Effects of Carbon-Based and Organic Nanoparticles in Advanced Dressings for Skin Regeneration: A Review
Funding: This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo, 2019/05856-7, 2021/09335-1, 2023-11117-8.
ABSTRACT
Chronic wounds may develop when there is a delay or disturbance in one of the stages of the healing process, presenting challenging financial, clinical, and quality-of-life costs. Therefore, continuous efforts have been made to develop dressings that optimise wound healing. In recent years, nanotechnology has revolutionised wound care, enabling the development of innovative materials with high efficiency that positively impact the healing process. Nanoparticles have been extensively used in wound dressings because of their specific properties, such as a high surface area-to-volume ratio, increased surface reactivity, and improved biocompatibility, representing a unique tissue repair tool. This review article addresses advances in the use of organic nanoparticles in the field of skin regeneration, considering papers published in the last 5 years, and highlighting the effects of this class of materials on the wound healing process. The analysis of the literature shows that the materials being considered are carbon-based and organic materials, including polymeric, cellulosic, lipid, and liposome nanoparticles, which are covered in this review (inorganic nanoparticles are not considered). Furthermore, important aspects to prevent the development of chronic wounds are presented, as well as general characteristics of wounds, the healing process, and their particularities.
Abbreviations
-
- C-NPs
-
- Carbon-based NPs
-
- CNC
-
- Cellulose Nanocrystals
-
- CNF
-
- Cellulose Nanofibrils
-
- EGF
-
- Epidermal Growth Factor
-
- erGO
-
- GO nanofilm enriched with epoxy groups
-
- GO
-
- Graphene Oxide
-
- HA
-
- Hyaluronic Acid
-
- Hg II
-
- Mercury Iodide II
-
- MIC
-
- Minimum Inhibitory concentration
-
- MRSA
-
- Methicillin-resistant Staphylococcus aureus
-
- NLCs
-
- Nanostructured Lipid Carriers
-
- NPs
-
- Nanoparticles
-
- PCL
-
- Polycaprolactone
-
- PCL NPs
-
- Polycaprolactone NPs
-
- PDT
-
- Photodynamic Therapy
-
- P-NPs
-
- Polymeric NPs
-
- PTT
-
- Photothermal Therapy
-
- PVA
-
- Poly(vinyl alcohol)
-
- rGO
-
- Reduced GO
-
- ROS
-
- Reactive Oxygen Species
-
- SBH
-
- Stingless Bee Honey
-
- SLNs
-
- Solid Lipid-NPs
1 Introduction
Tissue engineering integrates principles of biology, medicine, and engineering, employing functional biomaterials to develop technologies for repairing damaged tissue [1]. Functional biomaterials used in tissue engineering approaches must possess properties capable of stimulating tissue regeneration quickly [2]. Therefore, in the skin regeneration area, a functional biomaterial must promote the rapid and efficient healing of damaged tissue [3]. These properties are essential to avoid the development of chronic wounds, which can lead to severe consequences (e.g., amputation or even premature death) [4]. Moreover, chronic wounds can be caused by improper application of dressings that impair the healing process, resulting in an injury with prolonged recovery.
The treatment of chronic wounds incurs considerable costs and represents a burden for the public health care system [5]. The most severe problem in this scenario is that chronic wounds significantly impact patients' quality-of-life, as they generate discomfort and negatively interfere with the development of the individual's basic activities. Studies indicate that an initial improvement in the quality of life of a person affected by chronic wounds are often not sustained over long periods [6]. Therefore, efforts are being continuously made to advance technologies that include effective materials for improving or accelerating wound healing [7]. In this context, nanoparticles (NPs) have been widely referred to in the recent literature for the manufacture of advanced dressings to be applied in skin regeneration approaches [8].
To emphasise the relevance of NPs in the skin regeneration area, the number of related publications was evaluated in comparison with all articles published on skin regeneration in the last two decades (2006-April/2025). For this analysis, the Scopus database was used. Initially, the term “skin regeneration” was searched, resulting in 25 006 publications. Then, the search was refined to “skin regeneration” AND [“nanoparticles” OR “nanomaterials”], resulting in 13 333 publications. Based on these data, a graph (Figure 1) was plotted to show the annual distribution of relevant publications.
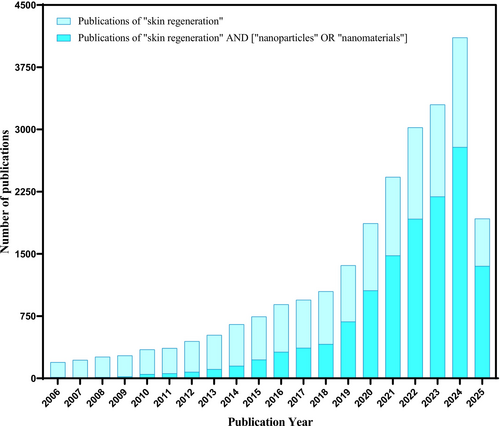
When analysing the number of publications related to the use of NPs in the skin regeneration area, a significant increase in studies has been observed. In recent years, highlighting the importance of NPs in this field. Additionally, a specific analysis of the literature was conducted over the last 5 years (2021-April/2025) to determine the percentage of publications that address the use of NPs in the skin regeneration area. The results showed 14 858 publications for the term “skin regeneration” and 9773 publications for the search “skin regeneration” AND [“nanoparticles” OR “nanomaterials”]. This ratio represents approximately 66%, emphasising the growing trend of research focused on the use of nanomaterials for skin regeneration.
From this perspective, this review aims to analyse studies published in the last 5 years, highlighting the effects of NPs with the potential to revolutionise wound care by producing advanced dressings. To classify the effects of carbon-based and organic NPs on skin regeneration, Table 1 summarises the relationship between the classes of NPs addressed in this review and their benefits. The main classes of NPs mentioned are carbon-based and organic materials, including polymeric, cellulosic, lipid, and liposome. Accordingly, this review compiles scientific evidence highlighting the synergistic integration of nanotechnology and skin regeneration strategies. The present study is organised in the following manner: Section 2 discusses the skin and its main characteristics related to wound dressing; Section 3 explores the different types of wound dressings; and Section 4 analyses the various NPs and their effects on the skin regeneration process.
| Nanoparticle class | Nanoparticle name | Scaffold type | Nanoparticle effect | References |
|---|---|---|---|---|
| Carbon-based | Graphene oxide | 3D by crosslinking and freeze-dried | Antibacterial activity and accelerated the closure of wounds | [9] |
| Multi walled carbon nanotubes | Hydrogel | Antibacterial activity and accelerated the closure of wounds | [10] | |
| Carbon nanotube | Carbon nanotube-loaded with chemical compounds | Antibacterial activity favoured epidermal growth factor growth and platelet-derived growth | [11] | |
| Graphene oxide | Nanofilm | Antibacterial and anti-inflammatory activity | [12] | |
| Graphene oxide | Hydrogel | Accelerated the closure of wounds | [13] | |
| Carbon dots | Carbon dots derived from mulberry | Antibacterial activity and effective tissue regeneration | [14] | |
| Polymeric | Chitosan-NPs | Hydrogel | Accelerated the closure of wounds, better collagen formation and arrangement | [5] |
| Chitosan-NPs | Nanocapsules | High efficiency of drug storage, sustained release capacity, and increased cell proliferation | [15] | |
| Poly(lactic-co-glycolic acid)-NPs | Nanocapsules | Improved the viability cell and protection of compound inside the nanocapsules | [16] | |
| Polycaprolactone-NPs | Hydrogel | Sustained release capacity and accelerated the closure of wounds | [17] | |
| Chitosan-NPs | Nanocapsules | Accelerated the closure of wounds | [18] | |
| Melanin-NPs | Hydrogel | Antibacterial activity and accelerated the closure of wounds | [19] | |
| Cellulose | Cellulose nanofibrils | Hydrogel | Great cytocompatibility and excellent mechanical integrity | [20] |
| Cellulose nanofibrils | Hydrogel | Improved hemostatic performance | [21] | |
| Cellulose nanocrystals | Electrospun nanofibers | Stimulated cell proliferation, improved structural integrity, and increased the biodegradability rate | [22] | |
| Cellulose nanocrystals | Hydrogel | Stimulate cell proliferation and improve, more efficient fibroblast migration and collagen deposition, and improve mechanical properties | [23] | |
| Cellulose nanocrystals | Hydrogel | Increase in storage modulus, higher cell viability, and proliferation potential | [24] | |
| Lipid and liposomes | Liposome-NPs | Film | High entrapment efficiency | [25] |
| Solid lipid-NPs | Nanocapsules | Sustained release capacity, antibacterial activity, and accelerated the closure of wounds | [26] | |
| Solid lipid-NPs | Hydrogel | Controlled delivery system and accelerated the closure of wounds | [27] | |
| Nanostructured lipid carriers | Film | Improved protection of compound inside and controlled delivery system | [28] | |
| Liposome-NPs | Membranes | High entrapment efficiency, controlled delivery system, and improved antibacterial properties | [29] |
2 Skin and Wound Dressings
The skin, the largest organ of the human body, acts as a physical, chemical, and biological barrier, playing vital roles in thermoregulation, hydration, immune defence, and vitamin D synthesis [30, 31]. Structurally, it comprises three layers: the epidermis, responsible for protection and cellular renewal; the dermis, which ensures vascularization and mechanical support; and the hypodermis, which regulates temperature and absorbs mechanical impact [32-35]. When damaged, this complex tissue initiates a cascade of events to restore homeostasis; however, depending on the nature and severity of the injury, the regenerative process may be impaired.
Wounds are typically classified based on their depth, aetiology, and healing potential [36-38]. While acute wounds follow an orderly progression through haemostasis, inflammation, proliferation, and remodelling phases [39-41], chronic wounds often stall in the inflammatory phase, particularly in pathological contexts such as diabetes [38, 42]. Therefore, the design of wound dressings that mimic skin function while actively supporting each healing phase is crucial to prevent complications and promote efficient regeneration.
Wound dressings are medical devices specifically designed to cover skin lesions, protect the wound from external contamination, control bleeding, manage exudate, and support the natural healing process [43]. They play a critical role in the treatment of wounds, especially in both acute and chronic cases. The choice of dressing depends on several factors, including the wound type, depth, exudate level, presence of infection, and stage of healing.
The basic characteristics of a dressing can be summarised as follows: (i) maintain a moist wound environment, (ii) effectively absorb excess exudate, (iii) thermally isolate the wound, (iv) allow gas exchange between the wound and the environment, (v) enable autolytic debridement, (vi) minimise the formation of scars, (vii) be impermeable to pathogens and dirt, (viii) be non-toxic, non-adherent, and comfortable, and (ix) promote mechanical support [8, 39]. Additionally, it should be cost-effective and easy to apply and remove without damaging newly formed tissue [44].
3 Advances and Classifications of Wound Dressings
Traditional dressings include gauze, cotton pads, and bandages, which offer mechanical protection but are limited in their ability to actively influence healing [44]. Modern conventional dressings have evolved to include more functional materials, such as hydrogels, hydrocolloids, films, foams, and hydropolymers. These categories are designed to manage moisture, reduce pain during dressing changes, and accommodate different wound exudate profiles. However, limitations remain, such as adherence to the wound bed, risk of maceration, and lack of antimicrobial or regenerative activity [45].
Moreover, conventional dressings often require the concomitant use of antibiotics to manage infections; however, these agents may be ineffective against the complex microbial environments found in chronic wounds [46]. Inappropriate antibiotic use also contributes to the emergence of drug-resistant bacterial strains, further complicating treatment [47].
On the other hand, advanced wound dressings can be broadly classified into three main categories: bioactive systems, smart dressings, and tissue-engineered scaffolds. Bioactive dressings incorporate compounds to actively modulate the biological phases of healing, such as silver NPs, essential oils, growth factors, and stem cells [48]. Smart dressings include systems capable of monitoring the wound environment (e.g., pH, temperature, or moisture sensors), as well as stimuli-responsive materials that react to external stimuli, such as light, electrical, or magnetic fields, to enhance therapeutic outcomes [43]. Lastly, scaffold-based dressings, often developed using tissue engineering principles, are designed to mimic the structure and function of the extracellular matrix, providing mechanical support while facilitating cell adhesion, migration, and proliferation [49].
In recent years, numerous research efforts have applied these strategies to develop innovative dressings that combine bioactivity, responsiveness, and structural support, often integrating multiple approaches into a single platform. Regarding the addition of a bioactive compound, Zhang et al. [50] developed a sodium alginate and type I collagen hydrogel loaded with human umbilical cord mesenchymal stem cells, which significantly enhanced wound closure and collagen remodelling in vivo. Concerning smart wound dressings, Wang et al. [51] developed a flexible bandage with a biocompatible liquid diode membrane and an ultrasensitive 3D polyaniline-based pH biosensor. The system enables unidirectional drainage of exudate and continuous monitoring of wound pH. The authors also proposed integrating this platform with additional therapeutic modules, targeting applications in home-based chronic wound care. Finally, as an example of scaffold-based dressings, Shang et al. [52] reported a carboxymethyl chitosan hydrogel enhanced with bioactive glass, titanium dioxide NPs, and mesenchymal stem cell-derived exosomes. This multifunctional system stimulated angiogenesis and enhanced cellular activity in human umbilical vein endothelial cells and L929 fibroblasts, facilitating in vitro wound repair [53]. Therefore, combining a malleable matrix doped with non-toxic NPs can satisfy the requirements for an ideal dressing, promoting adequate regenerative healing of the injured tissue.
Considering the complexity of the healing process, which involves sequential and overlapping molecular and physiological phases [43, 47], advanced dressings must address not only infection control but also the modulation of inflammation, tissue regeneration, and angiogenesis. In this context, tissue engineering, and especially nanotechnology, have emerged as important tools to create dressings that dynamically adapt to the wound microenvironment and accelerate regeneration, particularly in chronic or infected wounds [54].
Among the diverse strategies introduced by nanotechnology, nanomaterials have attracted increasing interest due to their ability to enhance biological performance [55]. Their incorporation into wound dressings has enabled the development of platforms with antimicrobial, antioxidant, and regenerative functions, factors for effective skin regeneration.
3.1 Nanoparticles and Nano-Effect
Nanotechnology is a field of science based on manipulating NPs, that is, particles with at least one of the material's dimensions varying between 1 and 100 nm [56]. NPs exhibit superior physicochemical properties, mainly due to the substantial increase in surface area and surface area-to-volume ratios [57]. Some examples of NPs are nanocapsules, nanowires, nanotubes, and nanosheets [58].
NPs can act significantly in the production of more efficient dressings, mainly due to the biological action that is induced in the extracellular matrix. The main effects of NPs that aid in the wound healing process are: (i) transport, protection, and controlled support for the active release of drugs; (ii) antimicrobial, anti-inflammatory, antioxidant, pro-angiogenic actions, stimulating cell proliferation, collagen deposition, and realignment; and (iii) ability to effectively interact with the cellular environment due to their size [56, 59].
Later sections of this review paper will address aspects related to the structure, properties, and effects of NPs in advanced dressings. The materials to be discussed are carbon-based and organic materials, including polymeric, cellulosic, lipid, and liposome. This review does not intend to comprehensively cover the literature; rather, it focuses on specific NP-based approaches, discussing their respective advantages and disadvantages and highlighting areas for further research. Figure 2 shows the different types of NPs addressed in this study.

4 Carbon-Based Nanoparticles
Carbon-based NPs (C-NPs) represent a group of materials that exhibit several special properties (e.g., mechanical resistance, large surface area, high chemical stability, adsorption capability, and high porosity), which make them great candidates for applications in different fields, from structural to biomedical materials [60]. Among the various relevant properties, the physicochemical ones make C-NPs widely explored for use in biomedical materials. The carbon allotropes that stand out in this type of application are graphene (most often in the graphene oxide (GO) or reduced GO (rGO) form), carbon nanotubes, and fullerenes, which are sheets of graphene containing hexagons and pentagons that give rise to their spherical shape [61, 62].
C-NPs applied to the wound healing process have led to significant advances in this type of treatment due to their particular characteristics, such as a high aspect ratio, large surface area, high stability, and high hydrophobicity [63]. Furthermore, the ability of C-NPs to accelerate the healing process is directly related to their anti-inflammatory and antibacterial potential, which contributes to the inhibition of inflammatory cell proliferation, prevents the harmful effects of these microorganisms, thus favouring the healing activity [64].
Regarding the use of C-NPs to promote biological properties, a study proposed a wound healing hydrogel composed of bacterial nanocellulose and GO derived from edge-functionalized charcoal [13]. Although bacterial nanocellulose displays excellent biocompatibility and mechanical properties, its lack of antibacterial activity limits its application as a wound dressing. To address this, the authors incorporated GO, known for its antibacterial potential and ability to promote cell adhesion and proliferation. The hydrogel with GO showed slightly enhanced L929 fibroblast cell viability (109%) compared to pure bacterial nanocellulose (96%), suggesting potential proliferative effects. Additionally, an antibacterial activity assay demonstrated that the inhibition zone against Escherichia coli (E. coli) was 17 mm for the GO samples, which was greater than that of the control counterpart (15 mm). Furthermore, the authors reported that the 3 T3 fibroblast cells migrated into the wound areas, and the rate of wound closure was high over 72 h. These findings suggest that GO incorporation into hydrogel has increased the biological activity of the hydrogels, indicating its potential as a candidate for improving skin regeneration.
In another study, GO was employed to load a hydrogel composed of chitosan and hyaluronic acid. Additionally, copper NPs were incorporated into the formulation, and sodium trimetaphosphate was used as a crosslinking agent [9]. GO was selected for its oxidative antibacterial mechanism, which damages bacterial membranes, while copper NPs enhanced the antimicrobial spectrum. The synergy between GO, copper NPs, and the hydrogel based on chitosan and hyaluronic acid resulted in improved physicochemical stability and biological performance of the dressing. Antibacterial tests against Staphylococcus aureus (S. aureus) and Staphylococcus epidermidis revealed significant reductions of the minimum inhibitory concentration (MIC) and minimum bactericidal concentration values, confirming bactericidal activity. The mechanism was associated with reactive oxygen species (ROS) generation and membrane disruption, favouring faster healing of infected wounds. The findings support the idea that combining C-NPs with biopolymers can amplify their therapeutic effects.
Expanding on the strategy involving GO, an eco-friendlier approach was proposed through the synthesis of a reduced GO nanofilm enriched with epoxy groups (erGO), obtained via a simplified MnO3+ intercalation method, as illustrated by the authors in Figure 3 [12]. The goal was to generate a high density of reactive groups to enhance ROS-mediated antibacterial effects while minimising synthesis waste. The erGO nanofilm demonstrated ~98% antibacterial activity against S. aureus and ~ 99.9% against Streptococcus pneumoniae. The mechanism involved bacterial encapsulation, isolation, and membrane degradation, as confirmed by electron paramagnetic resonance analysis. These results suggest erGO as a sustainable and highly effective antibacterial platform with potential applications in the treatment of infected wounds. Therefore, it presents a low-cost, green strategy aligned with the growing demand for sustainable biomaterials.

Still within the context of using C-NPs for the development of hydrogels aimed at promoting skin regeneration, carbon nanotubes were incorporated into a photothermal-responsive hydrogel composed of lignin, poly(vinyl alcohol) (PVA), and chitosan [10]. Lignin contributed to antioxidant activity, while carbon nanotubes provided mechanical reinforcement and potent antibacterial effects. Therefore, the hydrogel was prepared with good mechanical support, antioxidant activity, and antibacterial properties. The proposed hydrogels containing carbon nanotubes eradicated approximately 97% of E. coli and S. aureus, whereas control samples without carbon nanotubes reduced bacterial counts by less than 30%. Additionally, in vivo wound contraction studies further confirmed the bioactivity of the system, showing 61% closure in treated wounds versus 24% in controls. These findings suggest that carbon nanotubes can enhance both the antimicrobial barrier and the regenerative capacity of hydrogel-based dressings. The approach shows the positive effects of carbon nanotubes in supporting important skin regeneration processes, like antibacterial activity and wound contraction efficacy.
Carbon nanotubes were also explored for the treatment of infections caused by multidrug-resistant bacteria in burn wounds. In this study, Banihashemi et al. functionalised the nanotubes with carboxylic groups and loaded them with mercury (II) iodide at varying concentrations [11]. The composite demonstrated strong activity against clinical isolates of Acinetobacter baumannii, a pathogen frequently involved in antibiotic-resistant burn infections. The disk diffusion and MIC results confirmed its efficacy, highlighting carbon nanotubes as promising nanocarriers for antimicrobial agents. Despite the antibacterial results, the inclusion of Hg II raises concerns about biosafety and long-term toxicity, which must be addressed before clinical translation.
C-NPs have shown great promise in the development of wound dressings due to their multifunctional properties. Their ability to enhance epithelial regeneration is linked to controlled drug release, antibacterial and anti-inflammatory effects, and the promotion of cell proliferation. Additionally, C-NPs contribute to the mechanical reinforcement of scaffolds and can improve the overall stability and bioactivity of composite systems. Despite the notable advances, additional studies using complex wound models and long-term in vivo evaluations are required to consolidate the scientific basis for clinical application.
5 Polymeric Nanoparticles
Polymeric NPs (P-NPs) show advantages such as biocompatibility with tissues and cells, biodegradability, non-toxicity, sustained drug release, high encapsulation degree, and improved bioavailability. They have attracted attention in the biomedical and bioengineering areas because P-NPs can be used in wound dressings or delivery vectors. When used as a drug-delivery system, P-NPs can protect the drug from degradation and allow controlled and sustained release [63, 65, 66]. P-NPs can be composed of natural, synthetic, and semisynthetic polymers, such as albumin, alginate, chitosan, gelatin, poly(lactic-co-glycolic acid), polycaprolactone (PCL), and polyethylene glycol [65-67]. Figure 4 represents a schematic illustration, reproduced from Nunes et al. [68], showing the incorporation of P-NPs into hydrogels for biomedical applications.
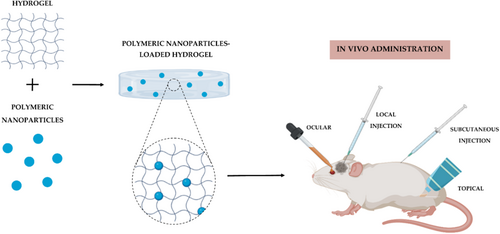
Among natural polymers explored for skin regeneration applications, alginate has gained attention due to its biocompatibility and gel-forming ability. In this context, one study proposed the use of alginate-based NPs to encapsulate stingless bee honey (SBH), aiming to enhance the wound healing process [69]. SBH is known to support skin regeneration by stimulating fibroblast proliferation and activating immune cells such as macrophages [69]. In addition, it helps reduce tissue damage caused by oxidative stress and exhibits antimicrobial properties. To enhance these biological effects, alginate NPs were used for SBH encapsulation. In vitro assays with human dermal fibroblasts demonstrated higher cell viability for SBH encapsulated in alginate NPs compared to free SBH. Scratch assays revealed improved tissue regeneration after 36 h in the encapsulated group, confirming the contribution of the nanoparticle system to enhanced wound closure and cell activation. The use of alginate NPs to deliver SBH improved both cell viability and scratch closure. The findings demonstrated that SBH-loaded alginate NPs exhibited regenerative effects, as confirmed by in vitro and in vivo analyses, including histological evaluation and gene expression studies [69]. In addition, the proposed material showed antioxidant and anti-inflammatory properties, highlighting its potential as a natural nanocarrier system for wound healing.
Still within the scope of natural polymer-based NPs, another study focused on chitosan NPs, which were used to encapsulate different concentrations of quercetin, a compound known for its anti-inflammatory, angiogenic, and proliferative effects on epithelial cells and fibroblasts [5]. The results in a rat wound model showed that NPs loaded with quercetin promoted better tissue granulation and collagen deposition after 7 days, as well as complete and mature epithelial layers by day 21. This group also exhibited the highest vascular endothelial growth factor and transforming growth factor beta 1 expression, indicating improved vascularisation and tissue remodelling. The encapsulation of quercetin in chitosan NPs enhanced its therapeutic performance by promoting wound closure and improving collagen organisation, particularly at lower doses, likely due to the controlled release provided by the NP system [5]. These findings showed that encapsulating quercetin into chitosan NPs aids in vascularisation, tissue remodelling, and collagen organisation, highlighting their potential for skin regeneration.
In a similar study, also employing chitosan-based NPs, the chitosan NPs were functionalised with hyaluronic acid and co-loaded with quercetin and curcumin for the treatment of chronic wounds [15]. The system showed high encapsulation efficiency and a controlled release profile. In vitro, the formulation stimulated cell proliferation in MC3T3-E1 preosteoblasts. In vivo, wounds treated with the proposed NPs exhibited a 98% closure rate after 28 days, significantly outperforming both untreated wounds (11%) and those treated with silver sulfadiazine (28%), demonstrating accelerated healing and infection control. The co-loading of quercetin and curcumin into the NPs further enhanced therapeutic efficacy, resulting in near-complete wound closure in vivo [15]. These results suggest that this approach represents a promising alternative as a basis for future studies involving P-NPs for skin regeneration.
Diabetic wound healing has long been a major clinical challenge due to impaired tissue regeneration and chronic inflammation. To address this challenge, researchers have focused on encapsulating insulin into chitosan nanoparticles to enhance its stability and therapeutic efficacy in diabetic wound healing [18]. Insulin promotes cellular proliferation, migration, and keratinocyte differentiation, but is unstable in physiological environments. Although it is very promising for producing materials for tissue regeneration, insulin is prone to easy degradation, and to attenuate such effects, P-NPs can be applied to encapsulate and protect the insulin. For this perspective, encapsulation in chitosan NPs preserved its activity and stability for up to 56 days. In vivo results demonstrated enhanced healing, with increased chemotaxis and activation of fibroblasts, keratinocytes, and endothelial cells, contributing to accelerated wound repair. Finally, chitosan NPs carrying insulin promoted fibroblast activation and faster wound healing in diabetic models [18]. Although the formulation showed good biocompatibility based on histological analysis, no specific immunogenicity assays, such as cytokine profiling or antibody detection, were conducted to fully assess systemic immune responses.
In addition to natural polymer-based systems, synthetic P-NPs have also been explored for skin regeneration applications, particularly in challenging scenarios such as diabetic wound healing. In this context, PCL-based NPs were used to encapsulate Asiaticoside, a bioactive compound derived from Centella asiatica known for its remarkable wound healing properties [17]. However, Asiaticoside has poor solubility, low lipophilicity, and a high molecular weight, which limit its pharmacological application. To overcome these barriers, PCL NPs were used to improve Asiaticoside delivery to the wound site. The release profile of Asiaticoside from PCL NPs was controlled over 24 h, in contrast to the burst release of free Asiaticoside. In vitro, the proposed wound dressing increased L929 fibroblast proliferation. In vivo, wounds treated with Asiaticoside-loaded PCL NPs showed faster closure (~27% improvement over Asiaticoside-free groups) and a thicker regenerated epidermis after 21 days. These findings demonstrate that encapsulating Asiaticoside in PCL NPs enhances its therapeutic efficacy by enabling controlled release and promoting superior wound healing outcomes, particularly in diabetic models.
In conclusion, P-NPs have emerged as promising tools for skin regeneration, offering protection and controlled release of bioactive compounds at the wound site. Systems based on polymers such as chitosan, alginate, PCL, and gelatin have demonstrated the ability to promote epithelialisation, granulation tissue formation, and collagen deposition, especially when loaded with agents like quercetin, curcumin, Asiaticoside, insulin, or natural substances such as SBH. Their effectiveness is closely linked to the physicochemical properties of the polymer, drug compatibility, and release kinetics tailored to the wound environment. Although advances have been made, further research is still needed to ensure long-term stability, biocompatibility, and therapeutic efficacy in more complex wound models and clinical conditions.
6 Cellulose Nanoparticles
Although cellulose is also a polymer, lignocellulosic materials exhibit promising applications for wound healing, and they are considered in this section. Cellulose NPs (i.e., nanocellulose) are highlighted due to their widespread interest in tissue engineering. Cellulose is the most abundant biopolymer in nature, as it can be obtained from bacteria and different biomass sources [70]. Nanocellulose is the generic name for two kinds of cellulose at the nanoscale: cellulose nanocrystal (CNC) and cellulose nanofiber (CNF). Both CNC and CNF can have similar diameter ranges (~3–20 nm), depending on their source and anatomical origins [71, 72]. Furthermore, CNC is a highly crystalline, rigid, and rod-shaped structure compared to CNF, which is flexible and has a greater surface area [73].
Due to their biocompatibility and ability to stimulate cell proliferation, CNC and CNF have emerged as promising fillers in bio-nanocomposites [74, 75]. In the context of wound healing, nanocellulose has been primarily explored to improve the mechanical performance of dressings, support cell adhesion and proliferation, and accelerate skin repair [76]. This section highlights recent advances in the use of CNC and CNF as fillers in wound dressings, emphasising their contributions to skin tissue regeneration.
To illustrate the potential of nanocellulose in skin regeneration, a PVA-borax hydrogel was reinforced with CNF and dopamine-grafted oxidised carboxymethyl cellulose to promote the healing of joint skin wounds [20]. According to the results, CNF improved the material's stretchability (elongation up to 3000%), and the dressing showed high cytocompatibility with the NIH3T3 fibroblast cell line, suggesting its potential for dynamic skin areas requiring both flexibility and support. The findings showed that the incorporation of CNF into the hydrogels provided remarkable flexibility and cytocompatibility; however, the study lacked in vivo validation to confirm its regenerative potential under physiological conditions.
Following this, another hydrogel system incorporating CNF was developed using chitosan and tannic acid for the treatment of traumatic haemorrhages and infected wounds [21]. CNF acted as a 3D scaffold mimicking the extracellular matrix, promoting fibroblast proliferation, while tannic acid enhanced haemostasis and antibacterial effects. The primary results indicated that the dressing could eradicate E. coli, S. aureus, and methicillin-resistant S. aureus (MRSA) bacteria by 99.9%. Additionally, it stimulated the proliferation of L929 fibroblast cells. Moreover, the hydrogel dressing exhibited high hemostatic performance, with the whole blood clotting index being up to 52.6% lower than that of gauze, the commonly used commercial dressing. The main results suggest that the proposed hydrogel demonstrates strong antibacterial, hemostatic, and regenerative properties, including enhanced collagen deposition and neovascularization, highlighting its potential as a multifunctional dressing for acute and infected wounds.
Beyond CNF, CNC has also been incorporated into various wound dressing systems, offering both structural reinforcement and biological benefits. For instance, CNC was added to electrospun gelatin/PCL nanofibers to enhance their mechanical strength and biodegradability [22]. The cell proliferation results indicated that, although control gelatin/PCL fibres initially performed better, the incorporation of CNC led to higher fibroblast proliferation after day 6, suggesting a delayed but beneficial effect on biocompatibility. In wound healing assays using mouse models, the CNC-containing nanofiber sample showed greater structural stability and enhanced hair growth. However, aside from collagen fibre formation and the presence of hair follicles, no significant differences were observed between the gelatin/PCL and gelatin/PCL/CNC groups in terms of overall wound healing. These findings suggest that, although CNC improved the mechanical properties of the nanofibers, its contribution to tissue repair was modest, indicating a primarily structural rather than a bioactive role.
In a further approach combining structural reinforcement and controlled bioactive delivery, CNC was incorporated into chitosan/ulvan hydrogels loaded with epidermal growth factor (EGF) [23]. The incorporation of CNC into chitosan/ulvan hydrogels improved mechanical stability, reduced swelling, and enabled a more controlled release of EGF. In vitro, CNC-enhanced hydrogels promoted L929 fibroblast viability and proliferation, likely due to improved dimensional stability. In vivo, the formulation accelerated wound closure, stimulated fibroblast migration, and increased collagen deposition. These results highlight its potential as an effective controlled-release system for bioactive molecules in skin repair applications.
On the other hand, CNC-based systems have also been explored for the development of magnetically responsive wound dressings. A recent investigation incorporated CNC into alginate/silk fibroin hydrogels, utilising magnetic alignment to enhance mechanical performance and support skin regeneration, as illustrated in Figure 5 [24]. CNC addition contributed to scaffold reinforcement, while the magnetic field induced anisotropic alignment, enhancing matrix–cell interactions. The hydrogel containing CNC showed superior cell viability, migration capacity, and upregulation of genes and proteins associated with tissue repair. These effects were confirmed through quantitative reverse transcription PCR, immunocytochemistry, and antibody array analyses. In vivo, the magnetically treated hydrogel promoted the formation of a structured neoepidermal layer and increased collagen deposition. These findings demonstrate that CNC, when combined with matrix alignment strategies, can actively enhance both the mechanical and biological performance of wound dressings, supporting its application in advanced skin tissue engineering. This implies that the CNC-based alginate/silk fibroin hydrogels aligned by magnetic fields demonstrated not only enhanced mechanical performance but also strong biological activity, including gene and protein expression linked to wound healing.
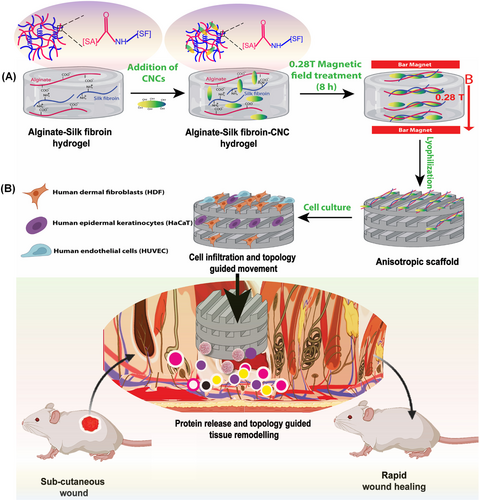
The incorporation of CNF and CNC into hydrogels and nanofibers plays a key role in enhancing the mechanical strength and structural stability of wound dressings, which are essential for supporting tissue regeneration. Beyond their reinforcing capacity, these nanocellulose components also contribute to improving biological performance. Together, these properties position CNC and CNF as multifunctional components in the development of advanced materials for skin regeneration.
7 Lipid and Liposomes Nanoparticles
Among NP-based drug carrier research, lipid-based nanocarriers are preferred for drug administration due to their non-toxic, biodegradable, and biocompatible properties. Furthermore, these nanocarriers exhibit a delayed profile over time, reduce adverse effects, and increase therapeutic adhesion. Therefore, such NPs are classified as vesicular systems and lipid-NPs.
Liposome-based NPs are classified as a vesicular system and were the first extensively studied drug delivery system due to their high biocompatibility and biodegradability. This system comprises phospholipid bilayers in which lipophilic drugs can be incorporated, and solubilised hydrophilic drugs [77, 78]. Meanwhile, the lipid-based NPs are classified into solid lipid-NPs (SLNs), which are an alternative to the traditional lipid-based formation (liposomes). These NPs are formed through distinct types of lipids and surfactants. The presence of surfactants can reduce the interfacial energy between the lipid and the aqueous phase by accumulating at the binding interface, thus favouring the physical stability of the dispersion [78]. The next generation of lipid-NPs is nanostructured lipid carriers (NLCs), which are formed by mixing solid and liquid lipids at ambient temperature; this blend improves drug payload and colloidal stability and offers better control over the release of active ingredients when compared to SLNs [79]. Figure 6, reproduced from Li, J. et al. [80], illustrates a schematic representation of the use of lipid NPs in the skin regeneration process.
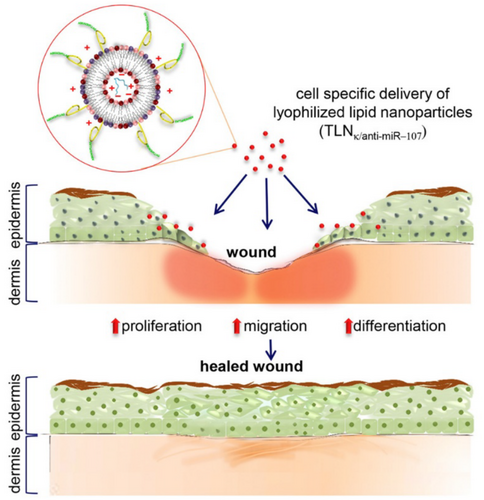
The use of phytotherapeutic agents such as essential oils in lipid-based NPs has gained increasing attention, particularly in the context of chronically infected wounds. For instance, a study focusing on a diabetic wound model infected with Proteus mirabilis (P. mirabilis) and Pseudomonas aeruginosa (P. aeruginosa) evaluated liposome-based NPs encapsulating cinnamon extract compared to chitosan NPs [25]. The comparison of the NPs was evaluated in vivo using albino rats, where diabetes was induced. The results indicated that liposome-based NPs were more effective in treating these wounds compared to the chitosan NPs. According to the authors, cinnamon exhibited the strongest anti-proteolytic activity, while the liposomes, due to their smaller particle size (< 200 nm) and greater stability, achieved higher entrapment efficiency, reaching up to 84%. The findings demonstrated that liposome-encapsulated cinnamon extract holds significant promise for promoting healing in infected diabetic wounds, showing superior results compared to chitosan NPs.
In line with the growing interest in phytotherapeutic compounds delivered via liposomal systems, another study developed a multifunctional wound dressing combining PVA, chitosan, taxifolin, and liposome-based NPs to accelerate healing in diabetic wounds [29]. The inclusion of liposomes significantly improved antioxidant and antimicrobial activities and enabled a more controlled release of taxifolin. In vivo studies confirmed that the liposomal formulation promoted faster healing in diabetic rats compared to non-encapsulated taxifolin. Liposome-encapsulated taxifolin enhanced antioxidant capacity, antimicrobial activity (against E. coli microorganisms), and wound healing; still, comparisons with conventional wound therapies were not included, which limits the translational perspective.
Expanding beyond liposomal systems, sesamol was incorporated into SLNs for diabetic wound healing [26]. This strategy similarly sought to harness the antioxidant and antimicrobial properties of natural compounds while improving bioavailability and targeting wound complications associated with diabetes. Sesamol is a natural phenolic compound known for its potent ROS scavenging abilities, along with anti-inflammatory, antifungal, and antibacterial properties. However, its rapid absorption and short residence time in skin layers limit its effectiveness in wound healing processes. To achieve a controlled release of sesamol, the compound was encapsulated in SLNs. The encapsulation led to a sustained 20-h release profile, enhanced antimicrobial activity against E. coli and P. aeruginosa, and complete wound closure in vivo within 18 days, an outcome not achieved with sesamol-free SLNs. Sesamol-loaded SLNs not only promoted complete wound closure but also demonstrated modulation of oxidative stress and inflammatory markers, providing a robust demonstration of therapeutic potential in diabetic wound healing.
Building on the potential of SLNs for delivering phytotherapeutic agents, another system was developed to incorporate curcumin-loaded SLNs into hydrogel formulations for wound healing applications [27]. This formulation accelerated wound closure to 11 days in a diabetic model, downregulated inflammatory markers, and reduced oxidative stress, highlighting the potential of SLNs to improve curcumin's stability and bioavailability in chronic wounds. Moreover, the results showed that the curcumin-loaded SLNs presented a significant antimicrobial effect against S. aureus. The findings showed that curcumin-loaded SLNs hydrogel demonstrated strong wound healing effects by modulating oxidative stress, inflammation, and angiogenesis, supported by both molecular and histological analyses, highlighting its therapeutic potential for chronic wounds.
In addition to SLNs, NLCs have also been investigated as advanced lipid-based systems to improve the transdermal delivery and stability of antioxidant compounds. One such formulation co-loaded α-tocopherol, quercetin, and tea tree oil, combining multiple bioactives with complementary therapeutic effects [81]. The results showed that tea tree essential oil increased the irritation score, unlike when it was encapsulated in the NLCs. Furthermore, NLCs were shown to preserve the essential oil and reduce its loss with increasing temperature, which could help maintain antimicrobial effects. This study used Franz diffusion cells and porcine ear skin as a model tissue to analyse the impact on antioxidant penetration into the skin. The results showed that the bio-adhesive polymer did not influence penetration into either superficial or deeper layers of the skin. Moreover, the authors concluded that independent of the type of polysaccharide employed, the NLC promoted cutaneous localization of antioxidants in damaged skin with ~74 to 180-fold higher delivery into the skin, compared to percutaneous delivery. The antioxidant-loaded NLCs demonstrated improved stability and skin penetration and also promoted cell migration ex vivo; however, in vivo wound healing assays were not conducted to confirm regenerative efficacy.
Lipid-based nanocarriers represent effective delivery platforms for wound healing due to their ability to penetrate the skin through intracellular and transfollicular pathways. These systems combine high biocompatibility, excellent physicochemical stability, and sustained drug release, allowing for greater drug retention at the wound site, reduced dosing frequency, and accelerated tissue repair. Their capacity to encapsulate and protect a wide range of bioactive compounds makes them especially promising for treating chronic or infected wounds.
8 Nanoparticle-Based Systems Activated by External Stimuli
Recent advances in nanotechnology have enabled the development of wound dressings that respond to external stimuli such as light and electrical signals, enhancing therapeutic precision and control [19, 82]. NPs integrated into these systems can be activated in situ, offering additional functionalities beyond passive protection or drug release [14, 83]. These stimuli-responsive approaches are particularly beneficial for chronic or infected wounds, where traditional dressings are often ineffective [84].
Photothermal therapy (PTT) and photodynamic therapy (PDT) are strategies explored in skin regeneration [12, 14, 85]. PTT acts through localised photothermal effects that induce cellular damage at the target site [86]; however, its broader clinical use remains limited due to the risk of unintended heat injury to surrounding tissues [85]. On the other hand, PDT relies on light-activated photosensitizers that generate ROS, effectively eradicating bacteria. For instance, carbon dots derived from mulberry leaves were used as biocompatible photosensitizers in a PDT system for MRSA-infected wounds, showing strong antibacterial activity and improved healing outcomes [14]. The use of naturally derived carbon dots for PDT demonstrated strong antibacterial activity and effective tissue regeneration, supported by in vivo healing and histological analysis, reinforcing its potential as a light-responsive therapeutic platform. As illustrated in Figure 7, the multifunctional design of this material enabled precise ROS generation, biofilm disruption, and targeted antibacterial action through dynamic borate ester interactions.
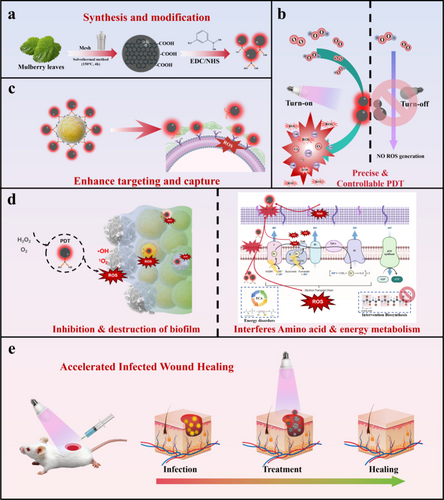
Similarly, a hydrogel embedded with photothermal melanin NPs and healing peptides demonstrated synergistic effects in infected wounds, combining bacterial clearance with enhanced re-epithelialisation [19]. The combination of photothermal melanin NPs and healing peptides showed synergistic effects in infected wounds, with in vivo studies confirming accelerated healing and bacterial clearance, highlighting the therapeutic integration potential of this approach.
Altogether, NP-based systems activated by light represent a promising frontier in wound care. By coupling responsiveness to external stimuli with antimicrobial, antioxidant, and pro-healing properties, these advanced dressings offer multifunctional solutions tailored to the complex demands of skin regeneration. Future studies exploring the integration of these technologies into clinically applicable formats may pave the way for a new generation of highly responsive wound healing materials.
9 Conclusions
This review highlights the growing potential of carbon-based and organic NPs in the development of advanced wound dressings, supported by evidence of their ability to enhance healing through antimicrobial, antioxidant, anti-inflammatory, and mechanical effects. These multifunctional systems also enable controlled and sustained drug delivery, promoting more effective and targeted tissue repair. Such attributes are particularly relevant for treating chronic or infected wounds, where conventional therapies are often limited.
Given their versatility, nanoparticle-based dressings emerge as promising tools not only for scientific research but also for industrial innovation in the biomedical field. Their scalable production and compatibility with bioactive compounds position them as strong candidates for future clinical translation. However, despite promising outcomes, challenges related to long-term safety, regulatory approval, and standardisation must be addressed. Continued research using validated in vitro and in vivo models is essential to support the safe integration of these technologies into new products for clinical wound care.
Acknowledgements
The authors would like to thank Aeronautics Institute of Technology, Friedrich-Alexander-Universität Erlangen-Nürnberg, University of Campinas, and São Paulo Research Foundation (FAPESP), grant number 2019/05856-7; 2021/09335-1; 2023-11117-8. Open Access funding enabled and organized by Projekt DEAL.
Conflicts of Interest
The authors declare no conflicts of interest.




