Safety and efficacy of botulinum toxin type A in preventing and treating scars in animal models: A systematic review and meta-analysis
Funding information: Jilin Scientific and Technological Development Program, Grant/Award Number: 20180414038; National Natural Science Foundation of China, Grant/Award Number: 81971842
Abstract
Previous studies have used botulinum toxin type A (BTXA) to improve postoperative and hypertrophic scars; however, there is lack of detailed verification on the safety and effectiveness of this approach. This study aimed to evaluate the therapeutic effect of BTXA on postoperative hypertrophic scars and its influence on cytokine expression in animal models. A computerised search of different databases was performed, including PubMed, Web of Science, Scopus, Cochrane, Embase, CNKI, and Wanfang, up to 10 March 2021. A meta-analysis was performed using R 4.0.0 based on hypertrophic index, epithelialisation time, wound area, and vascular endothelial growth factor (VEGF) expression. Eleven studies were included. The meta-analysis showed a significant difference in hypertrophic index (standardised mean difference [SMD] = −2.63, 95% confidence interval [CI]: −3.50 to −1.76, P < .01), wound area (SMD = −0.54, 95% CI: −1.24 to 0.16, P < .01), and VEGF expression (SMD = −2.56, 95% CI: −3.50 to −1.62, P < .01). This study shows that BTXA is safe and effective in preventing and treating scar hypertrophy in animal models, but excessive doses of BTXA and BTXA to treat large areas should be avoided.
1 INTRODUCTION
Both plastic surgeons and patients face the challenge of scars that result from wound healing.1, 2 During wound healing, abnormal proliferation of fibroblasts and excessive deposition of extracellular matrix lead to formation of pathological scars.3 The most common pathological scars are hypertrophic scars and keloids. Patients experience itching, pain, erythema, and social/psychological trauma, which are especially problematic in children.3, 4 Common treatment methods for pathological scars include steroid injection, silicone sheets and gel, pressure therapy, 5-fluorouracil, cryotherapy, and surgical resection.2, 4 However, there are currently no suitable guidelines for the treatment of pathological scars.
Botulinum toxin type A (BTXA) is a neurotoxin secreted by Clostridium botulinum. The US Food and Drug Administration approved the use of BTXA for the treatment of strabismus and blepharospasm.5 BTXA is now used in the clinic, and randomised controlled trials have gradually incorporated BTXA, but the evaluation criteria are limited to morphological indicators, including visual analogue scale (VAS) score, VSS, patient satisfaction, and adverse events.6-8 The mechanism of BTXA treatment and the stability of its action are still unclear. Therefore, we performed a literature search to identify studies using BTXA in animal models to obtain more detailed morphological and protein expression data and explore the mechanism of BTXA.
2 MATERIALS AND METHODS
We performed this systematic review and meta-analysis according to preferred reporting items for systematic reviews and meta-analyses guidelines.
2.1 Literature search
A computerised search of different databases was performed, including PubMed, Web of Science, Scopus, Cochrane, Embase, CNKI, and Wanfang, up to 10 March 2021. The following search terms were included: “keloid,” “scar,” “botulinum toxin,” “BTXA,” “botulinum toxins, type A,” and “model.” The PubMed search strategy was as follows: “(botulinum toxin) OR (BTXA) OR (BOTOX) OR (botulinum toxins, type a)” AND “(scar) OR (cicatrix) OR (keloid) OR (hypertrophic scar)” AND “(model) OR (modelling) OR (modelization) OR (modelized) OR (animal model) OR (rat) OR (mice) OR (mouse) OR (rabbit).” The equivalent Chinese terms were used in the Chinese database. The reference lists of these articles were also screened to identify other relevant articles.
2.2 Inclusion and exclusion criteria
The inclusion criteria were as follows: (a) studies using rabbit, rat, or nude mouse scar models; (b) studies including test groups to evaluate the effects of BTXA on scarring; (c) studies including control or placebo groups, regardless of additional treatment; (d) studies reporting at least one of the following outcomes: hypertrophic index (HI), scar thickness, vascular endothelial growth factor (VEGF) expression, wound epithelialisation time, and/or wound area.
The exclusion criteria were as follows: (a) in vitro only studies; (b) studies with no clear data results or studies in which the results are only presented in the form of pictures; (c) letters, commentaries, and guidelines.
2.3 Data extraction
Two researchers extracted data independently, including authors' names, publication year, case information, dose of BTXA, and treatment strategy. The primary outcome was HI. Any disagreement was discussed with a third researcher. Two researchers initially screened the articles by reading the titles and abstracts. If these researchers could not judge it by this method, full-text reading was performed. Finally, the researchers extracted the required data from selected articles according to the inclusion and exclusion criteria.
2.4 Quality assessment
The quality of included studies was assessed using a seven-item STAIR checklist. Items were scored 0 if they were answered “NO”; 1 if they were answered “UNCLEAR”; or 2 if they were answered “YES.” Article quality was assessed as follows: low quality = 1 to 4; moderate quality = 5 to 8; high quality = 9 to 12.
2.5 Statistical analysis
All statistical analyses were performed using “meta” packages in R 4.0.0. As the selected articles all measured outcomes using HI, we used the mean difference (MD) and 95% confidence interval (CI) of continuous indicators. Heterogeneity was assessed using the Q test and the I2 statistic. The random-effects model was used if heterogeneity was statistically significant (P < .1 or I2 > 50%). Otherwise, the fixed-effects model was used. A sensitivity analysis was performed to identify studies that deviated from the overall results. Funnel plots, Begg's rank correlation tests, and Egger's linear regression were conducted to evaluate publication bias.
3 RESULTS
3.1 Study identification and selection
We initially retrieved 152 articles, and 138 articles remained after deduplication. After screening titles and abstracts, 21 articles remained. Ten articles did not provide detailed data. Thus, 11 articles were included in the analysis9-19 (Figure 1).
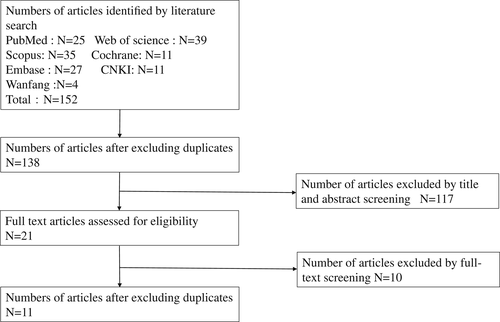
We summarised the characteristics of trials and performed the STAIR assessment (Table 1). The included studies originated from China, South Korea, and Turkey. The studies mainly used models of hypertrophic scarring in rabbit ears and hyperplastic wounds in mice. In addition, tumour-bearing models in nude mice were excluded because they failed to provide detailed data. There were eight high-quality articles and three medium-quality articles according to the STAIR checklist.
| Study | Year | Region | Type | Wound diameter (mm) | BTA dosage (U) | Observation time (days) | Outcome | STAIR |
|---|---|---|---|---|---|---|---|---|
| Na Zhou | 2020 | China | rabbit | 6 | 2 | 30,45 | HI, MVD, VEGF | 12 |
| Youjin Zhu | 2019 | China | rabbit | 7 | 2 | 60 | HI, collagen, fibroblast | 10 |
| Xueli Li | 2019 | China | rabbit | 10 | 3 | 60 | Thickness of scar, collagen, fibroblast | 10 |
| Jian Tao | 2018 | China | rabbit | 10 | 5 | 30 | HI, fibroblast | 8 |
| Dongqing Liu | 2018 | China | rabbit | 7 | 2 | 60 | HI, collagen, fibroblast | 8 |
| E. Çaliskan1 | 2016 | Turkey | rabbit | 8 | 2 | 60 | HI, fibroblast | 9 |
| Bingling Wang | 2013 | China | rabbit | 10 | 3 | 15,30,60 | HI, wound areas, wound epithelialisation time, VEGF, COX-2, Bcl-2,Bax | 12 |
| Zhibo Xiao | 2012 | China | rabbit | 8 | 5 | 90 | Thickness of scar | 7 |
| Dansheng Peng | 2010 | China | rabbit | 20 | 3 | 15,30,60 | HI, wound areas, wound epithelialisation time, a-sma | 12 |
| Byung-Joo Lee | 2009 | Korea | rat | 12 | 10 | 14,28,56 | Thickness of scar, wound areas, fibroblasts | 7 |
| Lin Wang | 2009 | China | rabbit | 10 | 5 | 30 | HI, wound epithelialisation time | 12 |
- Abbreviation: HI, hypertrophic index.
3.2 Comparison of HI
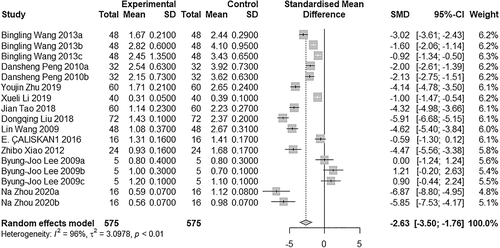
All 11 articles were included to compare HI between studies. We used the standardised MD (SMD) on account of the large gap between the research results. Heterogeneity was observed between studies (I2 = 96.1% > 50%, P < .01), so the random-effects model was adopted (SMD = −2.63, 95% CI: −3.50 to −1.76, P < 0.01; Figure 2). It is significantly different between the two groups, the combined results were located to the left of the invalid line. The postoperative effect of BTXA was significantly better compared with saline.
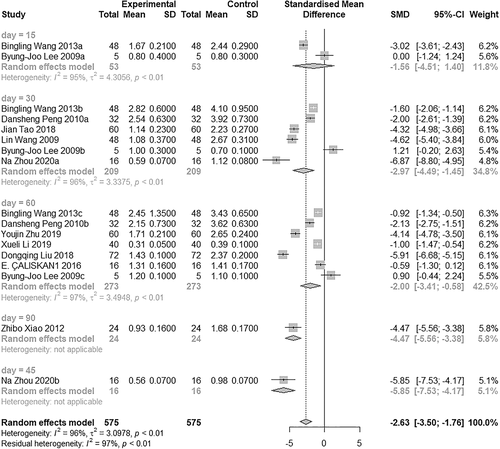
A subgroup analysis was conducted on the basis of sampling time. The results of each group were relatively stable. The HI in the BTXA group was lower compared with the control group. Due to the limited sample size, HI did not frequently change over time (Figure 3).
We conducted a sensitivity analysis to evaluate the influence of individual studies on the summary MDs and CIs. The results show that none of the individual studies influenced the corresponding MDs and CIs. This suggests that the results of our study were relatively stable and credible. Publication bias was assessed using funnel plots and quantified by Egger's test and Begg's test. The funnel plot was symmetrical, and Egger's test (P = .26) and Begg's test (P = .25) showed no publication bias.
3.3 Comparison of wound epithelialisation time
Three studies were incorporated in this comparison (Figure 4). There was heterogeneity between studies (I2 = 63% > 50%), so the random-effects model was used (SMD = 0.07, 95% CI: −0.33 to 0.48, P = .07). It is difficult to conclude whether BTXA prolonged or shortened wound healing time.

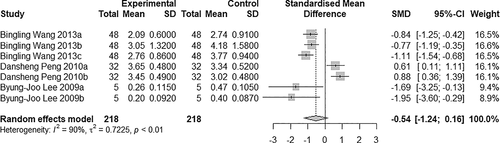
3.4 Comparison of wound area
Seven studies were included in this comparison (Figure 5). There was heterogeneity between studies (I2 = 90% > 50%; P < .05), so the random-effects model was used (SMD = −0.54, 95% CI: −1.24 to 0.16, P < .01). The results show that the wound area was significantly different between the two groups, and the wound area was smaller in the BTXA group compared with the saline group.
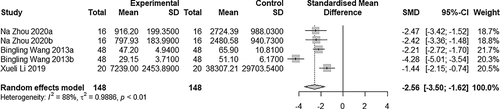
3.5 Comparison of VEGF expression
Five studies were included in the comparison (Figure 6). Heterogeneity was observed between studies (I2 = 88% > 50%; P < .05), and the random-effects model was adopted (SMD = −2.56, 95% CI: −3.50 to −1.62, P < .01). VEGF expression in the experimental group was significantly lower compared with the control group.
4 DISCUSSION
In 2000, Gassner et al first examined the effect of BTXA on the appearance of scarring in a primate model. They proved that BTXA can effectively improve the quality of scars. There is no clear conclusion on the effect of botulinum toxin on hypertrophic scarring and keloids in randomised controlled trials and clinical studies. Fanous compared BTXA with triamcinolone acetonide (TAC) and found that BTXA can effectively reduce the weight of keloids and relieve inflammation.20 A combination of local injections of TAC and BTXA had a similar effect on scar tissue compared with TAC alone. However, there was a significant effect on symptom control, such as control of pain and pruritus.21
BTXA has a positive effect on hypertrophic scars.16, 22, 23 Intralesional injection is the best option for hypertrophic scar treatment among the various available treatment options.24 Agents used for intralesional injection include TAC and 5-fluorouracil, which can be combined with silicon excipients and laser irradiation. One of the reasons BTXA is most popular among users of intralesional injections is that it has virtually no side effects, such as skin atrophy and telangiectasia, which are sometimes observed with steroid injections.25
Some studies show that BTXA has a significant effect on the prevention of postoperative scars.8, 26 Clinicians have achieved good prophylactic results with BTXA injection near the surgical incision after surgery. Laarakker reported that BTXA is used to salvage ischaemic hand injuries to avoid finger or limb amputation.27 However, some studies show the opposite conclusion with BTXA injection.28, 29 Therefore, more in-depth research is needed to confirm the role of BTXA.
This article examined previous studies using animal models to verify the role of BTXA in scar treatment, using HI or scar thickness as a morphological indicator of scar growth. Compared with the saline group, scars in the BTXA group were significantly less thick (SMD = −2.63, 95% CI: −3.50 to −1.76, P < .01). A subgroup analysis showed no regular changes in scars over time, which proved that the effect of BTXA in inhibiting scar hyperplasia is stable. In one of the included studies, scar hyperplasia in the BTXA group was more serious compared with the saline group. This may have occurred because the wound was too large or due to an excessive BTXA dose, which also occurs in clinical practice. A high concentration of BTXA (20 U/mL) inhibits angiogenesis, thereby affecting wound healing.30
We explored the effect of BTXA on wound healing, using epithelialisation time and wound area as measures. In this study, epithelialisation time was increased, but this was not significant. Since only three articles were included, more data are needed to support this observation. After BTXA injection, the wound area was reduced compared with the control group.
The mechanism of BTXA in the treatment of scars has not been clearly elucidated.31 BTXA is currently most widely recognised for its tension-reducing effects. Tension on the wound edge during healing is an important factor in determining the appearance of the final scar.32 Tension perpendicular to the edge of the wound mechanically pulls the wound muscles, impeding the normal healing process and leading to hypertrophic scarring and keloid formation.33, 34 BTXA inhibits acetylcholine secretion, blocking neuromuscular activation and causing muscle weakness. Botox is believed to reduce wound tension by preventing muscle contraction during the healing phase.35
Activation of the renin-angiotensin system is related to fibrosis of various organs.3 Compared with normal skin and scars, keloids and hypertrophic scars have more vascular tissue and occluded microvessels, a higher density of mesenchymal cells, more inflammatory cells and active fibroblasts, and thickened epidermis.36, 37 Topical application of angiotensin II stimulates angiogenesis and epidermal repair and may accelerate wound healing. Hikaru used angiotensin I receptor blockers to inhibit skin reepithelialisation, dermal repair, and angiogenesis to prevent wound healing in a rat model. Hedayatyanfard used losartan ointment to treat hypertrophic scars and keloids. Losartan caused a significant reduction in the vascular density of the lesion, and patients experienced significantly less itching around scar tissue. During blood vessel formation, endothelial cell migration and tube formation under the action of VEGF play an important role. In this study, we observed a significant decrease in VEGF expression after BTXA injection, suggesting that BTXA injection may inhibit scarring by reducing angiogenesis.
An excessive inflammatory response plays an important role in fibrosis.4 Increased expression of interleukin-6-based cytokines can promote extracellular matrix deposition and type I collagen content. It can also stimulate fibrotic mediators, including tumour growth factor (TGF)-β and tissue matrix metalloproteinase (TIMP) inhibitors to promote fibrosis.38 TGF-β isoforms 1 to 3 are related signal path. Matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs 1 to 4 (TIMPs) all affect scar formation.4, 31, 39, 40 However, we were unable to conduct a detailed analysis due to insufficient data in the literature.
Botulinum toxin is also used in combination with TAC,21, 41 5-fluorouracil,42 mesenchymal stem cells,43 and laser therapy.23 It has achieved good results, and serious side effects are rare. It can effectively relieve symptoms such as pain and itching, by using other treatment methods alone.
This study has some limitations that should be noted. First, at present, the most commonly used animal models of scar are the rabbit ear or rat back wound models. Some scholars propose a rat tail scar model,44 but further application lacks practical verification. Second, the number of included studies and the sample sizes were small. Third, no significant differences in the time of epithelialisation were observed between the BTXA and control groups. Fourth, there is a lack of uniform measurement methods to examine the expression of certain cytokines and fibroblasts, so this could not be analysed. Finally, wound area, as well as the products and doses of BTXA, differed between studies. Injections were used at the edge of the wound, with each injection point spaced 1 cm apart. Different BTXA products have different efficacies at the same doses; thus, dosage equivalence is a noteworthy issue.45, 46 The diffusion coefficient between different BTXA products and between different doses of each product may be different.47, 48
5 CONCLUSIONS
We conclude that BTXA is safe and effective in preventing and treating scar hypertrophy in animal models. Due to a lack of standardised treatment standards, the optimal time and dose of BTXA injection are worthy of further exploration by clinicians, but excessive doses of BTXA and treatment of large wounds should be avoided.
ACKNOWLEDGEMENTS
The present study was supported by the National Natural Foundation of China (grant no. 81971842) and the Jilin Scientific and Technological Development Program (grant no. 20180414038).
CONFLICT OF INTEREST
The authors declare no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
Data derived from public domain resources




