Assessment of Hard-To-Heal Lower Leg Wounds With Near-Infrared Fluorescent Imaging: A Pilot Cohort Study
Funding: This work was supported by the Fisher and Paykel Healthcare.
ABSTRACT
This case series explores the application of Near-infrared fluorescent (NIRF) imaging as a diagnostic tool for assessing hard-to-heal wounds. Our aim was to explore the potential of this modality to provide insights into wound perfusion, tissue viability and healing progress. We conducted a 12-week non-randomised observational study, a subset of an ongoing interventional study. Participants with hard-to-heal lower limb wounds received multi-disciplinary wound care. NIRF imaging using Indocyanine green contrast was performed prior to treatment, at six and 12 weeks. Visual assessment and quantitative analysis of fluorescent intensity within a region of interest were summarised. Seventeen participants were included, with a mean (SD) age of 64 (9) years. Forty-seven percent were diabetic and 45% had peripheral arterial disease. Wound size changes ranged from 23% to −95%. Changes in wound healing in patients whose intensity reduced had better observed effects (i.e., reduction in area and increase in granulation). Increasing intensity was observed in three patients requiring further treatment for pathology not visually observable. NIRF imaging allows visualisation of revascularisation and wound healing progress prior to observable changes in hard-to-heal wounds, providing insight into inflammation that may otherwise go unnoticed. Further research is needed to determine the utility of NIRF in wound management.
Summary
- NIRF imaging detects changes in wound perfusion and inflammation before they are visible through standard inspection, identifying early complications in hard-to-heal lower leg wounds.
- Quantitative analysis of fluorescence intensity offers a more objective assessment of wound healing progress, with trends suggesting that decreasing intensity correlates with better healing outcomes.
- NIRF imaging was feasible to integrate into routine wound care and provided additional clinical insights, but larger studies are needed to confirm its clinical utility and cost-effectiveness.
1 Introduction
Hard-to-heal wounds, including venous leg and diabetic foot ulcers, represent a significant burden to patients and healthcare systems. An estimated 3% of US citizens have hard-to-heal wounds [1], and this proportion is larger in elderly populations [2]. Hard-to-heal wounds do not follow the normal wound healing process of haemostasis, inflammation, proliferation and maturation [3]. They are often stalled in the inflammatory phase and are characterised by prolonged healing times, frequent recurrences and complications such as infections and amputations. Effective management and monitoring of these wounds can improve patient outcomes and reduce healthcare costs.
An important factor in wound healing is adequate tissue perfusion, which ensures the delivery of oxygen necessary for tissue repair. Traditional methods for assessing wound healing, such as visual inspection and measurement of wound dimensions, may not provide sufficient information on underlying tissue viability and perfusion [4].
Near-infrared fluorescent (NIRF) imaging can be used as a real-time assessment of tissue perfusion and vascularisation [5]. This imaging modality utilises near-infrared light to excite fluorescent dyes, such as Indocyanine Green (ICG), which bind to plasma proteins and emit fluorescence when illuminated. The emitted fluorescence can be captured using a near-infrared camera, allowing for the visualisation of blood flow and tissue perfusion in vivo.
Previous studies have demonstrated the potential of NIRF imaging to provide insights into the pathophysiology of various medical conditions, including cancer, cardiovascular diseases and wound healing [6-9]. However, few studies investigate the application of NIRF imaging in the assessment of hard-to-heal wounds.
This cohort study aims to investigate the utility of NIRF imaging using ICG in the evaluation of chronic lower leg wounds of various etiologies. By providing detailed visualisation of wound perfusion and tissue viability, NIRF imaging may offer a more objective and comprehensive tool for monitoring wound healing progress. This study explores the feasibility of incorporating NIRF imaging into routine wound care management and its potential impact on clinical decision-making and patient outcomes.
2 Methods
This was a prospective observational cohort study, which is a subset of an ongoing interventional study. Patients from Otago and Southland, New Zealand, were included if they were over 16 years old and had lower limb wounds over 10 cm in diameter and of a duration of more than 4 weeks. Patients were excluded if there was exposed bone or tendon, if they were on antibiotics for wound infection, if they required dressing changes more than every second day, or if wounds had slough covering more than 30% of the wound area. Additionally, for NIRF imaging, patients were excluded if they had severe kidney failure, defined as an estimated glomerular filtration rate (eGFR) of 15 mL min−1·(1.73 m2)−1 or a prior reaction to ICG, or were currently pregnant.
2.1 Treatment Protocol
Participants received wound care provided by a hospital wound care specialist. Care was provided three times weekly by a wound nurse specialist, with wound measurements taken using the Silhouette wound and skin assessment system (ARANZ Medical Ltd., New Zealand).
2.2 NIRF Imaging
Limbs were imaged at the first clinic visit, 6 weeks, and 12 weeks following the commencement of the treatment program. An infusion of ICG (Cardio- green, Sigma-Aldrich. Saint Louis, Missouri, USA) was administered via a cannula inserted into the anterior cubital fossa vein. A slow (10 to 12 s), continuous volume of ICG (0.1 mg/mL/kg in water for injection) was infused while imaging the limb with the Novadaq SPY Elite system (Stryker Corporation, Kalamazoo, MI, USA) with a laser wavelength of 805 nm. The camera was in a fixed, mounted position approximately 30 cm and 90° angulation from the focal point of the wound. The working distance was guided by laser sites that converge at 30 cm. The NIRF imaging sequence was 216 s and included the following distinct periods: [1] a 30 s pre-infusion (background buffering), [2] the entire ICG dye infusion and [3] a post-ICG saline “washout” infusion.
2.3 Data Collection
Wound size was recorded at each dressing change by the dedicated wound care nurse. Images from NIRF were compared qualitatively for changes in blood perfusion levels. SPYQ software (Stryker Corporation, Kalamazoo, MI, USA) was used to process the images for analysis. Each pixel in the image was assigned the average intensity of the corresponding pixel from every frame in the video sequence from start to finish and baseline-compensated for ambient light and skin reflectance. The possible levels for each pixel ranged from 0–255. Three values were recorded to measure the average dye intensity during the sequence: the highest value for dye intensity during the sequence including the wound bed and periwound; the highest value within the wound bed; and the lowest value within the wound bed. Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Otago, Dunedin. All participants provided informed consent, and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in approval by the Health and Disabilities Ethics Committee of New Zealand (2022 FULL 11479).
2.4 Statistical Analysis
Results are presented as n (%) for categorical data and mean (SD) or median (IQR) for continuous data depending on the distribution. We derived Tmax (Tmax = ) and approximated AUC (AUC = ) from the ingress and ingress rate provided by the Stryker software. The change in wound measures (area, volume, slough, granulation) were compared according to categorisation for participants whose imaging showed a higher or lower intensity according to where the intensity of the dye was measured. Wound measurements for the different categorisations (whole image, highest intensity in wound, lowest intensity in wound) were compared with Cohens Kappa and Wilcoxon rank sum tests. Correlation analysis using Spearman's rank correlation coefficient was performed to assess the association between changes in perfusion (AUC) and wound healing metrics such as area, volume, and slough reduction. Analyses were performed in R (V 4.3.0).
3 Results
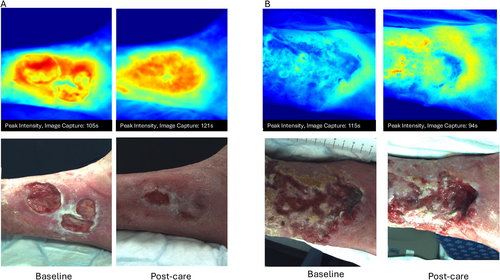
Seventeen participants were enrolled in this observational study. Patient characteristics are shown in Table 1. One patient was withdrawn after undergoing an above-knee amputation 2 weeks following enrolment. Thirteen patients (76%) completed 12 weeks of treatment for their ulcers. Four patients had imaging at baseline and 6 weeks, two due to wound healing. One patient was revascularised and was not further imaged, and one withdrew after 6 weeks due to lack of wound healing progress. There were no treatment or imaging-related adverse events. Wound area and volume reduced for 14 (82%) participants during the observation period. Dye intensity for the region imaged reduced for 10 patients during the study period and increased for seven. Figure 1 shows examples of images where the dye intensity increased (1A) and decreased (1B) on average over the imaging sequence.
| (N = 17) | |
|---|---|
| Sex | |
| Male | 16 (94.1%) |
| Age (years) | |
| Mean (SD) | 66.18 (11.5) |
| Range | 48.0–90.0 |
| Obese | |
| No | 14 (82.4%) |
| Yes | 3 (17.6%) |
| Smoker | |
| Ex | 5 (29.1%) |
| Non | 12 (70.1%) |
| Diabetic | |
| No | 9 (52.9%) |
| Yes | 8 (47.1%) |
| Primary Aetiology | |
| PAD | 7 (41.2%) |
| Mixed PAD, venous disease | 1 (5.9%) |
| Post op lesion removal | 1 (5.9%) |
| Trauma | 1 (5.9%) |
| Venous disease | 7 (41.2%) |
| Wound duration (weeks) | |
| Median (IQR) | 16 (7126) |
| Range | 4.00–1092.00 |
| Wound size, cm2 | |
| Mean (SD) | 23.39 (19.12) |
| Range | 3.10–68.00 |
The area under the curve for the fluorescent intensity was not significantly correlated with wound healing measures; however, wound volume and wound slough changes from baseline to 6 weeks were strongly and significantly correlated (Table 2).
| AUC change | p | Area change | p | Volume change | p | Slough change | p | Granulating change | p | |
|---|---|---|---|---|---|---|---|---|---|---|
| AUC change | 1 | 0.01 | 0.96 | 0.46 | 0.06 | 0.08 | 0.76 | 0.24 | 0.35 | |
| Area change | 0.01 | 0.96 | 1 | 0.05 | 0.85 | −0.54 | 0.02 | 0.39 | 0.13 | |
| Volume change | 0.46 | 0.06 | 0.05 | 0.85 | 1 | 0.23 | 0.37 | −0.25 | 0.34 | |
| Slough change | 0.08 | 0.76 | −0.54 | 0.02 | 0.23 | 0.37 | 1 | −0.54 | 0.02 | |
| Granulating change | 0.24 | 0.35 | 0.39 | 0.13 | −0.25 | 0.34 | −0.54 | 0.02 | 1 |
Figures 2-4 show the distribution of values for the change in wound healing measures for each method of categorisation. None of the measures significantly differ between patients whose imaging results are higher or lower in intensity, although across all methods of categorisation, patients whose intensity appears lower over time have better observed effects (i.e., reduction in area and increase in granulation).
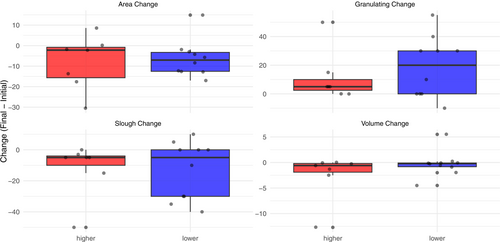

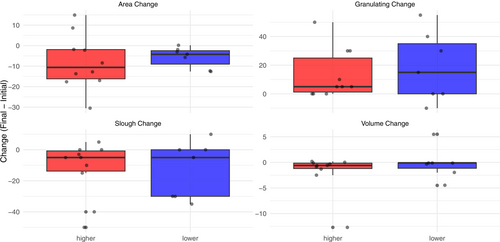
Categorisation based on the whole sequence and the highest intensity in the wound (kappa = 0.628, p = 0.009) agrees strongly for change in wound area, and their Wilcoxon p-values are similar (e.g., area p > 0.05 for both). Categorisation based on the lowest intensity value (kappa = 0.430–0.553) for measures, has the largest effect on change in area (p = 0.09). Wound volume and necrotic tissue changes were similar between groups.
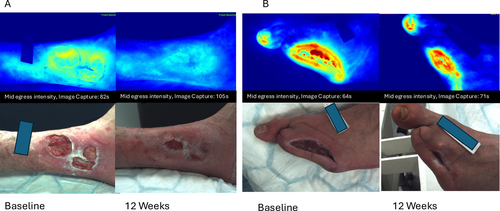
Near infrared images enabled detection of inflammation sites not visible to the naked eye, with two patients who had increasing dye intensity develop subsequent infections, and one patient with increasing intensity diagnosed with a wound malignancy. Figure 5A shows baseline and 12 week images for a wound that demonstrated decreased dye intensity and healing (50% reduction in wound area). Figure 5B shows a wound that retained areas of high dye intensity (red areas), however had a 92% reduction in wound area. The patient shown in Figure 5B was diagnosed with a left great toenail infection, subungal ulcer and received total nail ablation and clindamycin therapy 4 weeks following the capture of this image.
4 Discussion
This pilot cohort study provides preliminary evidence that Near-Infrared Fluorescent (NIRF) imaging with Indocyanine Green (ICG) can visualise tissue perfusion and detect subclinical changes in hard-to-heal lower leg wounds. By capturing dynamic changes in fluorescence intensity, NIRF imaging is a potential adjunct to traditional wound assessment methods, such as visual inspection and wound size measurement. Our findings suggest that changes in dye intensity may reflect underlying perfusion and inflammatory states, with patients exhibiting decreased intensity over time showing greater reductions in wound area and increased granulation tissue formation. Conversely, increased intensity was associated with inflammation and, in some cases, subsequent complications like infection or malignancy.
Inflammation not visible to the naked eye was observable from the NIRF imaging, as seen in two diabetic patients who later developed infections and one patient diagnosed with wound-related cancer. This aligns with prior studies, such as Arnold and Marmolego's work on infected limbs using near-infrared spectroscopy, and Hoven et al.'s visualisation of healing post-amputation [10, 11]. However, unlike qualitative literature [11, 12], our study quantified fluorescence intensity across imaging sequences, providing a less subjective framework for comparison. While no statistically significant correlation was found between intensity changes and wound healing metrics, trends, particularly when categorising by the lowest wound bed intensity, suggest a potential link. For instance, patients with a lowest wound bed intensity that had a higher intensity over time showed a median wound area reduction of −10.6 cm2 compared to −4.2 cm2 in those with lower intensity, with a medium effect size (p = 0.094), though this did not reach significance due to the small sample size. The lowest average pixel intensity within the wound area may reflect regions of hypoperfusion or necrosis, which are clinically relevant and may not correspond to the regions of highest intensity. Including highest and lowest intensity analyses allows us to explore the heterogeneity of perfusion within the wound bed and its potential relationship to healing outcomes.
The feasibility of integrating NIRF imaging into clinical practice was demonstrated, with the imaging process well-tolerated alongside standard wound care protocols. This suggests a practical role for NIRF in multidisciplinary settings. However, factors such as wound aetiology, comorbidities (e.g., diabetes, peripheral arterial disease), and individual healing trajectories may confound the relationship between fluorescence intensity and outcomes.
Measuring the average intensity of a sequence or region of interest allows for a less subjective comparison of imaging sessions, as has been demonstrated in patients with peripheral arterial disease [13]. While quantitative perfusion changes (AUC) did not show statistically significant associations with wound area or slough reduction in this small cohort, we observed a trend towards correlation with volume reduction (ρ = 0.46, p = 0.06). This may reflect preferential perfusion-driven changes in wound depth rather than surface area. The strong inverse relationship between slough reduction and granulation improvement (ρ = −0.54, p = 0.02) aligns with clinical expectations of tissue remodelling. An advantage of NIRF imaging is its ability to detect inflammatory changes invisible to the naked eye, as observed in three patients. These patients later developed clinically manifest infections or malignancy. The ability to detect inflammation is relevant in diabetic foot ulcers, where subtle changes in tissue perfusion and inflammation often precede clinical signs of infection. Prolonged inflammation delays wound healing, shown in animal [14] and human studies [15, 16].
Other researchers have used near infrared reflectance spectroscopy (NIRS) to quantify blood oxygenation (StO2), demonstrating that values obtained from this imaging modality may also predict wound healing [17]. NIRS, such as the MIMOSA system, offers practical benefits and is well-suited for routine or remote monitoring as they are non-invasive, and changes in StO2 may provide similar indications of inflammation [18]. However, NIRF imaging with ICG provides dynamic visualisation of tissue perfusion and can detect subtle changes in microvascular flow and inflammation, which may be less apparent with reflectance-based techniques [18]. Additionally, while NIRF imaging may signal early inflammation, its predictive value for complications like infection requires validation with larger cohorts and longitudinal follow-up.
While our results provide insights into the visualisation of wound healing, this study has several limitations. The small, all-male cohort (n = 15) from a single region limits generalisability, and the observational design precludes causal conclusions. Variability in wound aetiology and duration (median 16 weeks) may have influenced outcomes, yet these factors were not fully explored due to sample size constraints. Moreover, the absence of a control group and long-term follow-up leaves unanswered questions about NIRF's impact on wound closure rates or recurrence, critical metrics in chronic wound management. Cost-effectiveness and scalability in resource-limited settings also remain unaddressed.
The results of this study suggest that NIRF imaging with ICG can be used to assess perfusion and inflammation in hard-to-heal wounds, potentially complementing conventional methods. However, these findings are exploratory, and the technique's role in routine care requires further validation. Larger, randomised studies with diverse populations, modalities that utilise NIRS, and extended follow-up are needed to confirm efficacy, define clinical utility and assess practical implementation.
Acknowledgements
The authors would like to acknowledge Otago Vascular Diagnostics for their assistance with cannulating participants. Open access publishing facilitated by University of Otago, as part of the Wiley - University of Otago agreement via the Council of Australian University Librarians.
Ethics Statement
Health and Disabilities Ethics Committee of New Zealand (2022 FULL 11479).
Consent
All participants provided written informed consent for participation in this study.
Conflicts of Interest
This study presents a subset of participants from an interventional study funded by Fisher and Paykel Healthcare; however, the funder was not involved in the design, analysis, or interpretation of the results presented in this manuscript. The lead and corresponding author received no financial benefit from conducting this study.
Open Research
Data Availability Statement
The data used in this study are available upon reasonable request to the corresponding author.




