Hyperbaric oxygen therapy promotes the browning of white fat and contributes to the healing of diabetic wounds
Yue Yin and Shang-Yuan Wang contributed equally to this study and shared the first authorship.
Abstract
Non-healing wounds are one of the chronic complications of diabetes and have remained a worldwide challenge as one of the major health problems. Hyperbaric oxygen (HBO) therapy is proven to be very successful for diabetic wound treatment, for which the molecular basis is not understood. Adipocytes regulate multiple aspects of repair and may be therapeutic for inflammatory diseases and defective wound healing associated with aging and diabetes. Endothelial cell-derived extracellular vesicles could promote wound healing in diabetes. To study the mechanism by which HBO promotes wound healing in diabetes, we investigated the effect of HBO on fat cells in diabetic mice. A diabetic wound mouse model was established and treated with HBO. Haematoxylin and eosin (H&E) staining and immunofluorescence were used for the analysis of wound healing. To further explore the mechanism, we performed whole-genome sequencing on extracellular vesicles (EVs). Furthermore, we conducted in vitro experiments. Specifically, exosomes were collected from human umbilical vein endothelial cell (HUVEC) cells after HBO treatment, and then these exosomes were co-incubated with adipose tissue. The wound healing rate in diabetic mice treated with HBO was significantly higher. HBO therapy promotes the proliferation of adipose precursor cells. HUVEC-derived exosomes treated with HBO significantly promoted fat cell browning. These data clarify that HBO therapy may promote vascular endothelial cell proliferation and migration, and promote browning of fat cells through vascular endothelial cells derived exosomes, thereby promoting diabetic wound healing. This provides new ideas for the application of HBO therapy in the treatment of diabetic trauma.
1 INTRODUCTION
Wound management in diabetic patients is of an extreme clinical and social concern. The delayed and impaired healing renders research on it increasingly crucial.1 Approximately 50%–70% of all the limb amputations are due to diabetic wounds.2-5 Lack of oxygen, reduced vascularization, elevated oxidative stress and infection are critical clinical hallmarks of non-healing chronic diabetic wounds.6
Adipocytes are emerging as critical niche cells within multiple tissues including the skin.7 Adipocytes secrete cytokines to regulate regeneration of haematopoietic stem cells after injury and the antimicrobial peptide cathelicidin to combat bacterial infections. Zhou et al.8 reveal that dermal adipocytes regulate skin wound repair via release of fatty acids that promote macrophage recruitment and accelerated revascularization. Peri-vascular adipose tissue (PVAT) has been reported to undergo inflammatory changes in response to vascular injury.9 Vascular injury induces the beiging (brown adipose tissue-like phenotype change) of PVAT, which fine-tunes inflammatory response and thus vascular remodelling as a protective mechanism.10 Endothelial cells may be involved in the process of adipose browning, but the specific mechanism is unclear.
Extracellular vesicles (EVs) are small vesicles secreted by various types of cells and serve as tools for intercellular communication.11 During the wound healing process, EVs may affect and regulate different stages of wound healing by carrying and transmitting various bioactive molecules, such as proteins and RNA.12 For instance, EVs secreted by endothelial cells may carry and transmit bioactive molecules related to blood vessel formation and repair, thereby promoting wound healing.13 Additionally, EVs may also participate in wound healing by influencing processes such as inflammation, cell proliferation and migration.13
Hyperbaric oxygen (HBO) therapy is a physical procedure involving the topical application of 100% oxygen or a mixture of high-oxygen gases at elevated pressure, and it is often used to treat poorly healing foot ulcers in patients with diabetes.14 The currently available scientific evidence shows that there are statistically significant differences in favour of the use of HBO in the treatment of diabetic foot syndrome, compared with standard therapeutic management.15
Here we investigated whether browning of fat cells and vascular endothelial cell-derived exosomes are critical modifiers in the pathogenesis of diabetic wounds, which is closely linked to the mechanisms of HBO promoting diabetic wound healing.
2 MATERIALS AND METHODS
2.1 Ethics statement
All experiments referring to the use of animals in this study were approved by the Institutional Animal Care and Use Committee of Shanghai Xinhua Hospital affiliated to Shanghai Jiao Tong University School of Medicine (XHEC-F-2021-020). All animal protocols were conducted in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals.
2.2 Diabetes Induction with STZ
Male 8- to 10-week-old C57BL/6 mice were purchased from Shanghai Jihui Laboratory Animal Care Co., Ltd. All mice were housed at the Model Animal Research Center of Xinhua Hospital in standard conditions (12 h/12 h light/dark cycle, 68–72°F and a humidity of 30%–70%) in ventilated racks fitted with automatic watering systems and fed with standard chow ad libitum. We built the diabetic model by intraperitoneal (IP) injecting streptozotocin (STZ) (Sigma, St. Louis, MO, USA) with 50 mg/kg.16 Control mice received the solvent injected by IP. After finishing the injection, we tested the blood glucose level to confirm if the diabetic model was successful.
2.3 HBO therapy
We use a HBO chamber specifically designed for small animals and cells in our research. We used the gas with 100% oxygen to flush the chambers for 5 min to achieve the HBO condition. The group of control+ HBO and the group of STZ+ HBO mice achieved the HBO treatment at 2.4 atmosphere absolute (ATA) for 1 h. The chamber was compressed at a consistent compression rate of 0.5 ATA/min to a target pressure of 2.4 ATA, and this target pressure was maintained for 60 min. The chamber was then decompressed at a consistent decompression rate of 0.5 ATA/min to normal atmospheric pressure.17 This treatment is administered once a day, five times a week, for a total duration of 2 weeks. The group of control+ HBO and the group of HFD+ HBO mice were put into the chambers before the flushing stage. After finishing flushing the chambers, the HBO therapy proceeded as described earlier. The process of the HBO treatment lasts for continuously for 28 days.
2.4 Haematoxylin and eosin staining
The tissue was fixed in the 4% paraformaldehyde (PFA) for 24 h.18 The tissue was washed by PBS and then was soaked in either 10% sucrose solution, 20% sucrose solution, 30% sucrose solution or 30% ethanol, 50% ethanol, 75% ethanol for dehydration. Next, the tissue was embedded in optimal cutting temperature compound (OCT) or paraffin. The paraffin blocks were sectioned on the microtome at five-micron thickness. The tissue pieces can be stained with haematoxylin–eosin staining. And the OCT containing the tissue was frozen in the −80°C refrigerator and then cut on the freezing microtome at 10-micron thickness. And the pieces were stained with fluorescent dye.
2.5 Immunofluorescence
Chondrocytes were fixed with 4% PFA in PBS for 10 min at room temperature and then permeabilized and blocked in PBS containing 0.1% Triton X-100 and 5% FBS for 30 min.19 The fixed cells were washed with PBS and incubated overnight at 4°C with anti-collagen II antibody (1:200; lot no. ab34712; Abcam).
The cells were washed and incubated with rhodamine- or fluorescein-conjugated secondary antibodies, washed again and observed under a standard fluorescence microscope. Nuclei were identified with 4,6-diamidino-2-phenylindole staining.
2.6 HUVEC cell culture
Human umbilical vein endothelial cell (HUVEC) was cultured in the endothelial cell medium (ECM). Then the HUVEC was exposed to HBO.20 Stromal vascular fraction (SVF) cells were dissociated from the subcutaneous white adipose tissue (SWAT). And the SVF was formed in the DMEM/F12 medium.21 After 24 h, the SVF was washed by the medium. And when the SVF had grown to over 80% of the area of the petri dish, we started to differentiate the SVF into white adipocytes.
2.7 Co-culture of HUVEC and white adipocyte cells
The medium that was used to form the HUVEC for 48 h was collected, and the medium was used to co-culture with the white adipocyte for 48 h.
2.8 Cell HBO treatment
In order to perform the HBO treatment for HUVEC, we used the gas with 97.9% O2 and 2.1% CO2 to flush the chamber for 5 min at 3 L/min to achieve the HBO condition.20 Then, the atmosphere was pressurised to 2.4 ATA in 2 min and maintained the pressure at 2.4 ATA for 60 min. Finally, we reduced the pressure to the atmospheric pressure in 2 min.
2.9 Real-time PCR
We used the kit (EZB) to extract the RNA and used the reverse transcription kit (EZB) to reverse transcript the RNA to cDNA. Finally, we performed the real-time PCR by using the kit from EZB.
2.10 RNA sequencing
Total RNA was isolated from each sample using the RNA mini kit (Qiagen, Germany).22 RNA quality was examined by gel electrophoresis and with Qubit (Thermo, Waltham, MA, USA). For RNA sequencing, 1 μg of total RNA was used for library construction. Sequencing libraries were generated using VAHTS™ Stranded mRNA-seq Library Prep Kit for Illumina®. The libraries were sequenced as 150 bp paired-end reads using Illumina Novaseq6000 according to the manufacturer's instructions by the commercial service of Genergy Biotechnology Co. Ltd. (Shanghai, China). The raw data of all samples were submitted to the Sequence Read Archive at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/sra).
2.11 Data analysis
The raw data were handled by Skewer and data quality were checked by FastQC v0.11.2 (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). The read length was 2 × 150 bp. Clean reads were aligned to the Human genome hg38 using STAR. StringTie.23 The expression of the transcript was calculated by FPKM (Fragments Per Kilobase of exon model per Million mapped reads) using Perl. Differentially expression analysis between matched treatment and normal samples was performed by the R package DESeq2 with the likelihood ratio test option.24 Differentially expressed genes exhibiting two-fold changes and Benjamini and Hochberg-adjusted p-values ≤0.05 were selected.25 If the gene DESeq2 normalised read count value was close to 0, log2 transformation was performed after adding 1. The expression values were visualised by the R package pheatmap.26 Then DEGs were chosen for function and signalling pathway enrichment analysis using GO and KEGG database. DAVID was performed to do the GO enrichment.27 The significantly enriched pathways were determined when p < 0.05 and at least two affiliated genes were included. PPI analysis of differentially expressed genes was based on the STRING database,28 which includes known and predicted protein–protein interactions. Moreover, the network was established according to the known interaction of the selected reference species. Gene Set Enrichment Analysis (GSEA)29 is a computational approach to determine if a pre-defined Gene Set can show a significant consistent difference between two biological states. The genes were ranked according to the degree of differential expression in the two samples, and then the predefined Gene Set was tested to see if they were enriched at the top or bottom of the list. Gene sets enrichment analysis can include subtle expression changes. We use the local version of the GSEA analysis tool, Hallmark and KEGG dataset were used for GSEA independently.
2.12 Statistics
The photos were analysed by ImageJ 2.1.0, and the size of the wound was calculated by ImageJ 2.0.1. Statistical analysis was performed with GraphPad Prism Version 8.0.1. The data were analysed with one-way analysis of variance or two-way ANOVA. Results were considered statistically significant when p < 0.05.
3 RESULTS
3.1 HBO significantly improves diabetic wound repair
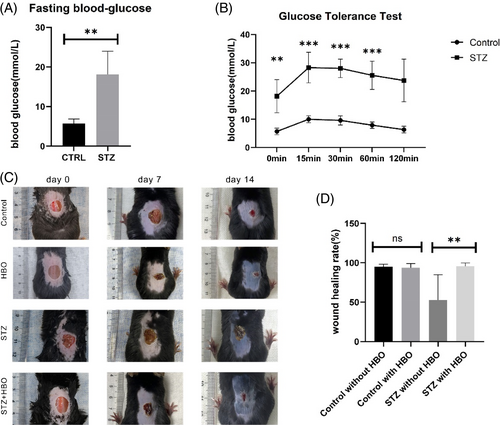
After 5 days of injection, both STZ and STZ + HBO groups showed significantly higher fast blood glucose levels compared to those in the control and HBO groups. The photographs showed that at day 28 post-wounding, the wound treated with HBO was clearly smaller in size compared with the wound treated without HBO (Figure 1A,B). We found that the wound healing rate was significantly higher in the diabetic mice treated with HBO, however, the healing rate showed no significant difference in both normal blood glucose groups (Figure 1C,D).
3.2 HBO promotes fat cell and adipose precursor cells proliferation in wounds of mice with diabetes
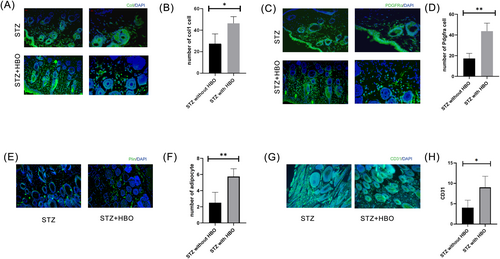
After being treated with HBO, we observed an increase in adipocytes labelled with Col1 in the group of diabetic mice (Figure 2A,B). Additionally, the fluorescent staining results demonstrated a significant increase in PDGFRα cells (Figure 2C,D). Endothelial cells marked by CD31 significantly increased in the group of STZ mice being treated with HBO than the group of STZ mice not being treated with HBO. The same results were observed for cells labelled with PLIN1 (Figure 2E–H).
3.3 Differential gene expression between the HBO treated group and the untreated group
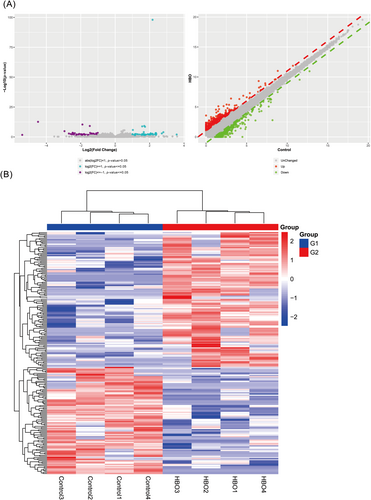
GEO analysis of the data reveals differential expression of various inflammation-related genes. This is demonstrated through the use of heatmaps and principal component analysis (PCA) dimension reduction plots. The research results indicate that compared to the control group, the expression of inflammation-related factors such as TLR5 is elevated in the wound healing in db/db female mice group (Figure 3A–C).
3.4 Functional and pathway enrichment of cellular components before and after HBO treatment
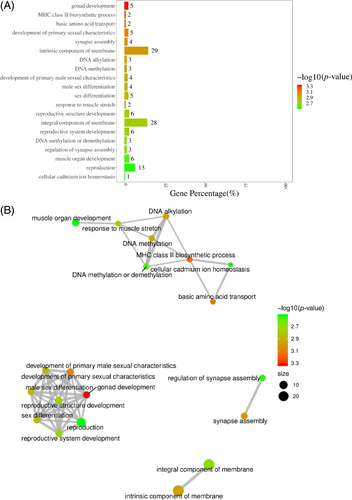
After being processed by HBO, the pathways of agonad development, major histocompatibility complex (MHC) class II biosynthetic process, basic amino acid transport, development of primary sexual characteristics, synapse assembly, intrinsic component of membrane, DNA alkylation, DNA methylation, development of primary male sexual characteristics and reproductive system development are enriched, with reproductive system development exhibiting the highest enrichment level (Figure 4A,B).
3.5 Functional and pathway enrichment of biological process before and after HBO treatment
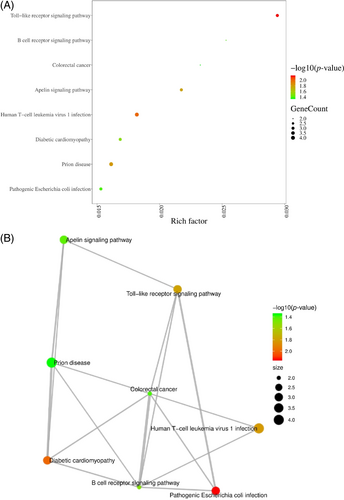
After HBO treatment, the pathways of toll-like receptor signalling pathway, B cell receptor signalling pathway, colorectal cancer, apelin signalling pathway, human T-cell leukaemia virus 1 infection and diabetic cardiomyopathy are enriched in endothelial cells (Figure 5A,B).
3.6 Functional and pathway enrichment of molecular function before and after HBO treatment
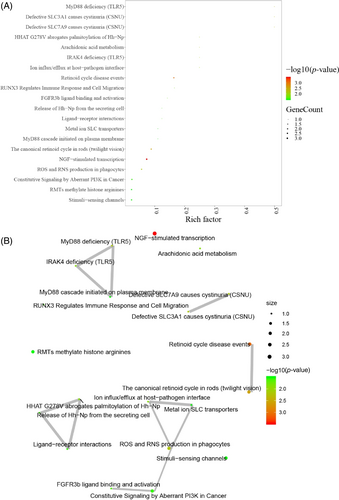
After HBO treatment, the pathways of MyD88 deficiency (TLR5) in endothelial cells, defective SLC3Al causing cystinuria (CSNU), defective SLC7A9 causing cystinuria (CSNU), HHAT G278V abrogating palmitoylation of Hh-Npl, arachidonic acid metabolism and IRAK4 deficiency (TLR5) were found to be enriched (Figure 6A,B).
3.7 GSEA enrichment analyses and PPI before and after HBO treatment
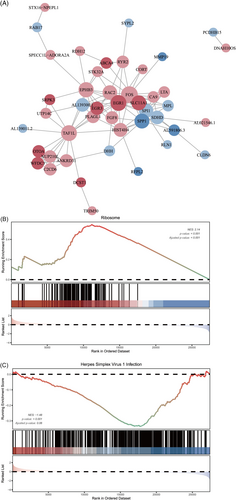
We performed functional and pathway enrichment analyses using DAVID to gain a comprehensive understanding of the roles played by the 14 differentially regulated pathways identified in the EVs dataset through GSEA. Additionally, these pathway such as EGFR1 pathway and cellular and inflammatory cytokine response pathway show significant enrichment in the network maps of intestinal immunity (Figure 7A–C).
3.8 The impact of HBO on the gene expression of endothelial cell-derived EVs, including TLR5
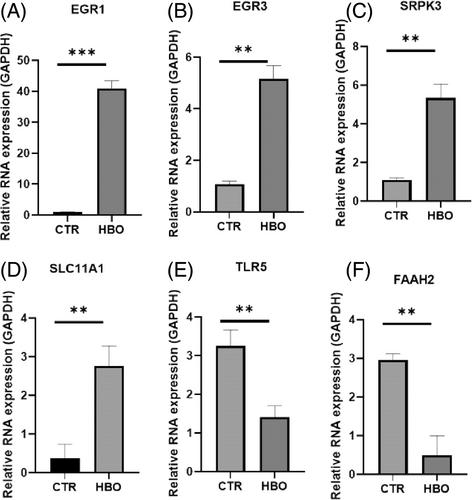
Figure 8A–D show that compared to the control group, the expression of EGR1, EGR3, SRPK3, and SLC11A1 decreased after HBO treatment, and the expression of TLR5 and FAAH2 also decreased compared to the control group.
3.9 The impact of EVs on fibroblast cells after processing by HBO
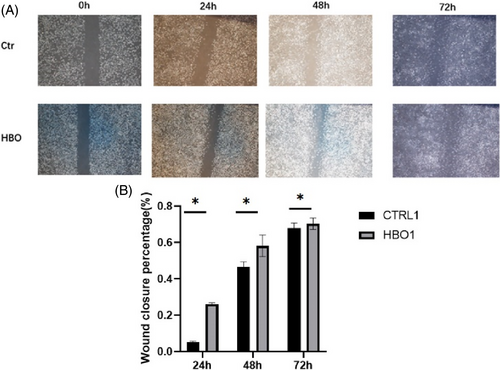
Figure 9A shows that compared to the control group, endothelial cell EVs pre-treated with HBO promoted fibroblast proliferation. The observation time points were 24, 48, and 72 h. Figure 9B presents a bar graph, indicating a higher wound closure percentage at 24, 48, and 72 h after HBO treatment. Compared to the non-HBO intervention group, the pore size of STZ mice decreased significantly after HBO intervention, with statistical significance (Figure S1A,B). The morphology and identification of EVs are shown in the Figure S2A,B.
4 DISCUSSION
Diabetes is an increasingly common chronic metabolic disease characterised by sustained high blood sugar and long-term health consequences.30 It is estimated that approximately 25% of diabetic patients experience impaired wound healing, often leading to lower limb amputation.31 This condition is accompanied by high economic and psychosocial costs. The high blood sugar environment promotes the formation of biofilms,32 making diabetic wounds difficult to treat. HBO therapy has been shown to improve diabetic wound healing. However, the specific mechanism has not yet been fully elucidated. Although there is a lack of large-scale high-quality trials on the use of HBOT, recent research emphasises the effectiveness of HBO therapy in treating patients with Wagner grade 3 and 4 ulcers, which has been shown to improve HbA1c, white blood cell levels and serum creatinine.15 While a recent study on rabbits receiving HBOT did not find significant changes in gene expression related to wound healing.33 A study using a diabetic mouse model found that HBO treatment can accelerate wound healing and significantly reduce MMP-9 levels.34 In our research, the therapeutic effects of HBO are particularly under-studied in diabetic animals. The present data suggest that HBO not only effectively promotes the proliferation of fat cells and adipose precursor cells but also enhances the phagocytosis of endotheliocyte-derived exosomes by fat cells and the browning of white adipocytes.
There is a growing realisation that adipocytes, once believed to act merely as local reservoirs of energy and to provide mechanical and thermal insulation, also have numerous other roles in in tissue repair.35 Adipocyte precursor cells are known to differentiate into mature adipocytes, and these appear to contribute to repair, as blocking their differentiation leads to defects in fibroblast migration and matrix deposition.36 We found that after HBO therapy, the rate of wound healing in diabetic mice was significantly increased, at the same time, there were more fat cells and adipose precursor cells at the wound site. It suggests that HBO therapy may improve wound healing in diabetic mice by promoting fat cell proliferation.
Recent studies have shown that EVs are one of the key secretory products mediating cell-to-cell communication to enhance wound healing.37 Endothelial cells secrete EVs that target adipocytes.38 Similar to previous results, we observed in vitro experiments that fat cells engulf endothelial cell-derived exosomes. Moreover, we found that endothelial cell-derived exosomes, after HBO treatment promote browning of adipose tissue. Browning of white fat is involved in the therapeutic effects mediated by HBO in diabetic wound healing.
In addition, we performed whole-genome sequencing on EVs treated with and without HBO. We found that MHC class II biosynthetic process, basic amino acid transport, development of primary sexual characteristics, synapse assembly, intrinsic component of membrane, DNA alkylation, DNA methylation, development of primary male sexual characteristics and reproductive system development pathways were enriched in the treated EVs. Furthermore, using RT-PCR, we observed a decrease in the expression of EGR1, EGR3, SRPK3, and SLC11A1 after HBO treatment compared to the control group. Additionally, the expression of TLR5 and FAAH2 was also downregulated in the HBO-treated group.
Our research has found that exosomes, derived from endothelial cells under HBO intervention, may improve the healing of diabetic wounds by regulating adipose tissue, thus providing a theoretical basis for the clinical use of HBO therapy in treating diabetic wounds. Furthermore, these exosomes, following HBO intervention, may facilitate clinical translation, serving as a new direction for the treatment of diabetic wounds.
5 CONCLUSION
HBO therapy is beneficial, promoting browning of white fat and assisting in the healing of diabetic wounds. As an effective treatment option, it holds potential in both fat browning and wound healing. However, further research is needed to explore underlying mechanisms of exosomes derived from endothelial cells following HBO intervention in diabetic wound healing. This will facilitate the discovery of new targets for clinical treatment of diabetic wounds.
ACKNOWLEDGEMENTS
We would like to convey our appreciation to the researchers who contributed to the creation of the DESeq2 package.
FUNDING INFORMATION
National Natural Science Foundation of China, No. 81971861, 82072207; Program of Shanghai Academic Research Leader 21XD1402200.
CONFLICT OF INTEREST STATEMENT
The authors have declared that no competing interest exists.
ETHICS STATEMENT
All animal experiments were performed in accordance with the protocols approved by the institutional Animal Care and Use Committees of the XinHua Hospital.
CONSENT TO PUBLISH
All authors agreed to publish.
Open Research
DATA AVAILABILITY STATEMENT
Data are available onrequest to the authors.




