Sub epidermal moisture measurement and targeted SSKIN bundle interventions, a winning combination for the treatment of early pressure ulcer development
The School of Nursing & Midwifery has a research collaboration with Bruin Biometrics. The SEM Scanner was donated by Bruin Biometrics for the duration of the study.
Abstract
This study aimed to investigate the impact of sub epidermal moisture (SEM) measurement and targeted pressure ulcer (PU) prevention, versus visual skin assessment and usual care, on mean SEM delta scores and early pressure ulcer development in acute hospital patients. A quantitative quasi-experimental observational approach was used. A total of 149 at risk acute hospital patients took part, 78 treatment, and 71 control. SEM deltas were recorded daily for a maximum of 5 days using the SEM Scanner (Bruin Biometrics LLC, Los Angeles, California), on three sites: the sacrum, the right heel, and the left heel, with enhanced and targeted PU prevention interventions occurring in those with an elevated SEM delta scores in the treatment group. Intention to treat analysis was used to guide the final composition of results. SEM PU represents PU development as identified by 2 days of sustained abnormal SEM delta scores, ≥0.5, after day one. The mean number of days completed by participants was just under 4 days, participants had many different comorbidities, with the most common being: hypertension, cancer, and chronic obstructive pulmonary disease. Results showed that following the introduction of SEM guided targeted treatments, participants in the treatment group yielded a statistically significant reduction in mean SEM delta scores (MD: 0.49; 95% CI: 0.59, 0.39; P < .0001), and in the odds of developing a SEM PU (OR: 0.59, 95% CI: 0.24 to 1.00; P = .05). In the treatment group, none of the participants developed a visual PU, whereas, in the control group, 1.41% (n = 1/71) developed a visual PU. Based on all the results, the following is concluded, (1). There was a greater reduction in mean SEM delta scores among those cared for using SEM measurement and targeted PU prevention, versus those cared for using visual skin assessment and usual care, and (2). the mean SEM delta scores was statistically significantly lower at the study end for those who received targeted treatments based on abnormal SEM scores. More research is now needed in other and larger at-risk groups to further validate what was found in this study.
1 INTRODUCTION
Pressure ulcers (PUs) are localised damage to the skin and/or underlying tissue, because of pressure, or pressure in combination with shear.1 There are a number of important papers that have addressed the extent of the existence and emergence of PUs in health care, giving an indication of the scale of this problem for patients, families, health care workers, and services. In an extensive systematic review by Moore, Avsar,2 79 articles were reviewed with a first-time in-depth exploration of the prevalence of PUs in Europe. They found that the median prevalence was 10.8% (SD: 7%; range: 4.6–27.2%) across the 79 reviewed studies. The incidence of PUs in observational studies was assessed in a global systematic review and meta-analysis by Afzali Borojeny, Albatineh.3 Inclusion criteria were met by 35 studies, and all were included in the review. The incidence rate of PUs was 12% (95% CI: 10–14). Additionally, a systematic review by Chaboyer, Thalib4 found that the 95% CI of cumulative incidence and prevalence of PUs were 10.0–25.9% and 16.9–23.8% among patients in intensive care units.
It is widely recognised that early and effective risk assessment and PU detection are important. Evidence indicates that decreased mobility/excess abnormal movement and the presence of pressure/shear are central to PU formation.5, 6 The European Pressure Ulcer Advisory Panel recommends a structured risk assessment and comprehensive skin assessment for each patient.1 The most common method of assessing skin is using visual skin assessment (VSA), which has been shown to vary greatly in terms of reliability, meaning that in practice, there is generally only moderate agreement among staff when assessing the same patient for evidence of PU development.7-12 Further, assessment of early evidence of PUs, before the skin is broken, is even more challenging, particularly among individuals with darker skin tones.10 This is a real problem for clinical practice, as skin assessment is fundamental to determine an individual's responses to pressure and shear, in addition to their responses to prevention strategies offered.
Considering the burden that PUs and their prevention place on a health care system and the individual, it seems illogical to use an evaluation tool that is inconsistent. This highlights the need for an alternative tool that can consistently predict the onset of PU. However, existing risk assessment tools include several risk factors, all weighted the same, diluting the importance of immobility as a risk factor. This poses a challenge in the clinical battle to prevent PU in the first place. Furthermore, visual skin assessment, the gold standard, is unable to detect damage which is manifesting beneath the skin, which, if left unnoticed, can progress to irreversible visible tissue damage.5, 6
SEM is a biophysical marker (product of the leak of plasma after the inflammation process increases local vasculature permeability). After tissue damage occurs, local microscopic oedema (or sub-epidermal moisture) and the local increase of moisture change the electrical capacitance of the tissues which can be measured using an electrical bio-impedance device. As SEM is related to deeper layers, it is not influenced by environmental changes, yet is directly related to inflammation.5, 6 Elevated SEM has been shown to be an indicator of early-stage PU damage and when detected can direct staff to anatomical areas of the patient which are at risk, thus enabling the targeting of interventions to combat this risk. Early PU detection can be performed with the use of an SEM Scanner.5, 6, 13-17 The SEM Scanner manufactured by Bruin Biometrics, is a low frequency, handheld bioimpedance device which uses measures of capacitance to assess changes in SEM in the tissues of patients. Research has demonstrated consistency in the detection of changes in SEM and the development of subsequent PUs 1 week later. Further, the use of the SEM Scanner has been shown to have high inter-rater reliability and accuracy in early PU detection.18-20
No previous study has investigated if identifying elevated SEM in patients at risk of PU, and correspondingly implementing enhanced skin care interventions, reduces the incidence of PU when compared with a control group receiving usual care. This study, therefore, sets out to assess the usefulness of measuring SEM in prompting targeted preventative wound care interventions, impacting PU incidence.
“What is the impact of SEM measurement and targeted pressure ulcer prevention versus visual skin assessment and usual care on mean SEM deltas and early pressure ulcer development?”
2 METHODS
2.1 Study outcomes
- Changes in mean SEM deltas from baseline to study completion, tracking SEM deltas as targeted interventions were implemented.
- PU development as identified by 2 days of sustained abnormal SEM delta scores, ≥0.5, after day 16
- Visual PU development, as graded by the EPUAP pressure ulcer grading system.1
2.2 Research design
A quantitative, quasi-experimental, interventional, and observational approach was employed.
2.3 Inclusion and exclusion criteria
- Been over 18 years of age and an in-patent in the study site hospital.
- Been at risk of PU development as identified by the Braden Scale and/or mobility assessment.
- Had an expected stay of two or more days.
- Consented to participate in the study.
- Patients who were unable to give their own consent.
- Outpatients.
- Patients under the age of 18.
- Patients with a predicted discharge date of less than 48 hours.
- Patients who did not have a predicted risk or developing a pressure ulcer whether that be as identified by the Braden Scale or mobility assessment.
2.4 Data collection
To ensure participants believed confident in their decision to become part of the study, the principal researcher approached each individual and supplied them with both verbal and written information about the study. Following a 24-hour cooling off period, the principal researcher approached the potential participants and if they were receptive, obtained informed consent. Then, the principal researcher recorded the participants' demographic details: gender, age, current medical condition, and past medical history.
- SEM measurements from right heel, left heel, and sacrum.
- VSA observed on right heel, left heel, or sacrum.
- Braden Score.
- Mobility assessment.
- Continence status; Application of barrier cream.
- Pressure relieving mattress use; Pressure relieving cushion use.
- Dietician, occupational therapist, and/or physio input.
- Increased repositioning schedule.
- Where the participant was (in bed or sitting out).

Both groups had SEM deltas and VSA recorded daily for a maximum of 5 days using the SEM Scanner (Bruin Biometrics LLC, Los Angeles, California), on three sites: the sacrum, the right heel, and the left heel, as these are the most common sites for PUs to occur.4, 21-28 Results of the SEM measurement were not shared with staff caring for participants in the control group.
2.4.1 Measurement tools
Recording measurements with the SEM scanner
SEM measurement detects changes in fluid contents of human skin and subdermal tissues, by measuring “capacitance.” The capacitance of tissues, called “biocapacitance,” is impacted by the amount of fluid in the tissue.15, 29 To take SEM measurement using the SEM Scanner the operator performs several readings from each site: six readings from the sacrum and three to four from the heel depending on the level of callous present. The SEM Scanner automatically compares the values in each set of measurements and pinpoints the maximal and minimal values within the set. The variance between the maximal and minimal values is displayed as the SEM-delta. The SEM-delta is a measure of the difference between the capacitance at a tissue site that may contain subsurface damage in comparison with healthy (reference) tissue sites. For this study, and in keeping with previous works,6, 30 an SEM delta was considered abnormal if the value was ≥0.5.
- Application or increase in pressure relieving mattresses/cushions.
- Application of barrier cream if sacral SEM raised and they were incontinent.
- Increase in reposition schedule and/or offloading of heels.
The Braden Scale
The Braden Scale31 was used from day one (baseline) and every day thereafter on all participants. The Braden PU risk assessment scale uses six subscales to assess an individual's risk of developing a PU: sensory perception, moisture, activity, mobility, nutrition, and friction/shear. Each subscale is marked from 1 to 4 apart from friction/shear, which is from 1 to 3. The scores are accumulated to give a total score that ranges from 6 to 23. A lower Braden Scale Score indicates a lower level of functioning and, therefore, a higher level of risk of PU development. A score of 19 or higher indicates that the patient is at low risk, with no need for immediate treatment.
There are numerous PU risk assessment tools including the Norton Scale,32 Gosnell Scale,33 Waterlow scale,34 Braden Scale,31 and the Risk Assessment Pressure Score Scale.35 Pressure ulcer risk assessment tools are varied and inconsistent in their inclusion of different risk factors,36 an issue that has prompted substantial research into their reliability and validity.37-40 The Braden Scale was selected as it has been shown to have moderate to high validity and reliability among varied patient cohorts including trauma/ICU patients,40-42 paediatric patients,43, 44 long term care patients,45, 46 patients with spinal cord injuries,47 and elderly patients.48 The inter-rater reliability of the Braden Scale was substantial in every ward versus not good in orthopaedic and spinal surgery wards for the Norton Scale and not good in the orthopaedic wards for the Waterlow Scale. These were the key reasons that underpinned the use of the Braden Scale in this study.
Mobility assessment
‘Does the person regularly reposition themselves without assistance, every few minutes (including during sleep, when seated and persons with sensation deficits).”
If Yes, the patient is not at risk, if No the patient is at risk.
Individuals who are immobile or unable to reposition effectively are susceptible to developing a PU despite any other traditional risk factor being present, as mobility is the highest predictor.6, 49-51
2.5 Sample size calculation
Following the guidance of Kirby, Gebski,53 the sample size was calculated with consideration of the incidence of the problem, the power of the study, the effect size, and the level of significance. The sample size calculation was undertaken using a sample size calculator https://clincalc.com/stats/samplesize.aspx, and the results were verified by a statistician. The expected incidence figure of SEM PU was 63% in the control group. This figure was derived from data in the published literature.6, 30 The expected incidence figure of SEM PU was 45% for the treatment group. The alpha is the probability of a type I error (0.05), the Beta is the probability of a type II error (0.2), and the power is the ability to detect a real difference in outcomes between the two arms of the study (80%). Thus, a total sample of 238 participants was required meaning two groups of 119 participants each.
2.6 Randomisation schedule
The two study wards were assigned as either control or treatment status by virtue of the flip of a coin undertaken by a member of the staff at the hospital site, not involved in the study. Both wards were acute medical wards, in a large urban acute hospital in Dublin, with a similar cohort of patients in both.
2.7 Data analysis
All data were recorded onsite onto data collection sheets, these data were then numerically recorded, tabulated, and entered onto a Microsoft Excel spreadsheet and finally stored, analysed, and presented using SPSS,54 Stata55 and RevMan.56
Descriptive analysis was used to summarise, describe, and explain the data, which are presented in numerical form using means and standard deviations as appropriate. An independent samples t test, or χ2 test was used to analyse differences between the study groups at baseline in terms of the key demographic data. Correlation analysis between Braden and Mobility was undertaken. SEM deltas for the study groups are displayed using box and whisker plots and differences between the mean SEM deltas per group are analysed using the mean difference (MD) and 95% confidence intervals (CI). Differences in the incidence of SEM PU per study group were analysed using the odds ratio (OR) and 95% CI. Similarly, the differences in resolution of abnormal SEM reading at end of the study are also explored using the OR and 95% CI. In keeping with statistical rigour, an alpha level of 0.05 was used to determine statistical significance. Intention to treat analysis (McCoy 2017) was used, meaning that results were based on the initial assignment of patients into treatment or control groups and did not omit participants lost to follow up. ITT was not used for the day 5 delta analysis but used for the odds ratio analysis of SEM PUs. In the ITT odds ratio analysis, those who left the study early were assumed to have not developed a PU after leaving the study.
2.8 Ethical approval
Ethical approval was sought from the study site's Research Ethics Committee on 4 February 2017 (Ref number: April 7, 2017).
3 RESULTS
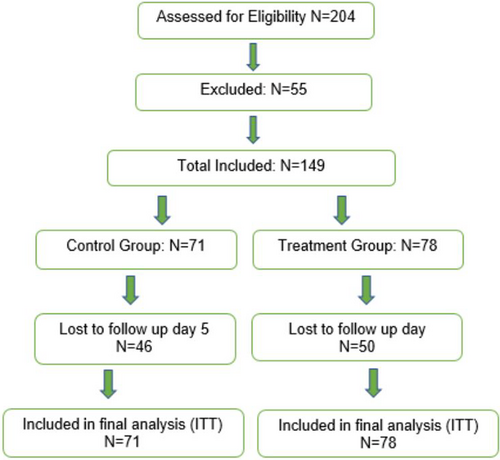
3.1 Flow of participants through the study
Following the application of the inclusion criteria, 204 people were invited to participate, 149 consented, an additional 50 people refused to consent, and 5 people were excluded. Postrandomisation the treatment group had 78 participants and the control group had 71 participants. Data were collected over several months, thus participants were commencing the study and left on many different dates. Both groups were followed up for a maximum of 5 days and only participants with an abnormal SEM delta reading in the treatment group received targeted treatments (See Figure 2).
3.2 Days completed in the study
The mean number of days completed by participants was 3.62 days (SD:±1.3). The minimum number of days completed was one, representing10.1% (n = 15) of participants. Further, 8.1% (n = 12) completed 2 days, 26.8% (n = 40) completed 3 days, 19.5% (n = 29) completed 4 days, and the highest percentage, 35.6% (n = 53), completed the full 5 days of follow up.
3.3 Demographics
Females accounted for 36.6% (n = 26) in the control group and 48.7% (n = 38) in the treatment group. The mean age for the control group was 68.61 years (SD: 14.2 years) and the mean age for the treatment group was 66.1 years (SD: 16.13 years). Using an independent samples t test, there was no statistical difference between the study groups in terms of mean age (t [147] = 1.001; P = .319) and gender (t [147] = 1.091; P = .961).
There was no statistical difference between the study groups in terms of the underlying comorbidities (P ≥ .05 for all comorbidities), except for the presence of cancer, where more of those in the control group had a diagnosis of cancer (P = .005) (see Table 1).
| Comorbidity | Frequency | Percentage | ||
|---|---|---|---|---|
| Control | Treatment | Control | Treatment | |
| Hypertension (HTN) | 31 | 30 | 43.7 | 38.5 |
| Cancer (Ca) | 35 | 21 | 49.3 | 26.9 |
| Chronic obstructive pulmonary disease (COPD) | 26 | 21 | 36.6 | 26.9 |
| Atrial fibrillation (AFib) | 13 | 10 | 18.3 | 12.0 |
| Diabetes mellitus (DM) | 12 | 17 | 16.9 | 21.8 |
| Cerebral vascular accident/transient ischaemic attack (CVA/TIA) | 10 | 10 | 14.1 | 12.8 |
| Osteoarthritis (OA) | 10 | 4 | 14.1 | 5.1 |
| Deep vein thrombosis/pulmonary embolism (DVT/PE) | 7 | 9 | 9.9 | 11.5 |
| Congestive cardia failure (CCF) | 8 | 7 | 11.3 | 9 |
| Ischemic heart disease (IHD) | 3 | 8 | 4.2 | 10.3 |
| Smoker | 5 | 5 | 7 | 6.4 |
| High body mass index | 0 | 6 | 0 | 7.7 |
| Peripheral vascular disease (PVD) | 2 | 3 | 2.8 | 3.8 |
| Osteoporosis | 0 | 4 | 0 | 5.1 |
| Multiple sclerosis (MS) | 0 | 2 | 0 | 2.6 |
| Liver cirrhosis | 0 | 2 | 0 | 2.6 |
| Crohn's disease | 1 | 1 | 1.4 | 1.3 |
| Parkinson's disease | 0 | 1 | 0 | 1.3 |
| Fracture | 10 | 11 | 14.1 | 14.1 |
Continence was higher among the treatment group participants at 62.8% (n = 62/78), with 53.5% (n = 38/71) of participants in the control group categorised as a continent. Using an independent samples t test, there was no statistical difference between the study groups in terms of mean continence status (t [147] = −1.148; P = .253).
3.4 Braden Score
Table 2 outlines the Braden risk categories for the study groups and as can be seen 59% (n = 42) and 58% (n = 45) of the control group and treatment group, respectively, were assessed as being at low risk or, not at risk of PU Development. Thus, there was no statistical difference between the study groups in terms of Braden risk status (P = .961).
| Braden risk category | Frequency | Percentage | ||
|---|---|---|---|---|
| control | Treatment | control | Treatment | |
| Severe risk | 1 | 2 | 1.4 | 2.6 |
| High risk | 15 | 16 | 21.1 | 20.5 |
| Moderate risk | 13 | 15 | 18.3 | 19.2 |
| Low risk | 30 | 29 | 42.3 | 37.2 |
| Not at risk | 12 | 16 | 16.9 | 20.5 |
| Total | 71 | 78 | 100 | 100 |
3.5 Mobility
Participants in both groups had a similar mobility assessment, with 66% (n = 47) in the control and 69% (n = 54) in the treatment group deemed to be at risk of PU development based on their inability to reposition themselves regularly without assistance. Using a χ2 test, there was no statistical difference between the study groups in terms of mobility status (P = .692).
Among the participants, 92% (80/87) of those classified as being low/not at risk according to Braden had mobility problems, according to the yes, no assessment of mobility. Thus, a correlation analysis was undertaken to explore the relationship between Braden risk assessment and mobility assessment. Results show a moderate positive correlation (rs = 0.63; P = .0001) (Ratner 2009), meaning that as Braden's score increases (reduced risk), more participants actually have mobility problems according to the yes, no mobility assessment Thus, Braden incorrectly classified 54% of the total participants (80/148).
3.6 SEM Scanner deltas at baseline
3.6.1 SEM
The mean SEM delta right heel at baseline was 1.02 (SD: 0.8) in the control group and 1.0 (SD: 0.8) treatment group. The mean SEM delta left heel was 0.9 (SD: 0.77) in the control group and 1.01 (SD: 0.75) in the treatment group. Finally, mean SEM delta sacrum was 0.6 (SD: 0.54) in the control group and 0.55 (SD: 0.48) in the treatment group (see Figure 3).
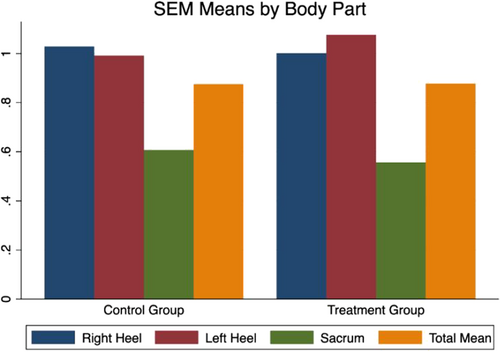
Using an independent samples t test at baseline, there was no statistical difference between the study groups in terms of mean SEM delta right heel (t [147] = 0.242; P = .80), left heel (t [147] = −0.778; P = .43), and sacrum (t [147] = 0.695; P = .48).
3.7 Results for the primary outcome
3.7.1 Difference in all mean SEM deltas from baseline to end of the study
Figure 4 outlines the mean SEM deltas at end of the study across each anatomical area, in addition, to the total SEM deltas. Analysis of the data determined the mean difference in SEM deltas from baseline to end of the study.
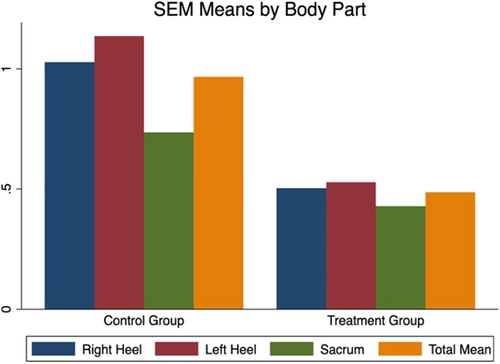
The mean SEM delta at baseline in the control group was 0.88 (SD: 0.42) and at end of the study, this mean SEM delta was 0.98 (SD: 0.36). The mean difference (MD) is 0.09 (95% CI: 0.22 to 0.04; P = .19). In the treatment group, the mean SEM delta at baseline was 0.88 (SD: 0.40) and at the end of the study, this mean SEM delta was 0.49 (SD: 0.28). The MD is 0.40 (95% CI: 0.29 to 0.51; P = .0001).
Further analysis determined the difference in mean SEM deltas at study completion between the control and treatment group. The MD is 0.49 (95% CI: 0.59, 0.39; P < .0001). These results indicate that the mean overall SEM delta was statistically significantly lower in the treatment group at the study end.
3.7.2 Incidence of SEM PU (sustained SEM ≥0.5, developed during the study period, any anatomical site)
SEM readings were recorded daily and were considered elevated if they were ≥0.5, and they were considered sustained if they were elevated for two or more days over any anatomical site, after day 1. Of the total participants, 30% (n = 45/149) had a SEM PU ≥0.5 from day two onwards on any anatomical site, 38% (n = 27/71) of the control group, and 23% (n = 18/78) in the treatment group.
The mean time to SEM PU ≥0.5 developing over any anatomical site was 2.44 days (SD: 0.69; min 2 days; max 4 days). In total, five participants developed more than one SEM PU ≥0.5, with 2 participants developing the SEM PU on both right and left heels, and 3 participants developing the SEM PU on the left heel and the sacrum.
Analysis of the data was undertaken to explore the odds ratio (OR) of SEM PU ≥0.5 between the study groups. The OR of SEM PU ≥0.5 was 0.59 (95% CI: 0.24 to 1.00; P = .05), indicating a 49% reduction in the odds of SEMPU ≥0.5 in the treatment group and this finding is statistically significant.
3.7.3 Abnormal SEM delta scores (≥0.5) at baseline and sustained through the study period
At baseline, there were 330 abnormal SEM deviations (≥0.5) recorded across all three anatomical sites. Of these, 50% (n = 166) were in the treatment group and 50% (n = 164) were in the control group. In total, 65% (n = 213) were sustained during the study follow-up period, of these, 36% (n = 76) were in the treatment group and 64% (n = 137) were in the control group.
By the end of the study, 35% (n = 117) of all readings returned to normal by the end of the study follow-up period, 54% (n = 90) in the treatment group, and 36% (n = 27) in the control group. The OR of readings returning to normal by the end of the study period is 6.01 (95% CI: 3.60 to 10.04; P = .0001). This indicates that the treatment group is 6 times more likely to have an SEM reading return to normal at the end of the study, and this finding is statistically significant.
3.8 Results for the secondary outcome: Pressure ulcer incidence as identified using VSA
Results for VSA were determined by identification of a visual PU on any day, after day 1. The anatomical sites assessed were the right heel, the left heel, and the sacrum, (See Table 3).
| PU incidence VSA | Total per group | Right heel | Left heel | Sacrum | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| Control | 1 | 0.67 | 1 | 0.67 | 1 | 0.67 | 0 | 0 |
| Treatment | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 1 | 0.67 | 1 | 0.67 | 1 | 0.67 | 0 | 0 |
In the treatment group, none of the participants developed a visual PU, whereas, in the control group, 1.41% (n = 1/71) developed a visual Grade 1 PU. The participant in the control group developed two PUs, one on the left heel and one on the right.
4 DISCUSSION
It is widely accepted that tissue damage begins at a cellular level.57 In individuals with normal sensation, mobility, and cognition, exposure to protracted pressure prompts a reaction that results in a change in body position.52 However, when the feedback response is deficient or missing, sustained pressure/shear ultimately occurs leading to ischaemia, cell deformation, and necrosis.58, 59 Ischaemic damage caused by occlusion of blood vessels can take several hours to develop, however, cell deformation occurs within minutes when tissue is exposed to high pressure strains.57, 60-62 This rapid onset of pressure/shear induced damage highlights the importance of prompt detection and initiation of effective prevention strategies.
The pathophysiological changes that result in PUs are now widely known; blood vessels adjacent to micro-damage sites become more permeable when the inflammatory response to cell death is triggered, allowing immune cells to flee the vasculature and transfer towards these cell death sites, aiming to repair the tissue.62 Subsequently, plasma also leaves the leaky vasculature and gradually builds up in the interstitial space, causing oedema. The dielectric constant of the affected tissues becomes like water biocapacitance because of the accumulation of plasma fluids.18, 62, 63 It is thus reasonable to conclude that pressure damage begins at a microscopic level.18, 20, 62, 63
The current “Gold Standard” approach of VSA is a macroscopic observation attempting to identify microscopic changes, which identifies existing damage rather than pre-empting it.6, 30, 64 A device measuring biocapacitance provides a quantitative assessment of early pressure damage allowing health care professionals to initiate interventions that will reduce or reverse the damage.61, 65 The SEM Scanner (Bruin Biometrics, LLC, Los Angeles, California) is a low frequency, handheld bioimpedance device designed for just this use.11, 17
In this study, the mean SEM delta for participants who received targeted interventions were significantly lower than those in the control group, further, the odds of having SEM pressure ulcer ≥0.5 were also lower in the group receiving SEM guided interventions. In addition, the intervention group was 6 times more likely to have a SEM reading return to normal at the end of the study. These findings provide exciting opportunities for improved PU prevention strategies benefiting the individual, the health care professional, and the health care system. PUs' are considered a key performance indicator (KPI) in health care settings, with low incidence representing an example of clinical care being performed well.66, 67 Further, PUs can negatively impact financially both the individual and the health care setting,68, 69 but more importantly, they can negatively impact a person's quality of life.6, 66, 70 Despite the onus on PU prevention, prevalence and incidence rates persist, particularly in hospitals and nursing homes, suggesting that there is a real need to re-think the current approach to the prevention of this prevailing health problem.71, 72
Early identification of pressure damage provides many benefits to care providers and to patients themselves. In terms of litigation, pressure damage can be diagnosed on admission or transfer, this can help attribute “ownership” over the pressure ulcer to the location the patient came from.6 Further, timely pressure/shear identification is key to successful PU prevention. When pressure or shear is in progress, the number of ischaemia/reperfusion cycles increases, and there is a corresponding reduction in skin blood flow and oxygenation to the skin.73 The longer durations of ischaemia, followed by reperfusion result in significantly increased tissue oedema (P < .005) and decreased tissue viability (P < .05).74 In addition, cell deformation causes immediate cell death, through cell rupture, eliminating the possibility of cell damage reversal. When cell death is present, the deprivation of oxygen and nutrient supply to the affected area serves to accelerate the damage, after 20 hours a large inflammatory response is evident.60 The longer the duration of compression, the greater the evidence of cell death, thus time under compression is an important variable.
Therefore, it is essential to know the individual's responses to pressure and shear so care providers can mitigate against allowing the patient to repeatedly load over an area that is adversely responding to pressure and shear. The results highlighted in this study support this concept, as 54% (n = 90) of participants in the intervention group who received targeted interventions had their SEM delta return to normal versus 36% (n = 27) in the control group. This demonstrates that the inflammatory response can be reversed when the original cause is eliminated.
Thus, from a clinical perspective, knowing the patient's individual responses to pressure and shear forces, using technology, such as SEM measurement, will enable the detection of anatomical areas responding adversely. The early detection of tissue damage is beneficial in two different ways. First, it allows practitioners to put interventions into place when the damage is still microscopic and reversible and, thus avoids the knock-on effect of inflammation, especially in the presence of tissue deformation. Secondly, cell death can be avoided when the problem is identified before the cell reaches the point when cell death is present, completely averting the development of a visual PU.
5 LIMITATIONS
Whilst this study has generated new knowledge relating to the identification of early-stage PU damage and the enhanced use of SSKIN interventions, in an Irish acute hospital setting, it does have limitations. The non-inclusion of a qualitative component is something that can be used in future larger scale studies across multiple sites replicating the current study. The use of Braden and counting visual PUs is also something that needs to be explored further, their inclusion in this study, on the one hand, could have been avoided, on the other had their inclusion further highlighted the need for a deeper conversation around there use in practice as the gold standard for risk and predictive assessment.
6 CONCLUSION
The overall purpose of this study was to ascertain if elevated SEM as determined by an elevated SEM delta, which triggered the application of enhanced SSKIN targeted interventions focused on at risk sites, reduced the incidence of PUs in comparison to usual care. In the current study, PU prevention strategies were implemented in the intervention group upon immediate identification of an elevated SEM delta (≥0.05). The interventions included the introduction or upgrading of a pressure redistribution device, the application of barrier cream (where appropriate), and the offloading of heels and/or increasing the repositioning schedule. The use of SEM measurement aided targeted interventions in this study and resulted in a statistically significant reduction in mean SEM delta in the intervention group at the study end in favour of the intervention group. Overall, the results have shown that targeting abnormal SEM deltas with appropriate interventions reduces the SEM delta, thereby reducing the chances of a visible PU occurring resulting in better patient and service outcomes.
ACKNOWLEDGEMENT
Open access funding provided by IReL.
CONFLICT OF INTEREST
The School of Nursing & Midwifery has a research collaboration with Bruin Biometrics.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request due to privacy/ethical restrictions




