RETRACTED: Effect of bevacizumab in combination with chemotherapy for ovarian cancer on wound healing in patients: A meta-analysis
Abstract
In this meta-analysis, we reviewed the findings and definitive findings of a new study that assessed the impact of bevacizumab on wound healing following combined chemotherapy for ovarian cancer (OC). The results of a controlled study that assessed the efficacy of bevacizumab in the treatment of ovarian cancer were retrieved from 4 databases, such as the Web of Science and EMBASE. The results of the adverse event associated with wound healing were determined by comparison of the controlled studies of bevacizumab plus chemotherapy in the treatment of ovarian cancer. A meta-analysis was conducted with either a randomized or a fixed-effect model in order to establish an odds ratio for time to event variables and for a binary outcome. In the research literature, 830 trials have been identified and seven have been chosen to be included in a definitive analysis of the trial. Among the 4134 cases who received chemotherapy after operation, 2098 received standard chemotherapy and 2036 received the addition of bevacizumab. A total of 7 trials have shown that the use of bevacizumab in the treatment of ovarian cancer patients has reduced wound healing (OR, 0.55; 95% CI: 0.37, 0.80, p = 0.002). Four trials demonstrated that there was no change in the incidence of haemorrhage in patients with ovarian cancer when administered with or without bevacizumab (OR, 0.48; 95% CI, 0.10, 2.34, p = 0.37). The combined use of bevacizumab and chemotherapy may have a negative effect on the healing of wound.
1 INTRODUCTION
Ovarian cancer continues to be the largest cause of female cancer mortality globally, with a significant number of people dying every year.1 Although the prognosis has been somewhat better, it is often late in life and has been linked to high rates of death and morbidity. The standard first-line therapy is primary surgery, followed by 6 courses of carboplatin and paclitaxel in the adjuvant setting.2, 3 Although initially responding well to surgical treatment and first-line chemotherapy, about 50% of the patients ultimately relapse because of drug resistance, resulting in poor long-term overall survival. In the last 30 years, there has been virtually no change in the survival rate of those who have been found to have had a poor 5 years of life in those who have been diagnosed with terminal ovarian carcinoma.4, 5 Adding a cytotoxic drug did not prove to be more effective but resulted in a worse prognosis.6 Thus, it is imperative to develop medicines to suppress angiogenesis, which is a critical route for cancer growth and progression.
Today, angiogenesis is critical for the development, invasion and metastasis of tumours.7 VEGF is one of the best known angiogenesis agents, which are thought to play an important role in angiogenesis.8 VEGF is related to the development of cancer in the late stage of the ovary.9, 10 Pre-clinical research on an ovarian carcinoma model has shown that VEGF can be inhibited only by inhibiting tumour growth and metastasis.11-14 Anti-VEGF agents can also improve the effectiveness of chemotherapy by normalizing tumour vessels, resulting in temporary decreases in interstitial pressure and improving the delivery of cytotoxic drugs.15 The effects of anti-VEGF medicines are unknown.
Nevertheless, there is no report on the impact of bevacizumab on wound healing following the use of bevacizumab in late stage ovarian carcinoma. Thus, the aim of this meta-analysis is to examine whether it is possible that the use of bevacizumab in the treatment of ovarian carcinoma may reduce the rate of wound healing.
2 METHODS
2.1 Search strategy
There are no linguistic limitations for testing in 4 databases, such as the Web of Science and EMBASE. The search strategy was based on the words “ovarian cancer” and “bevacizumab” in all areas, Table 1. Additionally, all related research papers, references and summaries are retrieved by Sep. 2023. The authors and researchers of the study were consulted if the information was not clear.
| No. | Query |
|---|---|
| #1 | Ovarian neoplasms[Title/Abstract] OR Ovarian cancer[Title/Abstract] OR Ovarian tumor[Title/Abstract] OR Ovarian tumour[Title/Abstract] OR Ovarian carcinoma[Title/Abstract] |
| #2 | Bevacizumab[Title/Abstract] OR Avastin[Title/Abstract] |
| #3 | Chemotherapy[Title/Abstract] OR Radiotherapy[Title/Abstract] OR Radiation[Title/Abstract] OR Irradiation[Title/Abstract] |
| #4 | Pain*[All Fields] OR Incision*[All Fields] OR Infection[All Fields] OR Dehiscence[All Fields] OR Haemorrhage[All Fields] OR Bleed*[All Fields] OR Haematoma[All Fields] OR Wound[All Fields] OR Complication*[All Fields] |
| #5 | #1 AND #2 AND #3 AND #4 |
2.2 Trial selection criteria
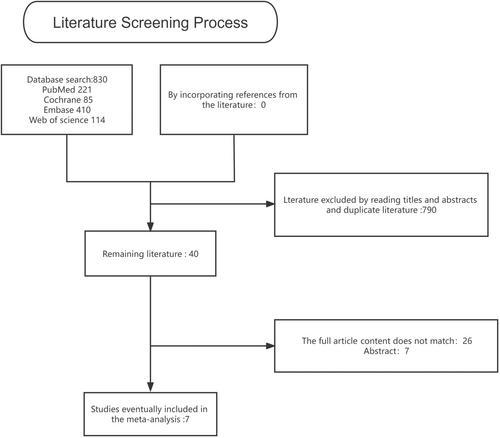
In all of the studies that have been published and not published, the results of which are comparable to those of bevacizumab combined with chemotherapy only in the treatment of advanced ovarian cancer are eligible. Trials that include other targeted drugs or immunotherapy have been ruled out. In case of duplication, only the latest or full study report has been included and analysed with updated data. Two evaluators reviewed the reference list and selected the studies separately. Figure 1.
2.3 Methodological quality assessment
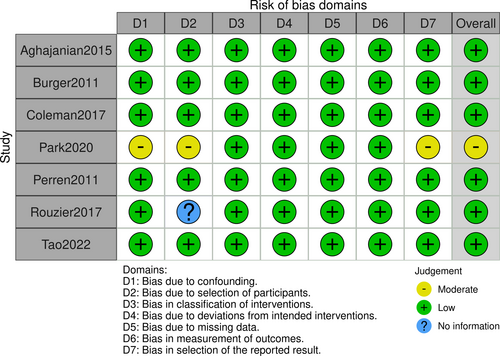
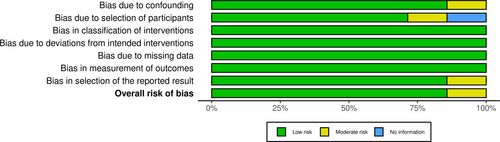
In order to evaluate the quality of the trial and to establish whether there is any potential for bias in the selection of studies, the selection of publishing practices was evaluated by two researchers. Independent evaluation of publications was carried out with ROBINS-I. Figures 2 and 3.
2.4 Data extraction
Two reviewers independently extracted data from the publication. The compliance of all the data with test protocols, statistics and publications was verified. The authors have been approached in order to find out if there was any lack of information. Any disputes are settled by negotiation and agreement. Disagreements, if they exist. A detailed record was made of the year, the number of patients, the therapy and the results.
2.5 Statistical analyses
A meta-analysis of nonhomogeneous studies was carried out with RevMan 5.3. The fixed-effect model was applied to estimate the odds ratio (OR) of binary variables with a two-sided 95% CI and p value. Forest plots were drawn for each adverse result. The diamonds in the lower part of the diagram summarize the best estimation of the total effective result. In both trials, efficacy was measured by the proportion of patients receiving bevacizumab versus those receiving chemotherapy alone. The statistical significance of p < 0.05 was regarded as significant. The chi-square method was used to assess the statistical heterogeneity, and it was represented as I index. I2 Values higher than 50% suggested greater nonhomogeneity. A random-effect model was applied if heterogeneous was found.
3 RESULTS
3.1 Study characteristics
The study found 830 research articles, and eventually, seven of them were chosen to be included in the final analysis. A qualitative evaluation of the seven articles is presented in Figures 2, 3. Among the 4134 cases who received chemotherapy after operation, 2098 received standard chemotherapy and 2036 received the addition of bevacizumab. Table 2 summarizes the features of patients with ovarian cancer.
3.2 Wound healing problems
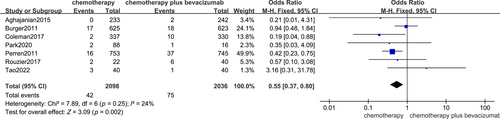
The impact of bevacizumab treatment on wound healing was investigated in 7 trials, and the outcome was analysed through a fixed-effect model, which showed that the administration of bevacizumab to patients with ovarian cancer decreased wound healing (OR, 0.55; 95% CI, 0.37, 0.80 p = 0.002), Figure 4.
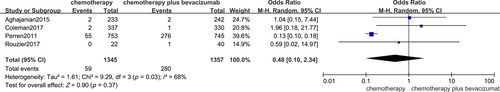
3.3 Bleeding
Four trials showed the impact of bevacizumab in the treatment of ovarian carcinoma patients on the incidence of haemorrhage, and a randomized effect model showed no impact on the rate of haemorrhage in patients with ovarian cancer (OR, 0.48; 95% CI, 0.10, 2.34 p = 0.37), Figure 5.
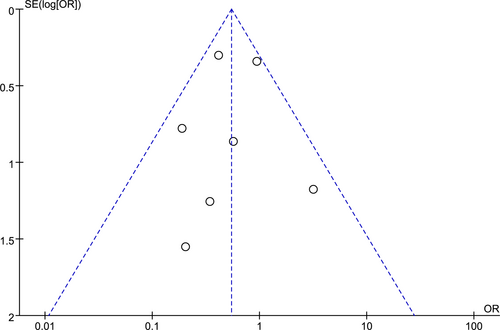
3.4 Publication bias
The publication bias was analysed on wound healing issues that occurred with bevacizumab during chemotherapy for ovarian cancer patients, Figure 6.
4 DISCUSSION
In this combined analysis, adding bevacizumab to pre-chemotherapy resulted in a statistically insignificant benefit for both wound healing. The study found 830 articles, and 7 of them were chosen to be included in the final analysis of the study. Among the 4134 cases who received chemotherapy after operation, 2098 received standard chemotherapy and 2036 received the addition of bevacizumab. Seven studies showed the effect of bevacizumab on wound healing in patients with chemotherapy for ovarian cancer, and the outcome was analysed using a fixed-effect model that showed that it reduced the rate of wound healing in patients with ovarian carcinoma receiving chemotherapy. Four trials demonstrated that randomisation models did not indicate any influence of bevacizumab or bevacizumab on the haemorrhage of patients with ovarian carcinoma.
In this paper, we analysed and confirmed the negative impact of the addition of bevacizumab in the treatment of ovarian cancer. There are no trials to evaluate the effect of bevacizumab plus chemotherapy alone in ovarian cancer patients wound healing. This is the reason for our initial study of the effect of bevacizumab on wound healing in ovarian cancer. Because bevacizumab is a growth factor-blocker, it interferes with the development of the tumour by interfering with angiogenesis in the tissues. In this regard, the creation of new vascularisation is necessary for the recovery of a patient's injury, and the administration of bevacizumab inhibits the development of new vascularisation in the patient, which in turn inhibits the speed at which the patient is healing. From this point of view, the explanation of the outcome is far more likely.
Our research has a few advantages. This review covers all of the research that is well-designed and of good quality.
This research, however, also has its own limits. First, it was established that only seven studies met the eligibility criteria. Studies with unpublished negative results may have been performed, even though all the relevant literature has been extensively consulted. Furthermore, the meta-analyses combined the data instead of the assessment of the individual studies.
5 CONCLUSION
In our meta-analyses, the combined use of bevacizumab and chemotherapy resulted in a significant increase in the risk of nonhealing of the wound. Despite the fact that adding bevacizumab to chemotherapy may provide a new choice for patients with terminal ovarian cancer. Possible adverse reactions must be taken into account in the decision to treat. The next step is to determine the best course of care, which will assist in choosing those who are most likely to benefit from the combination.
ACKNOWLEDGEMENTS
We thank Prof. Fang Zhao for his review of this study and suggestions for revisions.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request from the authors.




