Treatment of thoracic esophageal carcinoma invading adjacent structures
Abstract
T4 esophageal cancer is defined as the tumor invading adjacent structures, using tumor–node–metastasis (TNM) staging. For clinically T4 thoracic esophageal carcinoma, multimodality therapy, that is, neoadjuvant chemoradiotherapy (CRT) followed by surgery or definitive CRT, has generally been performed. However, the prognosis of patients with these tumors remains poor. Another strategy is needed to achieve curative treatment. In the present article, the treatment strategies employed to date are reviewed. Furthermore, the strategies for these malignancies are reassessed, based on our experiences. R1/2 and R0 resections are regarded as those with residual and no tumor after surgery. The present data show that patients who underwent R1/2 resection after neoadjuvant CRT experienced little survival benefit, while complete response (CR) cases after definitive CRT had comparatively better results. Therefore, curative surgery should not be attempted without down-staging, and definitive CRT should be the initial treatment. Then surgery is indicated for the eradication of residual cancer cells. Close surveillance is essential for early detection of relapse even after CR, because the operation will gradually become increasingly difficult due to post-CRT fibrosis. In conclusion, multimodality therapy consists of definitive CRT followed by R0 resection, which can be the treatment of choice for T4 esophageal carcinoma. These challenging treatments have the potential to constitute the most effective therapeutic strategy. (Cancer Sci 2007; 98: 937–942)
The incidence of stage T4, in which the tumor invades adjacent structures, is reported to be 8–30% among thoracic esophageal carcinomas.(1–9) A standard treatment for these diseases has not, however, been established. The variety of treatments currently available include surgery alone,(1,2) chemotherapy followed by surgery,(10) chemoradiotherapy (CRT) followed by surgery,(3–6,11–15) and definitive CRT.(6,7,16–20) The extremely poor prognosis of patients with these T4 tumors is well known, irrespective of the treatments employed.(1–21) Therefore, curative treatment for T4 esophageal carcinoma remains an elusive challenge.
The accurate diagnosis of T4 and the indications for combined resection of the invaded organs and additional surgery after definitive CRT are unresolved issues. The most serious problem is which treatment should be selected first. It is difficult to decide whether definitive or neoadjuvant CRT should be given initially to a patient with a potential T4 tumor. In the present article, these problems regarding the treatment of T4 thoracic esophageal carcinoma are reviewed. Furthermore, the strategy for managing these diseases is reassessed, based on results obtained by the authors’ institute hospital.
Diagnosis
Depth of invasion is usually diagnosed using computed tomography (CT)(1–5,10–21) and/or endoscopic ultrasonography (EUS).(1,9–11,13–18,20,21) The invaded structures are frequently vital organs, for example, the aorta, trachea, bronchus and lung.(1,2,7–9,12,14,15,20) These clinical findings of T4 disease are not, however, often coincident with operative or histopathologic findings. Because both radiologic and endoscopic examination have diagnostic limits, and microscopic examination of the invaded organ is impossible without the resected specimen, there have been few reports focusing on the accuracy of T4 diagnosis. A study showed the accuracy in identifying tumor invasion of EUS (87.5%) to be significantly higher than that of CT (43.8%) among surgical cases.(21) Occasionally, the histopathologic evidence of T4 can be obtained through bronchoscopic examination identifying marked invasion of the respiratory tract.(1,3,5,6,12–14,16,17)
A retrospective study in the authors’ institute revealed the true-positive rate in tumors preoperatively assessed as T4 by CT to be 51%.(2) The accurate diagnosis of true T4 is clinically difficult unless definitive evidence of invasion to adjacent organs is obtained. 1, 2 show CT examinations of the cases with and without invasion to the trachea. Discrimination between expansive growth and infiltration of tumor before surgery is actually hard (Fig. 3). Therefore, the treatment strategy must initially be decided according to the clinical findings (clinically identified T4 tumors: cT4).
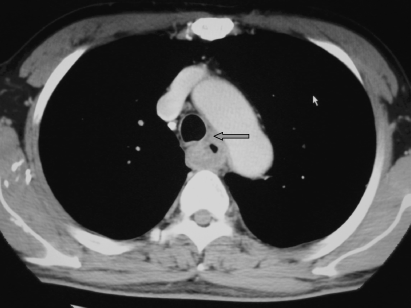
Computed tomography examination of cT4 case. Arrow shows tracheal invasion confirmed during surgery. Cancer cells were left after surgery.
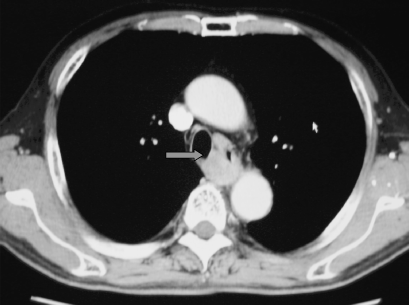
Computed tomography examination of cT4 case. Trachea was pressed by tumor (arrow). R0 resection was, however, done.
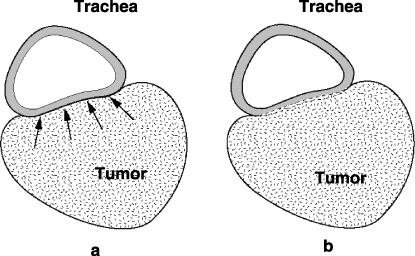
Schema of tumor growth. Discrimination between expansion and infiltration is difficult by clinical examinations. (a) Expansion, (b) infiltration.
Combined resection of the invaded organ
There is no doubt that R0 resection is strongly associated with longer survival compared with R1/2 resections.(1,2,12,13,22) R0 surgery for the true T4 carcinoma necessitates combined resection of the invaded organ. There are, however, only two reports describing such extended surgery.(1,2) Combined resections of the trachea, bronchus, lung and pericardium have been reported, but have not been shown to contribute significantly to prolonging survival. Most cases in those series did not undergo neoadjuvant therapy. The rates of R0 resection reported in those earlier studies were 18% and 44%.
Recently, the multimodality approach has come into widespread use for T4 esophageal carcinoma. CRT followed by surgery is the most commonly applied strategy.(3–6,11–15) There have, however, been no reports documenting benefits of combined resection of invaded organs after CRT. Therefore, the significance of extended surgery after CRT for T4 esophageal carcinoma remains unclear.
Additional surgery after definitive CRT
The CR rates after definitive CRT for cT4 esophageal carcinoma reportedly range from 15 to 42%.(16–19) The 3-year survival rates were, however, 7–24%.(16–20) These data suggest frequent recurrence even after CR is achieved using definitive CRT.
A discrepancy between the clinical response and histopathologic effect has been reported.(4) Furthermore, frequent local relapse (17–78%) after CRT, even with resectable tumors (T2,3) and even after CR, has been widely documented.(23–30) Therefore, clinical CR does not necessarily mean the absolute disappearance of viable cancer cells.
Esophagectomy after definitive CRT, so-called salvage surgery, has also been recently reported.(31–34) R0 resection has also been associated with long survival after definitive CRT.(32–34) However, few of those reports described any benefits of salvage surgery for primary cT4 esophageal carcinoma. Therefore, the significance of additional surgery after definitive CRT for cT4 tumor remains unclear. Furthermore, there have been no reports to date on planned surgery, that is, not salvage, after definitive CRT for the cT4 tumor.
Review of the results
Tumor invasion was assessed principally on the basis of CT scan examinations. The strategy for clinically T4 esophageal carcinoma in our institute was as follows. Patients presenting with a clinically T4 tumor were offered the multimodality approach.
Neoadjuvant CRT consisted of 40 Gy radiotherapy, and cis-diamminedichloride platinum (CDDP) and 5-fluorouracil (CDDP + 5-FU; FP) chemotherapy was administered when the pretreatment examinations showed no distant metastasis and that R0 resection was feasible after down-staging. Patients underwent the surgery 4 weeks after completion of this therapy. Definitive CRT consisted of 60 Gy radiotherapy, and FP chemotherapy was indicated when distant metastasis was present and/or there was no possibility of resection due to marked infiltration.
Neoadjuvant CRT was performed concurrently. Chemotherapy consisted of a combination of 5-FU and CDDP. From 1990 to 2001, 5-FU at 200 mg/m2 per day was administered as a continuous infusion on days 1–28. CDDP at 5 mg/m2 per day was administered as a bolus infusion on days 1–5, 8–12, 15–19 and 22–26. From 2002 to 2004, 5-FU at 400 mg/m2 per day was administered as a continuous infusion for 5 days/week for the first 2 weeks. CDDP at 40 mg/m2 per day was administered as a bolus infusion on days 1 and 8. The radiotherapy regimen consisted of 2.0 Gy daily, 5 days/week up to a total 40 Gy over 4 weeks. Radiation therapy was started on day 1 concomitantly with chemotherapy. The treatment volume extended 3 cm cranially and caudally beyond the gross tumor and generally included supraclavicular or celiac nodal regions, prophylactically, according to the primary tumor site. The operative procedure was an esophagectomy via a right thoraco-abdominal approach. Trans-hiatal esophagectomy was not performed. Lymphadenectomy involving the mediastinum and upper abdomen was usually performed, either with or without dissection of the cervical region. Combined resection of the potentially invaded organs was indicated for the en-block excision. Alimentary tract reconstruction was preferentially carried out using the gastric tube.
Definitive CRT was also administered concurrently. Chemotherapy consisted of infusion of 5-FU at 400 mg/m2 per day on days 1–5 and 8–12 combined with CDDP at 40 mg/m2 per day on days 1 and 8. This regimen was given twice with an interval of 5 weeks. The planning target volume of radiotherapy was the same as that of the neoadjuvant CRT. There was a 2-week break after a dose of 30 Gy. Radiotherapy was restarted on day 36 along with the previous chemotherapy schedule. Ultimately, a total dose of 60 Gy was administered, and the radiation dose to the spinal cord was no more than 50 Gy. Definitive CRT consisted of two courses separated by a 2-week interval.
All patients presented herein had histologically proven squamous cell carcinoma. Survivals were calculated from the date of initiation of CRT using the actuarial Kaplan–Meier method.
From 1990 to 2004, neoadjuvant CRT plus esophagectomy was performed in 59 patients. Potential invasion identified by pretreatment examinations involved the following organs: major respiratory tract (47 cases), aorta (16) pericardium (8) and lung (4). The effects of CRT that is, a decrease in tumor size, were observed in 40 of the 59 patients. Combined resections of the major respiratory tract, lung, and pericardium were performed in 10, six, and six patients, respectively. Aortic resection was not indicated. Forty-five patients underwent R0 resections (76.3%; 45/59). There were no viable cancer cells in the tumor bed based on histological observation in eight patients, although remnant lymphatic metastasis persisted in four of the eight. Complication-related deaths during hospitalization occurred in three patients (5.1%; 3/59). The overall 1- and 3-year survival rates of the 59 patients were 67.8% and 37.9% (Fig. 4). The 1- and 3-year survivals for R0 resection and palliative resection (R1/2) were 77.8%, 45.1%, and 38.5%, 0%, respectively (Fig. 4). The results of R0 surgery combined with resection of the invaded organs are shown in Table 1. The prognosis of patients who underwent tracheal resection was poor, even after R0 resection.
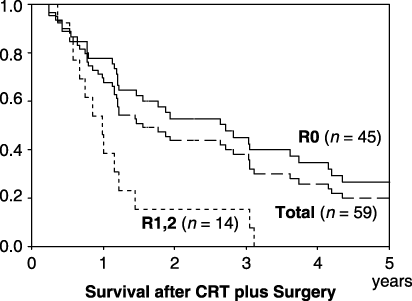
Survival after chemoradiotherapy plus surgery.
| Case no. | Resected organ | Outcome† | Recurrent site |
|---|---|---|---|
| 1 | Trachea | 2 y 5 m: dead | Local and brain |
| 2 | Trachea | 1 y: dead | Local |
| 3 | Trachea | 7 m: dead | Local and bone |
| 4 | Trachea | 7 m: dead | Local |
| 5 | Trachea | 6 m: dead | Lung |
| 6 | Trachea | 1 m: dead‡ | – |
| 7 | Lung/pericardium | 7 y 3 m: alive | – |
| 8 | Lung/pericardium | 2 y 10 m: dead | Lung |
| 9 | Pericardium | 1 y: dead | Local |
| 10 | Pericardium | 6 m: dead | Bone |
- † Postoperative outcome;
- ‡ ‡ operative death. m, month; y, year.
From 2000 to 2004, definitive CRT was performed in 29 patients. CR was observed in seven patients (24.1%; 7/29). A judgment of CR was made when the endoscopic examination confirmed tumor disappearance and the biopsy specimen histologically confirmed the absence of cancer cells. Neither spinal cord necrosis nor treatment-related death occurred. The overall 1- and 3-year survival rates for these 29 patients were 34.5% and 6.9% (Fig. 5). Survivals of CR and non-CR cases at 1 and 3 years were 83.3%, 33.3%, and 22.7%, 0%, respectively (Fig. 5). No surgical treatments were added to the management of these cases. Of nine patients who survived more than 1 year from the initiation of definitive CRT, four and two patients had grade 2 pericardial effusions and radiation pneumonitis. There was, however, no late toxicity-related death.
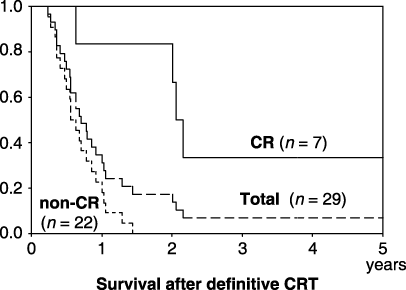
Survival after definitive chemoradiotherapy.
During this decade, five patients underwent esophagectomy after definitive CRT for the primary cT4 esophageal carcinomas. The operations were performed with the goal of eradicating residual or relapsing lesions. The results of these cases are shown in Table 2. In case 5, pretreatment bronchoscopic examination showed obvious infiltration to the trachea, which was confirmed histologically. However, the invasion disappeared and R0 surgery could be performed without combined resection after definitive CRT (Fig. 6a,b). Residual viable cells were histologically identified in the proper muscle layer of the resected specimen.
| Case no. | Invaded organ | CRT | Interval† | Combined resection | R0/2 | Outcome | Relapse site |
|---|---|---|---|---|---|---|---|
| 1 | Lung | 68 Gy/FP | 19 m | Lung | R2 | 8 m: dead | Local |
| 2 | Trachea | 66 Gy/CDDP | 5 m | – | R0 | 9 y 6 m: dead | – |
| 3 | Trachea | 60 Gy/FP | 18 m | – | R2 | 6 m: dead | Local |
| 4 | Trachea | 60 Gy/FP | 3 m | Trachea | R2 | 3 m: dead | Local |
| 5 | Trachea | 60 Gy/FP | 3 m | – | R0 | 1 y 2 m: alive | – |
- † Between the initiation of CRT and surgery. CDDP, cis-diamminedichloride platinum; FP, CDDP + 5-fluorouracil; m, month; y, year.
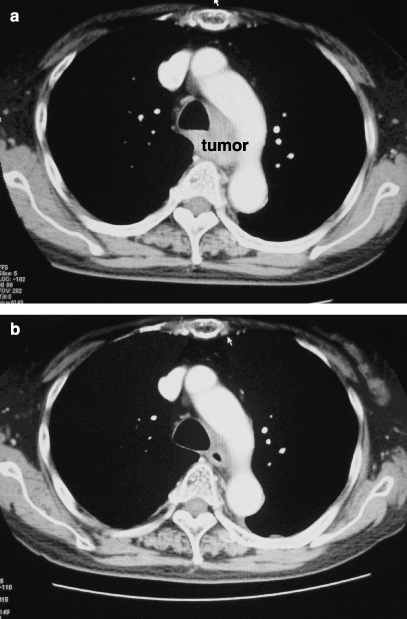
A case with evident invasion to trachea. (a) Trachea was obviously involved by tumor before treatment. (b) Reduction of tumor volume was observed after definitive chemoradiotherapy.
Development of an esophago-respiratory fistula makes curative resection impossible and prohibits oral intake. Bypass surgery has a benefit for those patients with this fistula after definitive CRT (Fig. 7).(35)
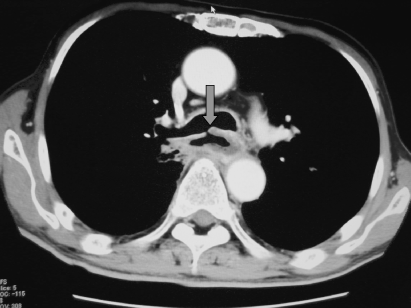
Arrow shows the esophago-bronchial fistula.
Should definitive or neoadjuvant CRT be the first treatment?
The major difference between neoadjuvant and definitive CRT is the radiation dose. Generally, a radiation dose less than 50 Gy is applied as neoadjuvant treatment. More than 50 Gy is regarded as the definitive dose. Radiation, as neoadjuvant treatment, was initiated in the 1960s.(36–40) Parker and Gregorie stated that 45 Gy preoperative radiation raised the resectability from 54%, in patients without radiotherapy, to 70%.(36) In 1970, Akakura et al. reported that a dose as high as 60 Gy of radiotherapy increased curative resectability from 26%, in a non-radiation treated group, to 65%,(38) although a dose of 45 Gy had been routinely used as neoadjuvant radiotherapy because of the risk of spinal cord necrosis resulting from high doses.(36) However, after the conclusion that there was no survival benefit from neoadjuvant radiotherapy,(41–45) neoadjuvant CRT followed by surgery came into widespread use for locally advanced esophageal carcinoma. Most of those neoadjuvant treatments consisted of 30–45 Gy radiation and FP chemotherapy, starting in the latter half of the 1980s.(46–62) The standard radiation dose for neoadjuvant treatment has not since been altered. However, improvement of radiation techniques has contributed to the elimination of spinal cord necrosis and rare treatment-related deaths even with definitive CRT.(6,7,16–20,63–67) Therefore, the neoadjuvant dose, that is, less than 50 Gy, despite being well established, should be reassessed.
The present data show that R1/2 resection after neoadjuvant CRT yielded worse results than CR after definitive CRT. Aggressive surgery, that is, esophagectomy after CRT, produces little survival benefit unless R0 resection is completed. Therefore, down-staging is essential before surgery for curative treatment of cT4 esophageal carcinoma. However, preoperatively judging the feasibility of R0 is difficult, particularly after inflammatory changes due to radiotherapy.
The authors thus conclude that the first treatment for cT4 esophageal carcinoma should be definitive CRT. Complete eradication using definitive CRT would appear to be the best option for patients. However, the possibilities for carrying out this treatment strategy are known to be limited. Thus, surgery should be considered when residual cancer cells are judged to be resectable. Definitive CRT has the potential to be the ultimate neoadjuvant treatment.
Additional surgery after definitive CRT
In actuality, the gap between the neoadjuvant and the definitive dose is closing. Frequent complications after salvage surgery have, however, been well documented(31–34) and surgeons must be concerned with the safety of this operation. Urschel et al. pointed out that the nature of radiation sequelae is determined by the time interval between completion of CRT and the performance of esophagectomy.(68,69) Radiation injures mediastinal structures, and causes early (measured in weeks) inflammation and late (measured in months) fibrosis. The intervals between CRT and salvage esophagectomy reported to date are usually many months.(31–34)
The interval from the completion of CRT to the operation is important when considering additional surgery. This is because salvage surgery gradually becomes more difficult with the passage of time after completion of CRT, as mentioned above. Therefore, close surveillance, including positron emission tomography (PET)–CT and endoscopy, is necessary even after CR is achieved using definitive CRT for early detection of residual or relapsed lesions. At the authors’ institute, the first surveillance is usually performed 4 or 6 weeks after the completion of CRT.
The present data suggest a survival benefit of additional surgery performed within several months after the completion of CRT if R0 resection is feasible (Table 2). Surgery following definitive CRT has the potential to be the ultimate neoadjuvant treatment.
However, the mortality and morbidity associated with salvage surgery are reportedly high.(31–34) Furthermore, several studies of preoperative high-dose CRT have also shown frequent complications related to subsequent surgery.(70,71) At our institute, salvage esophagectomy after definitive CRT via the right thoraco-abdominal approach has been performed in 27 patients, to date. Fortunately, there were no operative deaths among these patients (unpublished data, 2005). Conservative dissection is needed during salvage esophagectomy. Resection of all viable cancer cells is mandatory, but extensive mediastinal dissection and extensive lymphadenectomy are not indicated.
Benefit of combined resection
Our data show little benefit of combined resection of the trachea even when R0 resection is performed. These procedures are also associated with severe complications. Therefore, at the authors’ institute, tracheal resection is not presently advocated, nor is aortic resection performed. Partial resections of the lung or pericardium are feasible and are done with R0 surgery.
Radiotherapy
High radiation doses are well known to increase the risk of pulmonary function impairment.(72–74) However, a dose-escalation effect of radiotherapy has been reported.(75) As several studies have shown,(64–67,76) a dose exceeding 50 Gy is needed for definitive treatment of esophageal carcinoma. To decrease life-threatening complications, for example, acute lung injury, radiotherapy techniques that minimize the irradiated lung volume are required.
Recently, INT 0123 phase III trial reported that there was no significant difference in survival, or local/regional failure between the high-dose arm (64.8 Gy) and the standard-dose arm (50.4 Gy) with the same chemotherapy schedule.(67) Therefore, the radiation-dose of definitive CRT should be reassessed.
Chemotherapy
Recently, new neoadjuvant CRT regimens including paclitaxel have been reported.(77–79) Their role in the treatment of T4 esophageal carcinoma remain to be determined. The combination of CDDP and 5-FU is still the main regimen employed for definitive CRT.
Acknowledgment
The authors are indebted to Ms K Fujieda, who prepared the illustration.




