Extracorporeal shockwave therapy compared with standard care for diabetic foot ulcer healing: An updated systematic review
Abstract
Emerging evidence suggests that extracorporeal shockwave therapy (ESWT) may improve time to DFU healing. The aim of this review was to appraise the evidence on role of ESWT in DFU healing and impact of different ESWT doses. Databases were searched for trials comparing ESWT plus standard care to standard care alone in participants with DFUs. Search results were reviewed by two independent reviewers. The Cochrane Risk of Bias 2 tool and GRADE approach was used to assess bias and certainty. The primary outcome was time to healing. The search identified 345 papers after duplicates removed. Six trials consisting of 471 participants were included. There was unclear or high risk of bias across all domains. Time to ulcer healing was probably shorter in patients treated with ESWT compared with standard ulcer care alone (GRADE: low certainty). Patients treated with ESWT were more likely to heal at 20 weeks post-ESWT compared with those treated with standard ulcer care alone (GRADE: low certainty). There was significant heterogeneity. ESWT remains a promising new treatment but the translation into routine clinical practice is still limited by the low certainty of evidence surrounding its effectiveness, case selection and optimum dose.
1 INTRODUCTION
Diabetes mellitus affects 4.9 million people in the UK and is estimated to affect more than 422 million people worldwide.1, 2 One of the commonest complications are diabetic foot ulcers (DFU), which occur in 25% of patients.3 More than half of DFUs fail to heal within 12 weeks, and may lead to infection, limb amputation and even death.4-6 DFUs therefore have a huge impact on patient quality of life, healthcare resource use and society.7, 8 The annual healthcare cost of DFU wound care is £1billion in the UK and $237 billion in the US.8, 9 The cost of amputation is eight times higher than that of a healed ulcer.10
Extracorporeal shockwave therapy (ESWT) is a non-invasive intervention that is gaining traction in the treatment of DFUs. It is proposed to stimulate healing through the creation of shearing forces in tissues.11 The shearing forces cause hyperpolarisation of cell membranes, triggering the release of angiogenic growth factors and the upregulation of fibroblast and macrophage activity.12-14 A previous systematic review concluded that ESWT may potentially improve DFU healing, however, the methodology and outcome reporting in the included trials was of generally low quality.15
Another consideration is whether the number of shockwaves (dose) affects DFU healing. Wound models suggest a direct relationship between ESWT dose and angiogenesis.16, 17 The aim of this review is to assess the evidence concerning the impact of ESWT on DFU healing and to explore the relationship between ESWT dose and DFU time to healing.
2 METHODS
This was prospectively registered (PROSPERO ID: CRD42022312509) systematic review undertaken in line with guidance from the Cochrane Collaboration. The review is reported with reference to the PRISMA 2020 reporting guidelines,18 a copy of which can be found in the Appendix A.
2.1 Eligibility criteria
2.1.1 Study design
All randomised control trials (RCT) with a parallel design comparing ESWT with standard ulcer care to standard ulcer care alone or with sham ESWT were included. Cross-over design RCTs and cluster RCTs were eligible for inclusion as part of a narrative synthesis but not in any meta-analysis. There was no restriction on outcome assessor or participant blinding. Only studies with random treatment allocation were included with quasi-randomised trials excluded.
2.1.2 Participants
Participants must have had a diagnosis of diabetes, a diabetic foot ulcer, assessment of limb perfusion and over be 18 years old. There was no restriction on the diagnostic criteria of diabetes, minimum age of the DFU or DFU classification. If a trial population included all types of “chronic wounds”, it was considered for inclusion in the narrative synthesis if the DFU trial population met the inclusion/exclusion criteria.
2.1.3 Intervention
The trial intervention arm(s) must have included ESWT in addition to standard ulcer care and detail the ESWT dosing.
2.1.4 Comparator
The included trials comparator must have been either standard ulcer care or sham ESWT and standard ulcer care (with the method of providing sham ESWT detailed).
2.1.5 Outcomes
The primary outcome was time to ulcer healing in days. All trials must have reported an objective measure of ulcer healing to be eligible for inclusion, for example, proportion of ulcers healed at specific time points, reduction in ulcer size or ulcer recurrence. Secondary outcomes included quality of life (measured with a validated quality of life tool), adverse events (infection, amputation and mortality) and economic outcomes e.g. cost of treatment, incremental cost effectiveness ratio (ICER), net health benefit (NHB), net monetary benefit (NMB).
2.2 Information sources
Information sources were searched between 01/01/2000 and January 28, 2022. Ovid®MEDLINE®ALL, PubMed®, Cochrane Central Register of Controlled Trials (CENTRAL), Ovid Embase®, EBSCO CINAHL Complete, Web of Science, WHO International Clinical Trials Registry Platform and Clinical Trials Register were searched using the pre-defined MeSH and free text search strategy.
The following grey literature was searched for relevant publications: medrxiv.org, OpenGrey, Open Access Thesis and Dissertations and EThOS’ databases. The following ESWT companies were contacted: Soundwave Clinics Limited, Sanuwave, Elevation, Storz Medical and Venn. In addition, official source publications were searched (National Health Service (NHS), National Institute of Clinical Excellence (NICE) and the UK government).
Searches were restricted to English language only and manuscripts published after 01/01/2000.
2.3 Search strategy
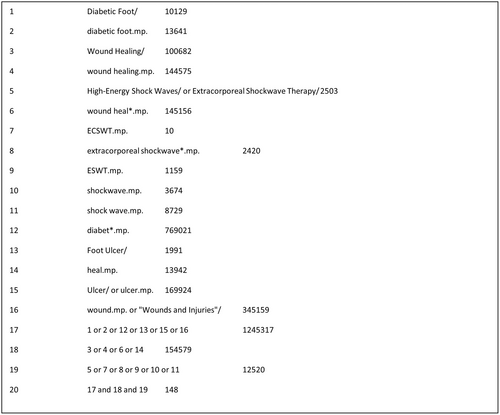
The full search strategy and the search uniform resource locators (URLs) for all databases searched are reported in Appendix A. Figure 1 shows the search strategy for OVID®MEDLINE®ALL database.
2.4 Selection process
Search results were uploaded to Rayyan,19 a bespoke online tool for conducting systematic reviews. Two assessors (LH, MM) independently reviewed search results with reference to the eligibility criteria. The reviewers were blinded to each other's decision. Search results were then unblinded and disagreements discussed. A senior researcher was consulted (ICC) when a decision could not be made. The reviewers based their decision on title, abstract or full manuscript review.
2.5 Data extraction
Data from included studies were extracted onto a pre-piloted Microsoft® Excel® spreadsheet. Two reviewers (LH, MM) independently extracted data from the full text manuscripts. The corresponding author of included manuscripts was contacted for missing or additional data not reported. Data were compared between reviewers for similarity.
2.6 Data items
- Study design: method of randomisation, blinding, number of treatment arms and presence of a power calculation.
-
Participants:
- Number of randomised participants, the number of participants in each arm and number of participants lost to follow-up and withdrawn.
- Patient demographics: age, sex, ethnicity, type of diabetes mellitus, HbA1c, comorbidities and ambulatory status.
-
Intervention:
- ESWT: number of shocks per cm2, depth of shockwave penetration, shockwave energy, number of pulses per second and frequency of treatment sessions.
- Standard ulcer care: dressing type, offloading footwear, glycaemic control, antibiotic use, adjuvant therapy and DFU guidelines followed.
-
Control:
- Standard ulcer care: dressing type, offloading footwear, glycaemic control, antibiotic use, adjuvant therapy and DFU guidelines followed.
- Method of delivering sham ESWT.
- Measures of Effect: Ulcer related outcomes, quality of life, adverse events and economic outcomes.
- Other data: funding source, country(ies) the trial took place in and clinical setting.
2.7 Individual trial risk of bias assessment
Two reviewers independently assessed each included trial for bias using the Cochrane Risk of Bias 2 tool and the overall quality of the evidence using the GRADE approach. Assessment was conducted with reference to the Cochrane Handbook.20
2.8 Effect measures
Time to ulcer healing is reported as (log) hazards ratio and standard error. Continuous outcomes are reported as mean differences with 95% confidence intervals (CI). Dichotomous outcomes are reported as risk ratios with 95% CI.
2.9 Data synthesis
Data items from the trials were tabulated and compared. There was significant heterogeneity in the included trial participants and shockwave regimens, therefore a meta-analysis was not undertaken. There was an insufficient number of trials to undertake a meta-regression21 and network meta-analysis was not deemed appropriate because of the significant heterogeneity in treatment regimens. Data are therefore presented as a narrative synthesis.
3 RESULTS
The search identified 582 records (Figure 2), of which 558 records were identified from database searches and 24 from the grey literature. Following removal of duplicates 345 record titles and abstracts were screened. Seven manuscripts were identified to meet the eligibility criteria. After full text review, one study was excluded as treatment allocation was not randomised.22 In total, six randomised controlled trials (RCTs) were included in the review.23-28
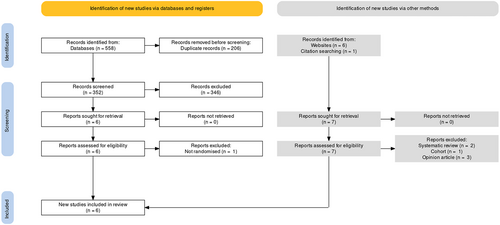
3.1 Included trials
The review included six trials published between 2009 and 2019 and including 471 patients. One included manuscript reported two separate RCTs, therefore is reported as two separate trials in this review (Snyder Trial 1 and Snyder Trial 2).25 The Galiano et al 201926 manuscript is a separate publication that reported the secondary outcomes from Snyder Trial 1 and Snyder Trial 2.25
All included trials were two arm parallel RCTs. Four trials compared ESWT (plus standard care) with standard care alone.23, 24, 27, 28 Two trials compared ESWT (plus standard ulcer care) with sham ESWT (plus standard ulcer care).25, 26
3.2 Study design
Four trials detailed the process of randomisation.25, 27, 28 Three trials blinded participants and/or outcome assessors to treatment allocation.25, 27, 28 Three trials included a sample size calculation,25, 27 and the primary outcome was stated in three trials.25, 27 The follow-up period ranged from 7 to 24 weeks. Participant eligibility criteria are presented in Table 1. Two trials were industry funded25 and another was supported by charity.28 Two trials were multi-centre (Snyder Trial 1, 19 centres in US, 1 in Germany and 1 in UK; Snyder Trial 2: 17 in US and 1 in Canada).25 The others trials were single centre in Denmark,28 Saudi Arabia,27 Italy24 and Egypt.23
| Trial | Inclusion | Exclusion |
|---|---|---|
| Jeppesen, 2016 | Diagnosed with a DFU Wagner 1 or 2 Over 18 years old |
Ulcer present less than 2 months Ulcer area < 0.25 cm2 Vascular surgery performed within last 2 months Planning vascular or orthopaedic treatment Wagner stage 3 to 5 Unable to consent Unable to speak Danish |
| Omar, 2014 | Diagnosis of T1DM or T2DM University of Texas grade 1A-2A DFU present for more than 3 months Ulcer area between 0.5-5 cm2 Peripheral neuropathy Willing to take part |
Evidence of local infection, cellulitis, gangrene Renal, hepatic, neurological or malignant diseases Severe malnutrition (albumin <2 g/dL) Severe anaemia Hb < 7 g/dL ABPI<0.7, absence of DP/PT pulse Pregnancy |
| Moretti, 2009 | Neuropathic ulcer below the medial malleoli present for 6 weeks Ulcer area > 1 cm2, ulcer size 0.5-5 cm Participant age between 30 to 70 years old ABPI >0.7, 1 palpable foot pulse Insensitivity to 10 g monofilament |
Peripheral vascular disease, coronary artery bypass grafting, pregnancy, coagulopathy, neoplasia “Based on the clinical judgement of the PI” |
Snyder, 2018 Trial 1 |
Male or female over 18 years old Females must use contraception and have a negative PT* within 2 weeks of visit 2 Female or post-menopausal incapable of pregnancy or postmenopausal for 1 year At least 1 DFU present for 30 days For toe ulcers the tip of the ESWT applicator must be able to be held perpendicular to target ulcer AND be applied to the entire surface including 1 cm beyond the surface of the ulcers in each direction at visit 2 HbA1c <12% Capable of wound care at home Target ulcer between 1-16 cm2 ABPI 0.7–1.2, TP >50 mmHg or TcPO2 > 40 mmHg Capacity to consent |
Pregnancy, trying to conceive BMI >40 On dialysis Prior ulcer in the same area as the target ulcer Target ulcer decreases by 50% or more at the end of week 2, Multiple foot ulcers connected by fistula, or other ulcers within 5 cm, target ulcers that tunnel into wound tracks that cannot be fully visualised PVD needing revascularisation Offloading for a reason other than target ulcer Lower extremity revascularsiation within 8 weeks Active Charcot's foot, surgical procedure to correct biomechanical abnormalities within 8 weeks of visit 1 DVT within 6 months of visit 1 Lymphoedema Chemotherapy within 60 days of screening visit Life expectancy <2 years Target ulcer treated with growth factors, prostaglandins, negative pressure or vasodilators within 2 weeks of visit 1 Receiving >10 mg steroids per day Sickle cell anaemia Immunodeficiency disorder Radiation of the treatment within 120 days of visit 1 Treatment with immunosuppressants or biologically active cellular products within 60 days of visit 1, treatment with acellular products within 30 days Current history of substance misuse History of major systemic infection requiring hospitalisation within 3 months of visit 1 Physical or mental disability or geographical concerns that inhibit adherence with required study visits Planning exclusionary treatment/procedure during the study Participating in another clinical investigation within 30 days before study visit 1 Subject believed by the investigator to be unwilling or unable to comply with study protocol requirements |
Snyder, 2018 Trial 2 |
As above |
As above Clinically significant renal disease or impaired renal function OM in foot or ankle Previous OM must have resolved >60 days before visit 1 PVD requiring vascular intervention at visit 1 or 2 Previously participated in a ESWT trial Current or history of malignancy within past 5 years of visit 1 |
| Nossair, 2013 | Diabetes Age 40–70 years old DFU for at least 3 months Area greater than 1 cm2 Ulcer grade 2 or 3 |
Malignancies, vascular insufficiency, renal failure, psychological problems, anaemia, hyperthyroidism, favism, alcoholic drinkers, pregnant, receiving radiotherapy/chemotherapy/immunosuppressants/anticonvulsants, participant in another clinical study, ulcer area > 20 cm2, ulcer grade 1 or 4 |
- Abbreviations: ABPI, ankle brachial pressure index; BMI, body mass index; DFU, diabetic foot ulcer; DP, dorsalis pedis; DVT, deep vein thrombosis; ESWT, extracorporeal shockwave therapy; Hb, haemoglobin; OM, osteomyelitis; PI, principal investigator; PT*, pregnancy test; PT, posterior tibial; PVD, peripheral vascular disease; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; TP, toe pressure.
3.3 Demographics of participants
The review included 471 participants (Table 2). Most participants were male (357/471; 76%) and the mean age was 58.9 ± 8 years. Ethnicity was only reported in the Snyder et al trials.25 Most participants were Caucasian (245/337, 73%), followed by African American (64/337, 19%).25 Other ethnicities included American Indian, Alaskan native, Pacific Islander, Asian, Mixed and Other.25
| Trial | Total (number of participants per arm) | Age (mean ± SD; years) | Sex (M:F) | Duration of DM (mean ± SD; years) | HbA1c (mean ± SD, mmol/mol) | Comorbidities | Hb (mean ± SD; g/dL) |
|---|---|---|---|---|---|---|---|
| Jeppesen, 2016 | 21 | ESWT: 8.6 ± 0.8 | |||||
| ESWT: 19 | ESWT: 65.3 ± 12.9 | ESWT: 5:5 | ESWT: 16.3 ± 12.2 | ESWT: 65.2 ± 20.0 | ESWT: HTN (n = 10), hypercholesterolaemia (n = 8) | ||
| Control: 12 | Control: 67.8 ± 9.7 | Control: 11:1 | Control: 25.1 ± 15.0 | Control: 71.4 ± 18.3 | Control: HTN (n = 1), hypercholesterolaemia (n = 8) | Control: 7.7 ± 1.2 | |
| Omar, 2014 | 44 | NR | |||||
| ESWT: 19 (21 ulcers) | ESWT: 59 ± 7.35 | ESWT: 14:5 | ESWT: 12.0 ± 3.66 | ESWT: 74 | ESWT: CKD (n = 8), HTN (n = 13), hypercholesterolaemia(n = 12) | ||
| Control: 19 (21 ulcers) | Control: 57 ± 5.39 | Control: 13:6 | Control: 13.14 ± 3.78 | Control: 66 | Control: CKD (n = 7), HTN (n = 14), hypercholesterolaemia (n = 12) | ||
| Moretti, 2009 | 30 | NR | NR | NR | NR | ||
| ESWT: 15 | ESWT: 56.2 ± 4.9 | ESWT: 9:6 | |||||
| Control: 15 | Control: 58 ± 7.5 | Control: 7:8 | |||||
Snyder, 2018 Trial 1 |
206 | NR | NR | ||||
| ESWT: 107 | ESWT: 60 ± 4 | ESWT: 83:24 | ESWT: 18 ± 10 | ESWT: HbA1c < 53: n = 33 | |||
| Control: 99 | Control: 56.2 ± 9.4 | Control: 83:16 | Control: 15.7 ± 11.1 | Control: HbA1c < 53: n = 33 | |||
Snyder, 2018 Trial 2 |
130 | NR | NR | ||||
| ESWT: 65 | ESWT: 59.1 ± 9.4 | ESWT: 54:11 | ESWT: 15.44 ± 11.4 | ESWT: HbA1c < 53: n = 23 | |||
| Control: 65 | Control: 56.8 ± 10.7 | Control: 49:16 | Control: 17.45 ± 13.3 | Control: HbA1c < 53: n = 13 | |||
| Nossair, 2013 | 40 | NR | NR | NR | NR | ||
| ESWT: 20 | ESWT: 56.1 ± 7.51 | ESWT: 15:5 | |||||
| Control: 20 | Control: 55.15 ± 6.3 | Control: 14:6 |
- Abbreviations: CKD, chronic kidney disease; DM, diabetes mellitus; ESWT, extracorporeal shockwave therapy; F, female; Hb, haemoglobin; HTN, hypertension; M, male; NR, not recorded; SD, standard deviation.
3.4 Demographics of DFU
Most trials reported the mean duration of ulcer and ulcer area (Table 3). Most studies included participants with an ulcer extending into the deep tissues (University of Texas Grade 1A to 2A,27 Wegner Grade 1 to 228) and excluded participants with serious infections.25, 27 Site of index ulcer was variably reported.
| Trial | Number of active ulcers per trial arm | Site of Ulcer | Mean duration of DFU (mean ± SD, months) | Ulcer area (mean ± SD, cm2) |
|---|---|---|---|---|
| Jeppesen, 2016 | ESWT: 11 | ESWT: Digital n = 3; Plantar n = 3; Dorsum n = 2; Malleoli n = 3 | ESWT: 22.6 ± 24.4 | ESWT: 2.34 ± 1.66 |
| Control: 12 | Control: Digital n = 4; Planter n = 5; Dorsum n = 0; Malleoli = 3 | Control: 15.2 ± 11.1 | Control: 2.37 ± 0.93 | |
| Omar, 2014 | ESWT: 24 | ESWT: Planter n = 16; Dorsum n = 3; Web n = 3 | ESWT: 11.97 ± 6.5 | ESWT: 7.89 ± 2.97 |
| Control: 21 | Control: Planter n = 13; Dorsum n = 3, Web n = 5 | Control: 10.81 ± 4.63 | Control: 8.62 ± 3.47 | |
| Moretti, 2009 | ESWT: 15 | NR | NR | ESWT: 29.78 ± 12.9 |
| Control: 15 | Control: 24.5 ± 10.09 | |||
Snyder, 2018 Trial 1 |
ESWT: 107 | NR | ESWT: 12.2 ± 16.7 | ESWT: 3.5 ± 5.2 |
| Control: 2 | Control: 17.4 ± 26.9 | Control: 2.8 ± 2.4 | ||
Snyder, 2018 Trial 2 |
ESWT: 65 | NR | ESWT: 11.2 ± 13.4 | ESWT: 3.71 ± 2.8 |
| Control: 65 | Control: 17.4 ± 26.9 | Control: 3.73 ± 2.8 | ||
| Nossair, 2013 | ESWT: 20 | NR | NR | ESWT: 8.86 ± 3.42 |
| Control: 20 | Control: 8.32 ± 3.88 |
- Abbreviations: DFU, diabetic foot ulcer; NR, not recorded; SD, standard deviation.
3.5 ESWT dosing
None of the included trials used the same treatment regime of ESWT (Table 4). The number of shocks per cm2 ranged from 100 to 500 and the number of weeks the intervention was given ranged from 1 to 10 weeks. ESWT was given between 1 and 3 times per week. No trials reported the depth of ESWT penetration. Jeppesen et al used a soundwave frequency of 5 Hz28; no other trials reported soundwave frequency. The Snyder et al trials used four shocks per second25; no other trials reported number of shockwaves delivered per second.
| Trial | ESWT Machine | Number of shocks delivered (a/cm2) | Shockwave energy (mJ/mm2) | Number of sessions per week | Total number of weeks |
|---|---|---|---|---|---|
| Jeppesen, 2016 | DUOLITH SD1 T-top (Storz Medical) | 250a | 0.2 | 2 | 3 |
| Omar, 2014 | NR | 100a | 0.11 | 2 | 8 |
| Moretti, 2009 | Minilith SL1 (Storz Medical) | 100a | 0.03 | 3 | 1 |
Snyder, 2018 Trial 1 |
dermaPACE (Sanuwave) | 500 | 0.23 | 2 | 2 |
Snyder, 2018 Trial 2 |
dermaPACE (Sanuwave) | 500 | 0.23 | 0–2b | 10 |
| Nossair, 2013 | BLT-5000 | 500a | 0.1 | 1 | 3 |
- Abbreviation: NR, not recorded.
- a Plus 500 “deep” shocks to the anatomical location of artery supplying the ulcer.
- b 4 applications every 3 ± 2 days, then one session every 2 weeks until 8 weeks.
3.6 Standard ulcer care
Two trials referred to published guidelines for standard ulcer care.24, 28 Four trials reported standard ulcer care consisted of offloading footwear,23, 24, 27, 28 three trials reported the use of anti-hyperglycaemia medication23, 27, 28 and three trials reported the use of antibiotics.23, 24, 28 Four trials reported the use of debridement.23, 24, 27, 28
3.7 Risk of bias
Figure 3 details the risk of bias for each domain.
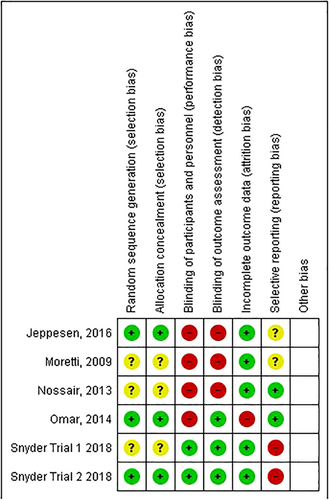
3.7.1 Selection bias
Selection bias risk was judged as low in three trials that used a computer based random generation sequence.25, 27, 28 Trial 1 by Snyder et al was judged as unclear risk as sealed envelopes are not as robust as computer generated sequences.25, 29 Two trials gave no details of randomisation processes.23, 24
3.7.2 Performance and detection bias
Performance and detection bias was judged as low in two trials who used sham ESWT and blinded outcome assessors.25, 26 Detection bias was judged as low in two trials who used blinded outcome assessors.27, 28 Four trials were judged as high risk of performance bias for lack of participant blinding.23, 24, 27, 28
3.7.3 Incomplete outcome data
Attrition rates were low in four trials.24, 25, 28 One trial excluded patients who developed an infection and was judged as high risk of attrition bias.27
3.7.4 Selective reporting
Two trials were judged as a low risk of reporting bias.23, 27 Both trials stated the primary outcome in the methods. Two trials were judged as unclear risk of reporting bias as the trials did not define the primary outcome (or secondary outcomes).24, 28 Two trials were judged as high risk of reporting bias.25 Although Snyder et al trials stated the primary and secondary outcomes these were selectively reported in the results, because of lack of statistical significance.25 In addition, the pooled analysis was not part of the original protocol and the justification was judged as weak.25
3.7.5 Other potential sources
There was judged to be a high risk of publication bias in the two commercially funded trials and did not present raw data or analysed data they make conclusions upon.25
3.8 Outcome
Four trials reported time to healing, four trials reported proportion of ulcers healed at specific time points and three trials reported reduction in ulcer size at specific time points.
3.8.1 Time to ulcer healing
Four trials reported time to healing.24, 26, 27 Moretti et al reported that the mean time to healing was 60.8 ± 5 days in the ESWT group compared with 82.17 ± 4.35 days in the control group (P < 0.001).24 Omar et al reported time to ulcer healing of 64.5 ± 8.06 days in the ESWT group compared with 81.17 ± 4.36 days in the control group(P < 0.05).27 Time to healing in days was not reported by Galiano et al, instead authors reported whether the differences between groups were statistically significant (defined as P < 0.05) or not for the two trials.26 The evidence was downgraded to low for imprecision and indirectness.
3.8.2 Proportion of healed ulcers
Four trials reported proportion of ulcers healed at 20 weeks.24, 25, 27 Moretti et al reported the proportion of healed ulcers at 20 weeks was 55.5% (8/15) in the ESWT group compared with 33.3% (5/15) in the control group (P < 0.001).24 Omar et al reported the proportion of healed ulcers at 20 weeks was 54.0% (13/24) in the ESWT group compared with 28.5% (6/21) in the control group.27 Snyder et al reported the proportion of healed ulcers at 24 weeks was 39.3% (42/107) in the ESWT group compared with 26.3% (26/99) in the control group in Trial 1 and 35.4%(23/65) in the ESWT compared with 26.2%(17/65) in the control group in Trial 2.25 The evidence was downgraded to low because of imprecision and risk of bias.
3.8.3 Reduction in ulcer size
Five trials reported reduction in ulcer size during follow-up.23, 25-28 Jeppesen et al reported a reduction in ulcer size at 7 weeks of 34.5 ± 14.9% in the ESWT group compared with 5.6 ± 21.4% in the control group.28 Nossair et al reported a reduction in ulcer size at 12 weeks of 83.3 ± 27.4% in the ESWT group compared with 48.7 ± 31.7% in the control group.23 Omar et al reported a reduction in ulcer area at 20 weeks of 83.3 ± 20.7% in the ESWT group compared with 36.2 ± 23.0% in the control group.27 Snyder Trial 1 reported a reduction in ulcer area of 54.3% in the ESWT compared with 6.9% in the control group.25 Trial 2 the reduction in wound area values were not reported but the manuscript commented differences were not statistically significant (defined as P < 0.05).26 The evidence is downgraded to low because of imprecision and risk of bias.
3.8.4 Ulcer recurrence
The pooled results of Snyder Trial 1 and Trial 2 reported ulcer recurrence rate of 7.7% in the ESWT group versus 11.6% in the control group.25 The evidence was downgraded to low certainty because of imprecision and risk of bias.
3.8.5 Adverse events
In the Snyder et al trials, the DFU infection rate ranged 28–36.8% in the ESWT group compared with 25.3%–35.4% in the control group.25 Moretti et al reported two incidences of DFU infection, one in each group, that resolved with oral antibiotics.24 The Omar et al trial withdraw three patients (one in the ESWT group, two in the control group) because of infection.27 In the Snyder trials, the pooled amputation rate was 2.3% (4/172) in the ESWT group compared with 3.0% (5/164) in the control group.25The evidence was downgraded to low certainty because of imprecision and risk of bias.
3.8.6 Quality of life
No trials reported quality of life outcomes.
3.8.7 Cost effectiveness
No trials compared the cost effectiveness of ESWT to standard ulcer care alone.
4 DISCUSSION
This systematic review supports previous research suggesting ESWT, along with standard ulcer care, improves time to DFU healing, when compared with standard ulcer care alone.15, 30 However, substantial gaps remain in the literature and the evidence presented in this review should be cautiously interpreted.
This review aimed to investigate the effect of different ESWT doses on time to healing. There was significant variation in number of shocks delivered per cm2, number of treatment sessions per week and length of overall treatment in the included trials. This variation was due to be explored in a planned meta-analysis and subgroup analysis. However, this was not performed for two reasons. Firstly, after tabulating results from the four trials that reported time to ulcer healing it became apparent that pooling of these data would result in a statistically significant result favouring ESWT, mainly driven by the Snyder et al trials. This would be misleading as heterogeneity between the trials would be unaccounted for and potentially lead to overestimation of ESWT treatment effect. Secondly, a high risk of bias in the trial methodology and low certainty in the evidence further risks a misleading result from a pooled analysis.31
A meta-regression was considered to address concerns about the heterogeneity in trial methods, included participants and ESWT regimens. However, the review included only four trials with suitable data for synthesis, which is well below the Cochrane Handbook recommended minimum of 10 trials.21, 31, 32 Other limitations in the included trials were the availability of only aggregate data leading a risk of ecological fallacy, multiple potential effect modifiers (arising from methodological and clinical heterogeneity) of which repeated testing may lead to regression to the mean and no individual patient data to validate interactions between ESWT dose and healing time.32 The lack of trials, and therefore data, further limited the application of more sophisticated statistical methods to counter these problems.21, 33
The quality of evidence remains a concern when applying the results of this systematic review to patient care. The review findings were all judged as low or very low certainty. The areas contributing to potential bias included treatment allocation, blinding and selective outcome reporting. Selective outcome reporting, along with sparce reporting of baseline patient demographics and ulcer characteristics, impede the generalisability of results. In 2016, recommended reporting standards for research of intervention studies in DFU were published, which included specific data points when reporting on participants, interventions and outcomes.34 The broad range of ulcer related outcomes reported in DFU research was highlighted as part of the COMET core outcome setting exercise.35 In the review by Dovell et al there were 714 different outcomes reported in the DFU literature.35 The lack of agreed and loosely followed reporting standards in DFU research makes evidence synthesis challenging and unreliable. This review echoes these findings with inconsistent reporting of time to healing, number of ulcers healed at various time points, and the use of surrogate markers of healing which have little clinical benefit or relevance to patients, that is, 90% healed rate, percentage of granulation tissue and number of unchanged ulcers. Other important clinical outcomes such as infection, recurrence and amputation rate were variability reported. Whether this was because of a short follow-up period or oversight is unclear. Overall, this makes the comparison of ESWT to different treatments difficult for patients, clinicians, economists and policy makers; especially when attempting to predict the clinical and cost effectiveness of ESWT to alternatives.
There is laboratory-based evidence of a dose dependant relationship between ESWT and healing.17 The included trials often referenced murine-based skin flap models for their choice of ESWT dose. Further translation work is required to investigate whether the positive effects of ESWT seen in murine models are also present in patients with DFUs who are treated with ESWT.
All trials included in this review excluded participants with infection or ischaemia. ESWT is hypothesised to aid healing through regulating inflammation, angiogenesis and stimulating fibroblast migration and differentiation.11, 14, 36-38 An increase in transcutaneous oxygen tension was observed in the Jeppesen et al trial and could suggest that angiogenesis is mediated by endothelial nitric oxide synthase leading to vasodilation as a potential mechanism of action.28 Poor perfusion is the most influential risk factor in DFU healing.6, 7 Patients with DFU and ischaemia classically develop extensive infra-popliteal and distal microvascular disease, which is challenging to treat. These patients may gain the greatest benefit from ESWT, and future trials should consider inclusion of this patient group. Ongoing infection is known to prolong DFU healing time35-37 and there is also emerging evidence that ESWT may increase bacteria sensitivity to antibiotics. Therefore, future ESWT research should consider a move away from targeting easier to heal neuropathic ulcers and investigate the application of ESWT in those with mild infection and microvascular disease.
The review has limitations. Although the search strategy was broad and applied to a number of sources, the search was limited to English language only, and randomised controlled trials, which may have excluded some potential important studies.
5 CONCLUSION
ESWT remains a promising new treatment but the translation into routine clinical practice is still limited by the low certainty of evidence surrounding its effectiveness. Any future trials must be undertaking in a scientifically rigorous way to prevent wastage of resources and improve DFU care.
FUNDING INFORMATION
LH is funded by the NIHR Doctoral Research Fellowship NIHR 301807. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
CONFLICT OF INTEREST
None.
APPENDIX A
PRISMA 2020 CHECKLIST
| Section and topic | Item # | Checklist item | Location where item is reported |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review. | |
| ABSTRACT | |||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | |
| METHODS | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | |
| Information sources | 6 | Specify all databases, registers, websites, organisations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | |
| Search strategy | 7 | Present the full search strategies for all databases, registers and websites, including any filters and limits used. | |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g. for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | |
| 10b | List and define all other variables for which data were sought (e.g. participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | ||
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g. risk ratio, mean difference) used in the synthesis or presentation of results. | |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g. tabulating the study intervention characteristics and comparing against the planned groups for each synthesis [item #5]). | |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | ||
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | ||
| 13d | Describe any methods used to synthesise results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | ||
| 13 e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g. subgroup analysis, meta-regression). | ||
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesised results. | ||
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias because of missing results in a synthesis (arising from reporting biases). | |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | |
| RESULTS | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | ||
| Study characteristics | 17 | Cite each included study and present its characteristics. | |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g. confidence/credible interval), ideally using structured tables or plots. | |
| Results of syntheses | 20a | For each synthesis, briefly summarise the characteristics and risk of bias among contributing studies. | |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g. confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | ||
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | ||
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesised results. | ||
| Reporting biases | 21 | Present assessments of risk of bias because of missing results (arising from reporting biases) for each synthesis assessed. | |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | |
| DISCUSSION | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | |
| 23b | Discuss any limitations of the evidence included in the review. | ||
| 23c | Discuss any limitations of the review processes used. | ||
| 23d | Discuss implications of the results for practice, policy, and future research. | ||
| OTHER INFORMATION | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | ||
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | ||
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | |
| Competing interests | 26 | Declare any competing interests of review authors. | |
| Availability of data, code and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | |
Source: Page et al.18
For more information, visit: http://www.prisma-statement.org/
APPENDIX B
SEARCH STRATEGIES
Uniform Resource Locators (URLs) for the database searches:
Ovid®MEDLINE®ALL
PubMed®
Ovid EMBASE®:
Web of Science
Medical Subject Headings (MeSH): Diabetic foot, wound healing, high energy shockwaves, extracorporeal shockwave therapy, foot ulcer, ulcer, wounds and injuries.
Free text: diabetic foot, wound healing, wound heal*, extracorporeal shockwave*, ESWT, ECSWT, shockwave*, shock wave, diabet*, heal, ulcer, wound.
Search Strategies
Ovid®MEDLINE®ALL
Ovid MEDLINE(R) ALL <1946 to January 19, 2022>
1Diabetic Foot/10129
2diabetic foot.mp.13641
3Wound Healing/100682
4wound healing.mp.144575
5High-Energy Shock Waves/ or Extracorporeal Shockwave Therapy/2503
6wound heal*.mp.145156
7ECSWT.mp.10
8extracorporeal shockwave*.mp.2420
9ESWT.mp.1159
10shockwave.mp.3674
11shock wave.mp.8729
12diabet*.mp.769021
13Foot Ulcer/1991
14heal.mp.13942
15Ulcer/ or ulcer.mp.169924
16wound.mp. or “Wounds and Injuries”/345159
171 or 2 or 12 or 13 or 15 or 161245317
183 or 4 or 6 or 14154579
195 or 7 or 8 or 9 or 10 or 1112520
2017 and 18 and 19148
21limit 20 to (english language and yr = “2000 -Current”)130
PubMed®
((diabetic foot OR foot ulcer OR wound OR ulcer) AND (high energy shockwaves OR extracorporeal shockwave therapy OR extracorporeal shockwave therapy OR ESWT OR ECSWT OR shockwave* OR shock wave) AND (heal OR wound heal* OR wound healing))
Web of Science
((diabetic foot OR foot ulcer OR wound OR ulcer) AND (high energy shockwaves OR extracorporeal shockwave therapy OR extracorporeal shockwave therapy OR ESWT OR ECSWT OR shockwave* OR shock wave) AND (heal OR wound heal* OR wound healing))
WHO International Clinical Trials Registry Platform
((diabetic foot OR foot ulcer OR wound OR ulcer) AND (high energy shockwaves OR extracorporeal shockwave therapy OR extracorporeal shockwave therapy OR ESWT OR ECSWT OR shockwave* OR shock wave) AND (heal OR wound heal* OR wound healing))
Medrxiv.org
“(((diabetic foot ulcer OR diabetic foot OR foot ulcer) AND (extracorporeal shockwave)))” and posted between “01 Jan, 2000 and 20 Jan, 2022”.
Ovid®EMBASE®:
Embase <1974 to January 19, 2022>
1diabetic foot/18230
2wound healing/127289
3high-energy shock wave/194
4shock wave therapy/1967
5foot ulcer/5608
6ulcer/41799
7wound/31342
8diabetic foot.mp.20481
9wound healing.mp.174753
10wound heal*.mp.175434
11extracorporeal shockwave*.mp.2716
12ESWT.mp.1666
13ECSWT.mp.16
14shockwave*.mp.5062
15shock wave*.mp.16157
16diabet*.mp.1275895
17heal.mp.18648
18ulcer.mp.246014
19wound.mp. or wound/376555
201 or 5 or 6 or 7 or 8 or 16 or 18 or 191827364
212 or 9 or 10 or 17188981
223 or 4 or 11 or 12 or 13 or 14 or 1518959
2320 and 21 and 22218
24limit 23 to (english language and year = “2000 -Current”)203
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article




