Retrospective real-world comparative effectiveness of ovine forestomach matrix and collagen/ORC in the treatment of diabetic foot ulcers
Funding information: Aroa Biosurgery Limited and Callaghan Innovation Limited (New Zealand) Growth, Grant/Award Number: MSMA1402
Abstract
The retrospective pragmatic real-world data (RWD) study compared the healing outcomes of diabetic foot ulcers (DFUs) treated with either ovine forestomach matrix (OFM) (n = 1150) or collagen/oxidised regenerated cellulose (ORC) (n = 1072) in out-patient wound care centres. Median time to wound closure was significantly (P = .0015) faster in the OFM group (14.6 ± 0.5 weeks) relative to the collagen/ORC group (16.4 ± 0.7). A sub-group analysis was performed to understand the relative efficacy in DFUs requiring longer periods of treatment and showed that DFUs treated with OFM healed up to 5.3 weeks faster in these challenging wounds. The percentage of wounds closed at 36 weeks was significantly improved in OFM treated DFUs relative to the collagen/ORC. A Cox proportional hazards analysis showed OFM-treated wounds had a 18% greater probability of healing versus wounds managed with collagen/ORC, and the probability increased to 21% when the analysis was adjusted for multiple variables. This study represents the first large retrospective RWD analysis comparing OFM and collagen/ORC and supports the clinical efficacy of OFM in the treatment of DFUs.
1 INTRODUCTION
The successful treatment of diabetic foot ulcers (DFUs) presents multiple challenges for clinicians and incurs a significant psychosocial toll on afflicted patients and their families. In addition to the negative impact on quality of life (QoL) measures, DFUs increase a patient's risk for infection,1 hospitalisation,2 and amputation.3 Current estimates demonstrate one in six patients with a DFU will undergo an amputation, making DFUs the leading cause of nontraumatic amputations in the United States (US).4 Additionally, there are significant financial burdens incurred by DFUs. A 2012 retrospective study of 7099 DFUs reported a mean cost to achieve closure of $3927 per DFUs.5The DFU related cost and burden to the US health care system has been estimated at $9 to 13 billion.3, 6 These factors, coupled with the increasing global incidence of adult type-2 diabetics, presents a significant unmet need in modern healthcare for readily accessible, affordable, and effective interventions for the treatment of DFUs.
Technical and procedural developments in modern wound care have produced numerous advances in the clinical management of DFUs. In parallel, the design of clinical studies to demonstrate therapeutic efficacy has evolved. Real-world data (RWD), as used routinely in other clinical specialties, is a recognised and validated methodology to support real-world evidence.7, 8 Meticulously designed and well-controlled prospective randomised control trials (RCTs), by definition, may not accurately reflect the real-world challenges that clinicians encounter when treating DFUs. For example, a review of 283 published RCTs found that individuals with co-morbidities common in the general population were excluded from 81.3% (n = 230) of RCTs.9 Cohort studies utilising real-world registry data can provide a more compelling and insightful perspective, while minimising bias as compared with RCTs. This is particularly evident in wound care studies where patient variability can be relatively large and RCTs would otherwise exclude patients commonly encountered in the typical Wound Care Centre (WCC). When comparing real-world patient cohorts to RCT cohorts, Fife et al. found that the initial wound area of DFUs selected for RCT studies were three times smaller as compared with the general real-world population.10 Additionally, the severity of DFUs included in RCTs was not reflective of the typical clinical practice, with 43.6% of real-world DFUs being Wagner 3 or higher, whereas many RCTs only included Wagner 1 and 2.10 With the exclusion criteria set by RCT's, it is estimated that these types of studies accurately represent only 4% of the real-world wound population.11 By matching key patient variables across cohort groups, RWD studies may offer clarity regarding the efficacy of specific treatment modalities, enabling meaningful evaluation across significantly larger sample sizes.
For many decades, reconstituted collagen wound dressings have been a commonly used treatment modality for acute and chronic wounds.12 These traditional technologies are comprised of collagen, isolated from animal tissues (including tendon and hide), using denaturing processes to fully or partially solubilise the collagen, with subsequent downstream fabrication into collagen foams. Many types of commercially available collagen wound dressings are available, including products comprising 100% reconstituted collagen (e.g., Puracol, Medline Industries), or collagen formulated with natural or synthetic polymers (e.g., oxidised regenerated collagen (ORC), Promogran, 3 M/KCI; carboxymethycellulose, Biostep, Smith and Nephew; or alginate, e.g., Cutimed Epiona, BSN Medical). These reconstituted collagen dressings contain types I and III collagen that rehydrate to a gelatinous form creating a moist environment in the wound bed. More traditional collagen-based dressings have been super-seeded by advanced bioscaffold technologies that support cellular infiltration, migration, and proliferation, providing biological ques to assist in tissue regeneration. One approach in the development of these technologies uses a subtractive manufacturing approach. Starting with a suitable source tissue, cellular components are selectively removed leaving only the proteinaceous tissue extracellular matrix (ECM). These “decellularized extracellular matrix” (dECM)-based technologies retain the structure and composition of soft tissue ECM and have been prepared from numerous source tissues including bovine, equine, porcine, piscine, and cadaveric.13 Being naturally derived from intact source tissue, dECM products are largely composed of collagen types I and III, but importantly also preserve and contain a diverse array of secondary proteins, polysaccharides, and proteoglycans that are known to play an important role in soft tissue repair through contribution to the milieu of wound healing.14
dECM-based products for wound care have largely remained inaccessible because of cost, prescribing habits and insurance coverage, and are therefore typically utilised as a “last resort” in modern wound care, being available only as “cellular or tissue-based product” (CTP, or “skin substitutes”). Ovine forestomach matrix (OFM) (Endoform Natural, Aroa Biosurgery), is the first dECM technology to be made widely accessible to wound care professionals, enabling increased accessibility and adoption into clinical practice. OFM is derived from ovine forestomach tissue using processes optimised to remove ovine cells while maintaining the structure and composition of the tissue ECM.15 OFM contains more than 150 different proteins, including elastin, fibronectin, glycosaminoglycans and various growth factors, such as vascular endothelial growth factor (VEGF) and platelet derived growth factor (PDGF).16 OFM has been shown to recruit mesenchymal stromal cells,17 stimulate cell proliferation,18 angiogenesis and vascularogenesis,18 while modulating wound proteases.19
Of the previously mentioned reconstituted collagen dressings, one of the most utilised and studied is the product, collagen/ORC (Promogran, 3 M/KCI). OFM and collagen/ORC are both available for first-line management of wounds. For example, both products are reimbursed in the US as A-code surgical dressings enabling immediate prescription, facilitating incorporation immediately in addition to standard of care (SOC) methods. These two wound care products have similar costs, clinical indications, application techniques, and are used to address wound chronicity (via modulation of wound proteases), support granulation tissue, and advance wound closure in complex soft tissue defects. Our goal was to undertake a retrospective analysis of RWD comparing the relative efficacy of OFM (dECM technology) versus the traditional reconstituted collagen dressing collagen/ORC in the treatment and outcomes of DFUs.
2 MATERIALS AND METHODS
This study was conducted in accordance with the Declaration of Helsinki. The study protocol was reviewed by the independent Institutional Review Board (IRB) (Advarra Institutional Review Board Services, MD, USA). The IRB concluded that the study was exempt from IRB approval as the study was retrospective and utilised de-identified wound data.
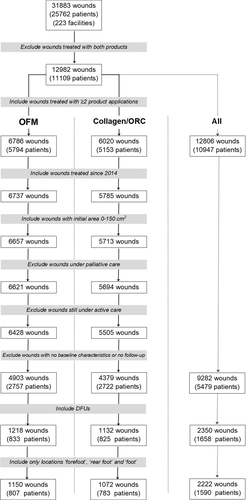
Data were extracted from the Net Health Wound Care (formerly “Wound Expert”) database (NetHealth, Pittsburgh, PA) during the period of January 1, 2014, to June 302 020, representing 449 WWCs across the United States. These are out-patient WCCs that are typically associated with a hospital system and receive patient referrals for specialised care in the management of complex wounds across a spectrum of etiologies and patient demographics. Wounds still under active management at the date of data acquisition were excluded from the study. Data were extracted from a pool of 31 883 wounds (25 762 patients) and filtered based on the inclusion and exclusion criteria represented in Figure 1 and Table 1. The data represented unique visits and associated treatments at the WCC only. Wounds with no baseline characteristics were excluded from the study, as well as wounds that included baseline characteristics but had no follow-up data. Wounds were further filtered based on DFU location and marked “forefoot”, “rear foot” or generically 'foot' in the absence of definitive anatomical location. Sequential treatment with either of the products was not specifically assessed in the study; for example, wounds receiving weekly treatment with OFM or collagen/ORC until closure were treated identically to wounds that may have only been treated for a period, then treatment ceased. All wounds were included in the study, including those that had been managed with other advanced therapies; hyperbaric oxygen (HOBT), negative pressure wound therapy (NPWT) and advanced biologic dressings (e.g., CTPs). All patients were assumed to have had proper offloading (TCC, nonweightbearing, padding) and workup and management of their underlying comorbidities.
| Inclusion | Exclusion |
|---|---|
|
|
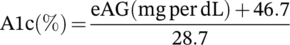
The number of WCC applications were calculated for all DFUs, as well as for DFUs receiving ≥4, ≥8 and ≥12 applications of either product in the WCC. These data were summarised using mean (SD) and median compared using nonparametric Mann-Whitney U test.
Time to closure was defined as the period between the first application of either product and subsequent wound closure, where closure was defined as a wound area of <0.25 cm2 or where wounds had been marked as “closed”, “healed” or “resolved” in the final reporting. The median time to wound closure and the percentage of wounds closed at 12, 24, and 36 weeks were estimated using the Kaplan-Meier method. The percentage of DFUs closed was statistically compared between treatment groups using Greenwood's standard error estimates.
Time to wound closure between the treatment groups was compared using Cox Proportional Hazards (CPH) regression analysis with the comparison summarised as the hazard's ratio (HR) with 95% confidence interval (CI). Adjusted analyses of the time to wound closure were undertaken using CPH regression to compare treatment groups, incorporating age, gender, initial wound size, wound type, and duration of wound as covariates in the model. Adjusted HRs for the treatment group comparison were estimated from these models for the total sample. All analyses performed by using SPSS v26 and a two-tailed P-value ≤.05 were taken to indicate statistical significance.
3 RESULTS
3.1 Patient demographics
The study followed a pragmatic design, with relatively open inclusion and exclusion criteria. Exclusion criteria consisted of DFUs managed with both products, those still under active management at the time of data acquisition, patients under palliative treatment and wounds with either no baseline characteristics or alternatively no follow-up (Figure 1 and Table 1). Only wounds that received 2 or more WCC treatments with either product, wounds treated since 2014 and wounds with an initial area of 0 to 150 cm2 were included in the study. A relatively large initial wound area (0-150 cm2) was included as all wounds were subsequently filtered and verified to ensure only DFUs were included, and wounds had an appropriate anatomic location (e.g., forefoot, foot, or rear foot). Of the initial wound records (n = 31 883), 25 762 patients were filtered (Figure 1) to yield final datasets for the two cohorts for OFM and collagen/ORC of n = 1150 (n = 807 patients) and n = 1072 (n = 783 patients), respectively, that met the study inclusion and exclusion criteria. This represented a total of n = 2222 wounds from n = 1590 patients. Patient demographics for both cohorts are presented in Table 2. The gender mix for the OFM treatment group was not significantly different (P = 0.137). OFM treated patients were similar in age (P = 0.725) to the collagen/ORC cohort (61.8 ± 12.9 and 62.0 ± 13.0, respectively). Haemoglobin A1c, estimated from the reported patient glucose concentrations (mg/dL) were equivalent between the OFM and collagen/ORC cohorts (7.2 ± 3.4% and 7.3 ± 3.5%, P = .930).
| OFM | Collagen/ORC | P value | Total | |
|---|---|---|---|---|
| Patients, n | 807 | 783 | 1590 | |
| Patients, gender specified, n | 805 | 778 | 1583 | |
| Male, n (%) | 580 (72.0%) | 534 (68.6%) | .137 | 1114 (70.4%) |
| Female, n (%) | 225 (28.0%) | 244 (31.4%) | 469 (29.6% | |
| Gender NS, n (%) | 2 (0.2%) | 5 (0.6%) | 7 (0.4%) | |
| Patients, age specified, n | 800 | 708 | 1508 | |
| Mean ± SD (years) | 61.8 ± 12.9 | 62.0 ± 13.0 | .725 | 61.9 ± 12.9 |
| Median (years) | 62.0 | 63.0 | 62.0 | |
| Age NS, n (%) | 7 (0.9%) | 75 (9.6%) | 82 (5.2%) | |
| Patients, glucose specified, n | 562 | 459 | 1021 | |
| A1c, mean ± SD | 7.2 ± 3.4% | 7.3 ± 3.5% | .930 | 7.3 ± 3.4% |
| A1c, median | 7.0% | 6.9% | 6.9% | |
| A1c NS, n (%) | 245 (30.4%) | 324 (41.4%) | 569 (35.8%) |
- Abbreviations: n, sample size; NS, not specified; SD, standard deviation.
3.2 Baseline wound characteristics
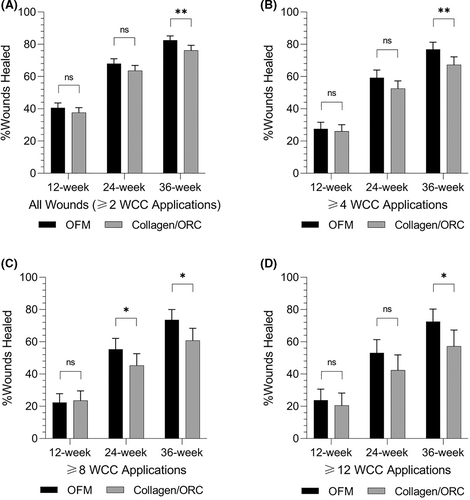
Baseline wound characteristics for the two cohorts are presented in Table 3. Total DFUs (n = 2222) included in the study and receiving ≥2 WCC applications of either product and consisted of 1150 DFUs treated with OFM and 1072 DFUs treated with collagen/ORC. Mean baseline wound areas for the OFM cohort (2.0 ± 5.5 cm2) were statistically larger (P = .013) than the collagen/ORC cohort (1.5 ± 3.8 cm2), but wounds in both cohorts were of comparable age (15.8 ± 41.7 and 14.5 ± 41.3 weeks, respectively) (P = .471). There was no difference in the number of wounds per patient between OFM and collagen/ORC (1.4 ± 0.9 and 1.4 ± 0.8 wounds per patient, respectively). Total wounds could be further segmented based on the number of product applications occurring at the WCC (Table 3), into sub-groups of ≥4, ≥8 and ≥12 WCC applications. Sub-group analyses were undertaken in order to assess the relative efficacy of the products for DFUs that were more challenging to close, hence requiring more visits to the WCC for product application. The smallest sample size for the sub-group analyses were DFUs that received ≥12 WCC product applications with n = 155 and n = 110 DFUs for the OFM and collagen/ORC cohorts, respectively. Comparing the location of the wounds showed a similar distribution between the cohorts, with the majority of DFUs being reported in the forefoot and foot locations.
| OFM | Collagen/ORC | P value | Total | |
|---|---|---|---|---|
| Baseline wound characteristics (All wounds, ≥2 WCC applications) | ||||
| Number of wounds (n) | 1150 | 1072 | 2222 | |
| Mean wound area ± SD (cm2) | 2.0 ± 5.5 | 1.5 ± 3.8 | .013 | 1.7 ± 4.7 |
| Median wound area (cm2) | 0.6 | 0.5 | 0.6 | |
| Mean wound age ± SD (weeks) | 15.8 ± 41.7 | 14.5 ± 41.3 | .471 | 15.2 ± 41.5 |
| Median wound age (weeks) | 3.9 | 4.4 | 4.1 | |
| Mean wounds per patient ±SD | 1.4 ± 0.9 | 1.4 ± 0.8 | .077 | 1.4 ± 0.9 |
| Median wounds per patient | 1.0 | 1.0 | 1.0 | |
| Wounds by location (All wounds, ≥2 WCC applications) | ||||
| Forefoot (%) | 505 (43.9%) | 535 (49.9%) | 1040 (46.8%) | |
| Rear foot (%) | 163 (14.2%) | 125 (11.7%) | 288 (13.0%) | |
| Foot (%) | 482 (41.9%) | 412 (38.4%) | 894 (40.2%) | |
| Wounds by WCC visit number sub-group analysis | ||||
| All wounds (≥2 WCC applications) | 1150 | 1072 | 2222 | |
| ≥4 WCC applications | 494 | 475 | 969 | |
| ≥8 WCC applications | 244 | 197 | 441 | |
| ≥12 WCC applications | 155 | 110 | 265 | |
- Abbreviations: n, sample size; SD, standard deviation.
3.3 Median healing time
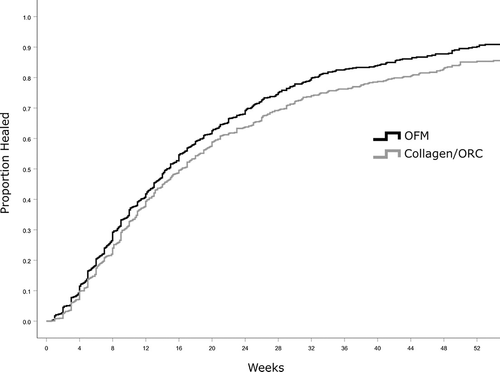
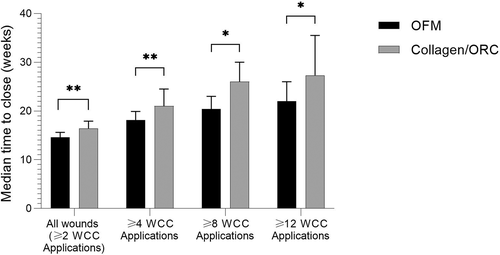
Kaplan-Meir survival curves were generated based on the time to close for wounds in each treatment cohort (Figure 2). Median time to heal was determined based on the Kaplan-Meier method for all wounds receiving ≥2 WCC product applications, and separately sub-group analyses for wounds receiving ≥4, ≥8 or ≥12 WCC applications of either product. Median time to close for all wounds (≥2 WCC product applications) in the OFM cohort were significantly shorter (P = .0015) than wounds receiving collagen/ORC, 14.6 ± 0.5 weeks and 16.4 ± 0.7 weeks, respectively (Table 4, Figure 3). This represented a difference in median time to close of 1.9 weeks, or a 11.3% reduction relative to collagen/ORC (Table 4). As expected, as the number of WCC visits and product applications increased, the median time to closure increased for both cohorts as wounds required more weeks of intervention to heal. Additionally, as the number of WCC visits and product applications increased the difference in median time to close between the two cohorts increased. For example, the sub-group that received ≥8 WCC applications of OFM healed 5.6 weeks faster than the collagen/ORC sub-group (20.4 ± 1.3 weeks vs 26.0 ± 2.1, P = .0118). For the more challenging wounds captured in the sub-groups receiving ≥8 or ≥12 WCC applications, the median time to close for OFM treated DFUs was reduced by ~20% relative to collagen/ORC.
| OFM | Collagen/ORC | Difference | P value | Overall | |
|---|---|---|---|---|---|
| Median time to close (weeks ± standard error) | |||||
| All wounds (≥2 WCC Applications) | 14.6 ± 0.5 | 16.4 ± 0.7 | 1.9 (11.3%) | .0015 | 15.3 ± 0.4 |
| ≥4 WCC applications | 18.1 ± 0.9 | 21.0 ± 1.8 | 2.9 (13.6%) | .0040 | 19.9 ± 0.9 |
| ≥8 WCC applications | 20.4 ± 1.3 | 26.0 ± 2.1 | 5.6 (21.4%) | .0118 | 23.0 ± 1.3 |
| ≥12 WCC applications | 22.0 ± 2.1 | 27.3 ± 4.2 | 5.3 (19.4%) | .0355 | 24.0 ± 2.0 |
| Percentage of wounds closed, 12 weeks [95% CI] | |||||
| All wounds (≥2 WCC applications) | 40.6%[37.7%, 43.6%] | 37.6%[34.6%, 40.7%] | .1695 | ||
| ≥4 WCC applications | 27.5%[23.4%, 31.6%] | 26.0%[21.9%, 30.1%] | .6093 | ||
| ≥8 WCC applications | 22.4%[17.1%, 27.8%] | 23.5%[17.5%, 29.5%] | .8011 | ||
| ≥12 WCC applications | 23.8%[17.0%, 30.6%] | 20.5%[12.9%, 28.2%] | .5272 | ||
| Percentage of wounds closed, 24 weeks [95% CI] | |||||
| All wounds (≥2 WCC applications) | 68.0%[64.9%, 71.0%] | 63.6%[60.3%, 66.9%] | .0571 | ||
| ≥4 WCC applications | 59.3%[54.5%, 64.0%] | 52.5%[47.6%, 57.3%] | .0500 | ||
| ≥8 WCC applications | 55.4%[48.7%, 62.1%] | 45.3%[38.0%, 52.6%] | .0468 | ||
| ≥12 WCC applications | 53.1%[44.8%, 61.4%] | 42.4%[32.8%, 51.9%] | .0961 | ||
| Percentage of wounds closed, 36 weeks [95% CI] | |||||
| All wounds (≥2 WCC applications) | 82.5%[79.8%, 85.2%] | 76.2%[73.1%, 79.4%] | .0033 | ||
| ≥4 WCC applications | 76.9%[72.5%, 81.3%] | 67.3%[62.4%, 72.2%] | .0046 | ||
| ≥8 WCC applications | 73.6%[67.3%, 80.0%] | 60.8%[53.2%, 68.4%] | .0113 | ||
| ≥12 WCC applications | 72.5%[64.7%, 80.3%] | 57.2%[47.0%, 67.3%] | .0191 | ||
3.4 Percentage of wounds closed
The percentage of wounds closed was estimated using the Kaplan-Meir method (Figure 2). The percentage of wounds closed were increased in the OFM cohort at 12-, 24-, and 36-weeks (Figure 4 and Table 4), and these differences were significant at 36 weeks. For example, where wounds received ≥12 WCC applications, 72.5% of OFM treated wounds were closed at 36 weeks versus 57.2% of the collagen/ORC wounds (P = .0191).
3.5 Cox proportional hazard (CPH) analysis
The time to wound closure were further analysed using CPH regression to compare treatment groups and to identify differences as the number of WCC visits and product applications increased. Without adjustment, the OFM cohort had a 18% greater probability of wound closure compared with the collagen/ORC cohort (P = 0.001) (Figure 5 and Table 5). When the adjusted CPH model incorporated age, gender, initial wound size and wound age the adjusted hazards ratios represented a 21% greater probability of wound closure for the OFM cohort (P = .001). When sub-divided by number of WCC applications the OFM treatment significantly increased the probability of wound closure by up to 36% and 38% in the unadjusted and adjusted models (Figure 5 and Table 5).
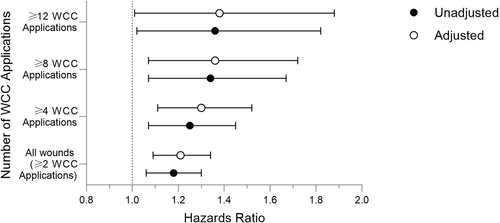
| Unadjusted | P value | Adjusted | P value | |
|---|---|---|---|---|
| All wounds (≥2 WCC applications) | 1.18[1.06, 1.30] | .001 | 1.21[1.09, 1.34] | .0004 |
| ≥4 WCC applications | 1.25[1.07, 1.45] | .004 | 1.30 [1.11, 1.52] | .001 |
| ≥8 WCC applications | 1.34[1.07, 1.67] | .012 | 1.36[1.07, 1.72] | .012 |
| ≥12 WCC applications | 1.36[1.02, 1.82] | .036 | 1.38[1.01, 1.88] | .045 |
- Note: Hazard ratios [95% CI].
3.6 Number of product applications
The mean number of WCC reported product applications for all wounds (≥2 WCC applications) were 6.8 ± 16.3 and 5.5 ± 8.6 applications for OFM and collagen/ORC cohorts, respectively (Table 6). The number of product applications was further analysed based on the sub-groups ≥4, ≥8, and ≥12 WCC applications. There were no significant differences between the cohorts with respect to the mean product applications, except for the sub-group receiving ≥4 WCC applications, where the mean number of OFM applications (13.4 ± 23.2 applications) was higher than the collagen/ORC sub-group (10.1 ± 11.3 applications) (P = .019).
| OFM | Collagen/ORC | P value | Overall | |
|---|---|---|---|---|
| All wounds (≥2 WCC applications) | ||||
| Mean ± SD | 6.8 ± 16.3 | 5.5 ± 8.6 | .257 | 6.2 ± 13.1 |
| Median | 3.0 | 3.0 | 3.0 | |
| ≥4 WCC applications | ||||
| Mean ± SD | 13.4 ± 23.2 | 10.1 ± 11.3 | .019 | 11.8 ± 18.4 |
| Median | 7.0 | 6.0 | 7.0 | |
| ≥8 WCC applications | ||||
| Mean ± SD | 22.0 ± 30.8 | 17.2 ± 14.8 | .059 | 19.9 ± 25.0 |
| Median | 14.0 | 12.0 | 13.0 | |
| ≥12 WCC applications | ||||
| Mean ± SD | 29.5 ± 36.6 | 23.4 ± 17.5 | .073 | 30.3 ± 19.0 |
| Median | 20.0 | 17.5 | 19.0 | |
4 DISCUSSION
In this retrospective RWD study, 2222 DFUs were sourced from 223 facilities and 1508 patients (Figure 1). The primary study outcome was median time to wound closure; DFUs treated with OFM closed significantly faster (14.6 ± 0.5 weeks) compared with the collagen/ORC cohort (16.4 ± 0.7 weeks), a difference of 1.9 weeks. As the number of WCC product applications increased the difference between the median time to closure increased. For example, the median time to closure of the OFM sub-group receiving ≥8 WCC applications was 5.6 weeks shorter than the collagen/ORC sub-group (Table 4). OFM treated DFUs had a higher percentage of wound closure at 12-, 24- and 36-weeks (Table 4), that was statistically significant at 36-weeks, and an increased the probability of healing by up to 38% (Table 5). A previous published RCT comparing the efficacy of collagen/ORC to SOC in the treatment of DFUs reported a 12-week incidence of healing of 37.0%, consistent with our findings (37.6%, Table 4).20 It is interesting to also compare these findings to the large retrospective analysis of RWD taken from the US Wound Registry that determined the percentage of DFUs closed at 12 weeks using SOC alone was ~30%.10
The data captured by the EMR reflect only those visits to the outpatient WCC, and as such, wound parameters (e.g., area) and associated treatments at interim dressing changes outside the WCC (e.g., home health) are not reflected. While this limitation does not necessarily impact the overall reporting of the healing outcomes (e.g., median time to close, percentage of wounds closed), product applications are only reported for the WCC visits. This is especially important when considering the number of product applications. Across all DFUs, the mean number of product applications for OFM and collagen/ORC were 6.8 ± 16.3 and 5.5 ± 8.6, respectively, with a median of 3.0 product applications for both cohorts (Table 6). However, this underestimates the actual product usage for the collagen/ORC group. Collagen/ORC becomes a gel in the wound bed, and as such must be re-applied every 2 days, or daily in the case of moderately exudating wounds.21 Thus, actual clinical usage of collagen/ORC are likely to be up to seven times greater than the application rates reported in Table 6. In comparison, OFM remains in the wound bed for up to 7 days depending on the chronicity of the wound and associated concentrations of wound proteases. Several studies have described tailoring the re-application of OFM to match wound chronicity, typically starting treatment with twice weekly re-application for the first 2 to 4 weeks, then reducing the re-application frequency to weekly as wound chronicity is corrected.22, 23 As WCC visits typically occur weekly, the OFM application rates presented in Table 6 are expected to approximate actual clinical usage.
There is a growing body of evidence to support the use of OFM in healing a variety of wound types.22-28 However, to the authors' knowledge there has not been a large, retrospective RWD study of OFM making comparisons to a reconstituted collagen product. In this instance we selected collagen/ORC for the comparison given the products long-established use in wound care. A related study used RWD to compare the efficacy of OFM to collagen/ORC/silver.29 However, it is difficult to compare findings from this study with our own, as most notably, the study compared OFM to a silver-based antimicrobial collagen dressing. The antimicrobial properties of silver are well known in wound care and, further, the detrimental impact of bacterial contamination and biofilm on wound healing is well understood.30-32 As such, it is difficult to draw any conclusions from a study comparing two products with different mechanisms of action. Additionally, the study did not assess changes in wound area or wound closure, but instead utilised non-traditional outcome measures.29
The argument that all collagen-based devices are not the same has been well documented and highlights differences between the semi-synthetic reconstituted collagen type products and dECM bioscaffolds.33-35 While both OFM and collagen/ORC are readily accessible for modern wound care, there are significant differences in composition. OFM contains over 150 different proteins that naturally occur in tissue ECM, including a variety of growth factors,16 while collagen/ORC comprises only oxidised regenerated cellulose (ORC) and 55% type I bovine reconstituted collagen.36 These compositional differences have been demonstrated to result in measurable differential performance outcomes. Comparative in vitro testing of OFM has demonstrated greater cellular bioactivity,18 more potent inhibition of relevant wound proteases19 and greater retention of native matrix structure,37 relative to collagen/ORC.
As a dECM, OFM represents a newer class of advanced wound care technology that have otherwise had limited clinical adoption because of financial barriers. The main feature of these tissue derived products, also termed CTPs, is the preservation and inclusion of secondary ECM proteins, particularly growth factors and additional signalling molecules that aid and promote healing. As the only dECM technology that is widely available for the day-to-day management of acute and chronic wounds, OFM represents a step change in the accessibility of these types of technologies to a wider patient population. For example, OFM and collagen/ORC are similarly priced at $USD 8-12/unit, while alternate dECM products, available as CTPs command prices of up to $1000 to $2000/unit. Where CTPs have traditionally only been used in wounds that have failed to respond after 4 to 8 weeks of SOC treatment, OFM can be initiated much earlier in the continuum and integrated into SOC. An additional benefit of being an “A-code” surgical dressing is the ability for OFM to be prescribed for US patients through a Durable Medical Equipment (DME). Globally, given the ease of application, the product can be applied by patients, caregivers, or home health care providers. The “proactive and early, aggressive”' utilisation of OFM, along with optimal wound bed preparation and secondary treatment modalities to disrupt the pathophysiology of chronic wounds was first proposed by Bohn et al38 as a protocol to improve wound closure rates. In the pragmatic design of this study, we have been deliberate in not defining inclusion/exclusion criteria to preclude DFU managed with additional advanced wound therapies, for example, hyperbaric oxygen therapy, negative pressure wound therapy, and CTPs. This approach was taken so as to not imply that OFM could replace any of these advanced therapies, but rather serves as a synergistic adjunctive therapeutic option in the armament of wound care professionals. For example, Ferreras et al demonstrated that while upfront management with OFM to correct wound chronicity reduced CTP usage, importantly, this approach also improved overall healing outcomes when CTPs were utilised later in the continuum.28
Our findings demonstrate that OFM can reduce the time of DFU closure by up to ~20% relative to collagen/ORC. This outcome has significant implications for patient QoL, but it is also valuable to consider the financial implications for other key stakeholders across the continuum of care. For patients, this represents a direct savings of up to ~20% incurred for any out-of-pocket expenses relating to their treatment including insurance co-payments, loss of income because of time off work or additional treatment related costs (e.g., transportation expenses).39 One consistent finding from the literature is that the main contributor to the overall cost to wound closure is professional time related to dressing changes, while material costs (e.g. primary and secondary dressings, saline, offloading pads) comprise ~10% of total cost.5, 40, 41 Thus, a significant reduction in the median time to wound closure could have direct positive impacts for payors, private insurers, and/or government agencies by reducing the total cost to wound closure. It is also important to consider those sites of care (e.g., Home Health Agencies) that receive a bundled payment for each episode of care. In these instances, reducing the time to wound closure by ~20%, or increasing the probability of closure by up to 38%, improves the likelihood that expenses incurred during wound treatment will not exceed the payment cap. In an era of increasing pressure on healthcare systems to remain financially viable, reducing the time to wound closure improves efficiency and productivity by increasing the number of new patient encounters, while maintaining resource allocation near neutral. New patient encounters have a positive impact on the financial health of a facility by enabling downstream revenue associated with new consultations. For example, new consultations undergo initial evaluation and management (E/M), wound debridement as well as advanced interventions, for instance diagnostic and interventional arterial and venous procedures or HOBT.
4.1 Limitations
By undertaking a large retrospective RWD study, we have been able to compare the relative efficacy of OFM and collagen/ORC across a significantly large number of wounds representing a complex patient demographic using previously defined recommendations.42, 43 As with other RWD studies, a limitation is that EMR databases are not typically developed for retrospective research purposes so there is inherent variability in the day-to-day documentation practices. Uniform data reporting is not typically monitored leading to the potential for inconsistent or “Missing Completely at Random” (MCAR) data. In this study, MCAR data would include fasting glucose readings, plantar or dorsal wound location, use of total contact casts (TCCs), adjunctive vascular intervention, and patient demographics (e.g., age, gender). One approach to potentially control for MCAR and other RWD reporting variability is to use matched-cohorts whereby treatment groups are carefully selected to identify two statistically equivalent cohorts. In this current study we instead used a pragmatic approach to derive two cohorts that were essentially equivalent based on demographics and baseline wound characteristics. Rather than using matched cohorts, adjusted CPH analysis using key variables (e.g., wound age, wound size) were used to offset any differences between the two cohorts. RWD analysis also assumes that each patient had a proper diagnostic workup and any intervention to optimise healing. This limitation though is assumed to be controlled for as there are multiple guidelines on the recommendations for revascularization requirements, offloading, treatment of infections, metabolic control, and local ulcer care.44, 45
5 CONCLUDING REMARKS
This retrospective RWD analysis demonstrates that the use of OFM reduced the median time to closure, increased the percentage of wounds closed, and increased the probability of closure relative to wounds managed with collagen/ORC. Differences between OFM and collagen/ORC were most apparent for wounds that required a greater number of WCC visits. This RWD study further substantiates the growing body of evidence to support the use of OFM as a first-line intervention to improve wound closure rates.
ACKNOWLEDGEMENTS
This work was supported by Aroa Biosurgery Limited and Callaghan Innovation Limited (New Zealand) Growth Grant (MSMA1402).
CONFLICT OF INTEREST
BB, SGD and BCHM are employees of Aroa Biosurgery Limited (Auckland, New Zealand). BCHM is a shareholder of Aroa Biosurgery Limited. TM is an employee of Net Health (Pittsburgh, Pennsylvania). AEC, GAB, KW, BDL, MMM have received educational grants from Aroa Biosurgery Limited. CF, CDL and TM provided consulting services to Aroa Biosurgery Limited.
Open Research
DATA AVAILABILITY STATEMENT
Data subject to third party restrictions. The data that support the findings of this study are available from Net Health. Restrictions apply to the availability of these data, which were used under license for this study. Data are available Net Health with the permission of Net Health.




