Downstream of signals from ligand-occupied GPCR receptors, Gαi is activated by Girdin, which induces simultaneous dissociation of Gβγ and Girdin from the Gαi-Giridin complex (2). Subsequently, the release of phosphorylated Girdin enhances actin remodeling at the leading edge and promotes cell migration (3), whereas released Gβγ activates PI3-K-dependent Akt signaling (4). These processes may work as a positive feedback cycle of Akt signaling.
Heterotrimeric G proteins are present in various intracellular organelles such as the Golgi apparatus, endosomes, endoplasmic reticulum, and cytoskeleton.(42–47) Gαi3 has been found at the pseudopod, a spherical protrusion of the membrane at the leading edge of migrating cells.(25) Here, the Gαi3/Girdin interaction has a pivotal role in sensing migratory cues and switching them to actin remodeling and cell polarization during cell migration. Gαi3 interacts with Girdin at the plasma membrane in the leading edge, which induces activation of Gαi3 and phosphorylation of Girdin by Akt, thereby promoting cell migration by enhancing actin remodeling (Fig. 3). Accordingly, RNAi-mediated depletion of Gαi3 resulted in the alteration of Girdin localization and the decrease in Akt and Girdin phosphorylation, which leads to the attenuation of macrophage chemotaxis and tumor cell migration.(25) Although the underlying mechanism here has not been thoroughly investigated, it is possible that the binding of Gαi3 to Girdin promotes Akt-induced phosphorylation of Girdin via a change in the conformation of Girdin that can facilitate the interaction between Girdin and Akt. All of these observations indicate spatially regulated localization and activity of GPCR, Gαi, and that Girdin is required for proper directional migration.
Roles of Girdin in angiogenesis
Girdin is expressed in primary endothelial cells, hinting at the involvement of Girdin in the motility-related cell events such as angiogenesis. Angiogenesis is regulated by a balance between pro- and anti- angiogenesis factors, and the disruption of this balance contributes to the pathogenesis of numerous disorders including wound repair and cancer progression.(48) Without blood vessels, cancers cannot grow beyond a critical size or metastasize to other organs. Because of the necessity of angiogenesis in tumorigenesis, inhibiting angiogenesis as a cancer treatment strategy has several advantages compared with conventional treatments.(49) First, cancers treated with angiogenic inhibitors, which target normal, genetically stable endothelial cells, do not become resistant upon repeated drug applications. Second, angiogenic inhibitors do not interfere with normal physiological activities because angiogenesis is not a required activity in mature adult tissues. Third, this treatment strategy should be broadly effective against many different types of cancer that presumably employ the same mechanism of angiogenesis. At present, however, our understanding of angiogenic mechanisms especially in a postnatal contexts, including tumorigenesis, is far from complete.
Among various factors involved in angiogenesis, members of the vascular endothelial growth factor (VEGF) and angiopoietin (Ang) families have predominant roles.(48,50) What has been established is that VEGF-dependent activation of Akt is essential for the motility of endothelial cells, which is necessary for complex morphogenetic events, such as tubular sprouting and network formation.(50) Knowledge of Akt regulation of these processes was greatly increased by the observation that Girdin expression in human umbilical vein endothelial cells (HUVECs), and its phosphorylation by Akt, regulate VEGF/Akt-dependent angiogenesis in vitro and in vivo.(17) Generation and phenotypic analysis of Girdin knockout (Girdin−/−) mice showed that Girdin is dispensable for embryonic vascular development but is required for postnatal vascular formation. For example, the development of the retinal vascular plexus, one of the most thoroughly studied sites of postnatal vascular remodeling, was severely inhibited in Girdin−/− mice: both vascular sprouts and the area were smaller than those in normal mice (Fig. 4). It is of great interest that the vessels in intermediate and deep retinal layers were poorly developed in Girdin−/− mice, suggesting a role for Girdin in the response of endothelial cells to migratory cues and signaling pathways in three-dimensional contexts (Fig. 4C). Further experiments proved that Girdin is also involved in postnatal angiogenesis in the neocortex of the brain.(17) All of these observations are indicative of the importance of Girdin for vascular formation/remodeling during postnatal periods rather than during embryonic angiogenesis.(17)
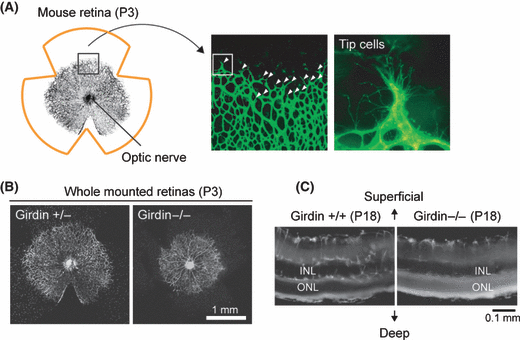
Role of Girdin in angiogenesis. (A) Schematic image of whole-mounted view of developing retinal vessels in mouse, showing that blood vessels radiate from the optic nerve to the periphery. Middle and right panels show isolectine B4 staining of P3 retinas of wild-type mice (a box in middle panel is magnified in right panel). White arrowheads in middle panel indicate migrating tip cells. (B) Isolectin B4 staining of whole-mounted P3 retinas of Girdin+/− or Girdin−/− mice. Retinal angiogenesis was impaired in Girdin−/− mice. (C) Cross sections of whole-mounted P18 retinas. In wild-type mice, superficial endothelial cells migrate to and form the area of the further deep and intermediate plexuses, which is in contrast to Girdin−/− mice that exhibited a poor vascular network in the deep and intermediate plexuses. INL, inner nuclear layer; ONL, outer nuclear layer. Figure 4A–C are reproduced from ref.(17) with permission from Nature Publishing Group.
Other factors that have been demonstrated to be to be involved in postnatal angiogenesis include the Angiopoietin-2/Tie2 and the Akt1-eNOS (endothelial nitric oxide synthase) signaling pathways.(51,52) Gale et al., in a milestone study using Angiopoietin-2-dificient mice, first reported that Angiopoietin-2, unlike VEGF and Angiopoietin-1, is not requisite during embryonic vascular development but, instead, is necessary during subsequent postnatal vascular remodeling.(51) Another study reported by Schleicher and colleagues found that Akt1-deficient mice show defective postnatal angiogenesis, which was confirmed in vivo in an experimental model of hind-limb ischemia. They also showed that Akt1-dependent postnatal angiogenesis is mediated by eNOS phosphorylation.(52) It remains unknown whether the signaling pathways mediated by these proteins are connected with Girdin, which should await further research.
An experimental challenge is to examine the involvement of the Akt/Girdin pathway in tumor angiogenesis. In addition, considering that Girdin function is applied to postnatal microvascular remodeling, one can speculate the Akt/Girdin signaling pathway is pivotal for neovascularization in adult life under various physiological and pathological conditions such as wound healing and proliferative retinopathies, and, as such, is a potential target for the development of anti-angiogenic agents.
Life without Girdin
Girdin−/− mice are not embryonic lethal and do not show any gross abnormalities at birth. However, they display weight loss after postnatal day (P) 7–8 and died by P25 without any serious pathological changes. Although it is possible that defects in angiogenesis contribute to the short-life span of Girdin−/− mice, they do not exhibit any angiogenesis-related severe symptoms and diseases such as hemorrhage, edema, and inflammation. These observations lead one to wonder about the cause of the death of Girdin−/− mice. Recent studies from our own and other laboratories shed some light on this issue;(18,53) Girdin−/− mice show severe defects in the cytoarchitecutre of the dentate gyrus of the hippocampus, which is known to develop postnatally, unlike other regions in the brain. Histochemical analyses have revealed that the defect is due to abnormal migration and/or positioning of newborn neurons in the dentate gyrus. It was a surprise that, in contrast to a positive role of Girdin in cell migration in cancer cells and endothelial cells, the loss of Girdin resulted in overextended migration of newborn neurons in the dentate gyrus,(18) suggesting that the role of Girdin in cell migration depends on complex physiological regulation including the availability of chemoattractants and niche components in addition to the intracellular machinery. One thing that all of these different cell types have in common is that they use Girdin and its regulatory proteins to gird for and respond to migratory cues derived from their microenvironment and neighboring tissues. Interestingly, the migration and positioning of newborn neurons in the dentate gyrus are regulated by the interaction of Girdin with Disrupted-In-Schizophrenia 1 (DISC1), a susceptibility gene for major mental illness including schizophrenia,(54) indicating the involvement of Girdin in the pathoetiology of these psychiatric diseases. DISC1 also has opposing migratory effects on neural progenitor cells in the developing neocortex and newborn neurons in the dentate gyrus.(55,56) A major explanation is that DISC1 and Girdin might relay positional signals to the intracellular migratory machinery rather than act as a direct mediator of neuronal migration. It is of interest that Akt might participate in the regulation of neural migration in the dentate gyrus. In a model presented by Kim et al., (53) Girdin augments the activity of Akt in newborn neurons, the dysregulation of which results in their mismigration and mispositioning. The Girdin/Akt pathway is modulated and fine-tuned by the interaction of DISC1 with Girdin.(53) In view of these complex findings, the intracellular signal cascade involving Girdin provides an attractive new paradigm for understanding the molecular mechanisms that underlie the migration of various types of cells, especially those of immature endothelial cells, neural progenitors, and cancer cells.
Another face of Girdin: possible involvement in membrane transport
Despite crucial roles in actin remodeling and cell motility, it remains a mystery how Girdin synergistically interacts and collaborates with other migratory mechanisms. A study using affinity column chromatography and mass spectrometric analyses identified Girdin as one of the binding proteins of activated dynamin, a large GTPase that is involved in the scission of a wide range of vesicles and organelles, and ultimately regulates various membrane transport processes including endocytosis. The authors of the paper referred to Girdin as HkRP1 (Hook-related protein 1), based on the fact that the N-terminal region of Girdin showed significant homology to the microtubule-binding domain of the members of the Hook family of proteins, which links membrane compartments to microtubules (Fig. 1A).(21,57) Immunofluorescent studies using Girdin antibody, produced against recombinant Girdin expressed in E. coli, showed the colocalization of Girdin with sorting-nexin 1 (SNX1) and early endosomal autoantigen 1 (EEA1), markers of early endosomes; dynamin; and the late endosomal marker lysosomal-associated membrane protein 1 (Lamp1). Expression of the C-terminal domain of Girdin leads to changes in the distribution of epidermal growth factor receptor (EGFR) and SNX1, a regulator of EGFR endocytosis. Further basis for the interpretation of physiological relationships between Girdin and membrane transport has been derived from a hypothesis that Girdin may associate with the microtubule cytoskeleton, which is considered to be the route of cellular vesicle traffic, through its N-terminal domain. All of these data and features strongly imply the involvement of Girdin in membrane transport processes, particularly endocytosis,(21) that play an important role in cell migration.
Future prospects
As is always the case, additional insights into Girdin function generate more questions than answers. What is the significance of postnatal angiogenesis regulated by the Akt/Girdin signal pathway? What is the role of Girdin and its interaction with DISC1 in the pathoetiolgy of major mental illness? An additional issue is the distinct cause of death or shortened life span of Girdin−/− mice. These questions will be solved by studies on conditional knockout animals in which the Girdin gene is disrupted in a temporal or tissue-specific manner.
Accumulated evidence suggests that Girdin sits at the crossroads of multiple cellular processes, and our appreciation of Girdin function is still in the most nascent of stages. The mechanism(s) behind Girdin regulation of cell migration remain obscure. One of the most relevant questions is how Akt phosphorylation of Girdin changes its function and, in turn, how Girdin augments Akt phosphorylation independently of growth factor stimulation. Also, regarding the interaction of Girdin with dynamin and heteromeric G proteins, it is imperative that we understand how Girdin regulates and connects membrane transport and intracellular signal transduction, which converge to form the cell migratory response.
Many facets of Girdin function await clarification. However, it is of note that Girdin differs from other promigratory proteins in that it may be dispensable for embryonic development but vital in some postnatal contexts and several migration-related diseases, including adult cancer. Future work will be required to reveal a comprehensive picture of Girdin function and determine if Girdin is a promising target for cancer therapy.
Acknowledgments
This work was supported by Grants-in-Aid for Global Center of Excellence (GCOE) Research, Scientific Research (A), and Scientific Research on Priority Area ‘Cancer’ (to M.T.); the Program for Improvement of Research Environment for Young Researchers from Special Coordination Funds for Promoting Science and Technology (SCF) (to A.E.) commissioned by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (to A.E.).
Conflict of interest statement
We declare that none of the authors have a financial interest related to this work.




