Chaetal loss and replacement in Pseudopotamilla reniformis (Sabellida, Annelida)
Abstract
Chaetae are continually being replaced during the life of a polychaete, but the process of chaetal degeneration during replacement is still poorly known. Chaetal loss occurs either one at a time through special degenerative sites, or all chaetae in a chaetal sac are lost simultaneously in the absence of degenerative sites. Old chaetae can be either resorbed inside the coelom or shed. This study describes chaetal degradation, loss, and subsequent replacement during experimentally induced regeneration in the sabellid polychaete Pseudopotamilla reniformis. During post-traumatic regeneration in P. reniformis, abdominal-type chaetae fall from the chaetal sac simultaneously without degenerative sites, and are replaced by thoracic-type chaetae. Chaetal sac degradation occurs in three main stages: (1) formation of numerous lysosomes and small electron-transparent vesicles in follicle cell cytoplasm; (2) disintegration of follicular cell membranes; and (3) disintegration of follicular cell cytoplasm. Description of changes during parapodial transformation allows identification of signs of degradation that can serve as histological indicators of chaetal degeneration in a wide range of polychaetes. Our study suggests that chaetal degeneration and loss in polychaetes can occur either with degenerative sites or without them, depending on the type of chaetal replacement.
Chaetae, epidermal extracellular structures that lent their name to the now-defunct taxon Polychaeta, play an important role in life of these annelid worms (Woodin & Merz 1987). Chaetal morphology and functions are extremely variable: in different species, chaetae are used for defense, crawling, swimming, burrowing, and body anchoring within tubes (Woodin & Merz 1987; Fitzhugh 1991; Merz & Woodin 2006). In spite of the functional importance of chaetae, many aspects of chaetal development are still unclear.
Annelid chaetae are continually and routinely replaced during the life of a worm. During settlement and metamorphosis, larval chaetae are replaced by juvenile chaetae (Eckelbarger 1975, 1976, 1977; Amieva & Reed 1987; Eeckhaut et al. 2003; Gibson & Gibson 2004; Gibson & Smith 2004; Pernet & McArthur 2006; Smart & von Dassow 2009; Budaeva & Fauchald 2010). As juveniles grow, chaetae of one morphological type may be replaced by those of another, as shown for, e.g., Lumbrineris fragilis (O.F. Müller 1766) by Tzetlin (1990), Scolelepis squamata (Müller 1806) by Hausen & Bartolomaeus (1998), and Dipolydora concharum (Verrill 1880) by Radashevsky & Fauchald (2000). In large polychaetes that grow dramatically in their lifetime, small juvenile chaetae are replaced by larger adult chaetae (Pilgrim 1977; Bhaud 1988; Duchene & Bhaud 1988). In addition, worn-out and broken adult chaetae are replaced by new chaetae (Hausen 2005).
There are, however, two unusual types of chaetal replacement. Probably the most remarkable of them is epitoky, metamorphosis of an immature worm into a sexually mature reproductive form. Epitokous transformation results in atokous chaetae of benthic worms being replaced by modified epitokous swimming chaetae (see, e.g., Clark 1961; Simpson 1962; Hofmann 1974; Daly 1975; Kuper & Westheide 1998; Petersen 1999; Chatelain et al. 2008). Another unusual type of chaetal replacement takes place during regeneration of Sabellidae and Serpulidae. The body of these worms is subdivided into thorax and abdomen, and the thoracic chaetal arrangement differs from the abdominal one. A part of an abdomen can regenerate into a complete worm; during regeneration, anterior abdominal parapodia are transformed into thoracic ones by losing their old abdominal-type chaetae and replacing them with thoracic-type chaetae (Berrill 1931, 1977, 1978; Berrill & Mees 1936a,b; Pernet 2001; Murray 2010; Licciano et al. 2012).
Each chaeta develops from an individual epidermal follicle. Several follicles are usually organized in one or several rows situated inside a common chaetal sac, an invagination of epithelial basal lamina (Bartolomaeus 1995; Hausen 2005; Kieselbach & Hausen 2008). Usually one parapodium has two chaetal sacs, the notopodial sac and the neuropodial one (Mettam 1967; O'Clair & Cloney 1974). According to a widely accepted general model of chaetal replacement, formation of new chaetae occurs at a special formative site at one end of the chaetal row, while degeneration takes place at a degenerative site located at the opposite end of the row (Pilgrim 1977; Duchene & Bhaud 1988; Hausen 2005; Hoffmann & Hausen 2007). Newly formed chaetae enter the row and push the older ones toward the other end; the oldest chaetae are either gradually resorbed (Thomas 1930; Pilgrim 1977) or are shed (Duchene & Bhaud 1988; Hausen 2005).
The outlined chaetal replacement model implies that each chaetal row should contain both formative and degenerative sites. Formative sites are easy to find, and chaetal formation (chaetogenesis) in annelids has been extensively studied by both light and electron microscopies (Bartolomaeus 1995; Meyer & Bartolomaeus 1996; Bartolomaeus & Meyer 1997; Hausen & Bartolomaeus 1998; Hausam & Bartolomaeus 2001; Bartolomaeus 2002; Hausen 2005). However, degenerative sites have been described only for Clymenella torquata (Leidy 1855) by Pilgrim (1977), Capitella capitata (Fabricius 1780) by Schweigkofler et al. (1998), and Owenia fusiformis Delle Chiaje 1844 by Meyer & Bartolomaeus (1996).
It is not clear why degenerative sites are so elusive. One possible explanation is that degenerative sites are difficult to observe because chaetae are lost so quickly that observing degeneration of an individual chaeta is nearly impossible. Alternatively, the chaetal replacement model may not be universal, and in some species, chaetal degenerative sites might not form at all. To address this question, we examined morphogenetic events during chaetal degeneration and replacement in the sabellid polychaete Pseudopotamilla reniformis (Brugière 1789). Chaetal replacement as a result of thoracic region regeneration in this species can be easily induced artificially (Kolbasova et al. 2013). Such experimentally induced regeneration provides a convenient experimental system not only for studies of chaetal regeneration (Berrill 1977, 1978; Murray 2010; Licciano et al. 2012), but also for examination of degenerative sites.
Methods
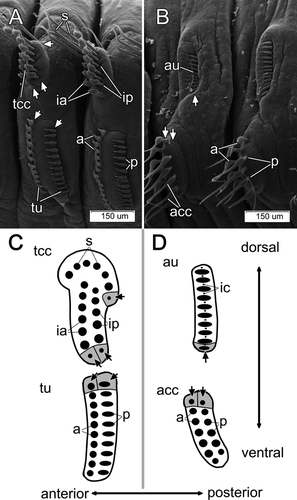
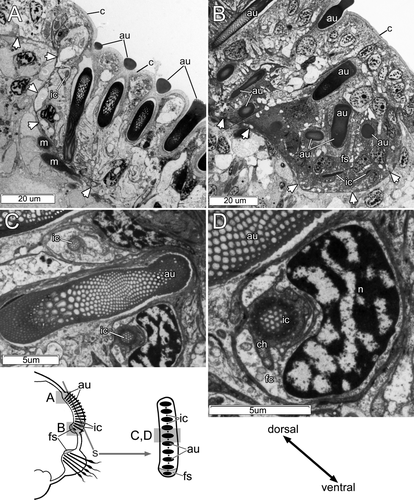
The adult body length of Pseudopotamilla reniformis is 50–70 mm. The thorax consists of 7–14 (typically 8–9) segments, and the abdomen consists of 80–120 chaetigers. Both thoracic and abdominal parapodia are illustrated in Fig. 1A–D. The first (collar) segment lacks neuropodia, while the subsequent thoracic segments bear dorsal notopodia and ventral neuropodia (Fig. 1A). The thoracic notopodium bears two clusters of capillary chaetae: a single semicircle of 8–10 narrowly hooded capillary chaetae in the superior (dorsalmost) cluster, and two parallel transverse rows of 10–12 paleate capillary chaetae in the inferior (ventralmost) cluster (Fig. 1A,C) (note that all chaetal terminology used is sensu Fitzhugh 1989). Each thoracic neuropodium bears two parallel tori with 19–28 acicular uncini in the anterior row and 18–24 avicular uncini in the posterior row (Fig. 1A,C). Each abdominal notopodium bears one transverse row of 15–20 avicular uncini, and each neuropodium bears two parallel transverse rows, each consisting of 7–12 broadly hooded capillary chaetae (Fig. 1B,D).
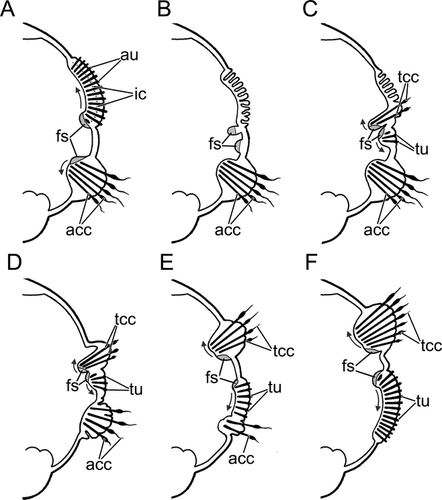
Adults of P. reniformis were collected by scuba divers in June 2010 from Kandalaksha Bay near the White Sea Biological Station (66°34′ N, 33°08′ E). Worms were cut into 2–4 pieces with scissors and placed into aquaria with running seawater to allow regeneration. During regeneration, the branchial crown, pygidium, and the first two thoracic chaetigers are formed de novo from the apical blastema, while the remaining thoracic segments (typically 6–7) transform from the nearest 6–7 abdominal segments (a phenomenon known as morphallaxis). The transforming segments lose abdominal-type chaetae, which are replaced by new thoracic-type chaetae (Figs. 2, 3A–F). The reorganization takes about 100 d (Kolbasova et al. 2013). To establish the course of morphogenetic events following the parapodial reorganization, every 3–5 d, 5–8 worms were photographed and fixed for further examination using both scanning and transmission electron microscopy (Kolbasova et al. 2013). For scanning electron microscopy (SEM), the animals were fixed in 2.5% glutaraldehyde in PBS buffer with 1% OsO4, dehydrated in an increasing ethanol series, critical point dried with CO2 in a Hitachi HCP-2, mounted on aluminum stubs, and sputter-coated with a Au-Pd mixture using an Eiko IB-3 Ion Coater. Specimens were examined using a Cam Scan S-2 scanning electron microscope. For both light and transmission electron microscopies (TEM), worms were fixed in 2.5% glutaraldehyde in 0.1 mol L−1 PBS buffer for 3–6 h (depending on their size), postfixed with 1% osmium tetroxide (OsO4 1%) for 1 h, dehydrated in an ethanol-acetone series, and embedded in Epon. Semi-thin sections (900 nm) were cut with Dupont MT 5000 and LKB-III microtomes, stained with a mixture of methylene blue and toluidine blue, and examined with compound microscopes. Digital images were captured with a Leica DFC 290 camera and Leica Application Suite software. Ultra-thin sections (50 nm) were cut with LKB-III and Leica UC 6 microtomes, collected on formvar-covered single slot copper grids, stained with uranyl acetate and lead citrate, and examined with a JEOL JEM-1011 electron microscope. Digital images were captured with a Gatan ES500W Erlangshen camera and SIS Mega View Software.
Results
The loss of both of capillary chaetae and uncini occurred in the same way, but uncini were more convenient for observations because they were smaller and were replaced more rapidly. Therefore, only the degeneration of uncinal follicle cells is described in detail below.
Morphology of parapodia and chaetal sacs
Structure of thoracic parapodia
Each thoracic parapodium had two chaetal sacs, one notopodial and one neuropodial. The chaetal sac of the notopodium extended deep into the epithelium and contained three chaetal rows having ventral formative sites (Fig. 1A,C). In contrast, the neuropodial uncinal sac was not deep and contained two rows of uncini (Figs. 1A,C, 4A). Darkly staining cells clearly indicated dorsal formative sites in both uncinal rows. No signs of degenerative sites, such as broken chaetae or a high density of lysosomes inside follicle cell cytoplasm, were found in either thoracic or abdominal chaetal sacs.
Structure of abdominal parapodia
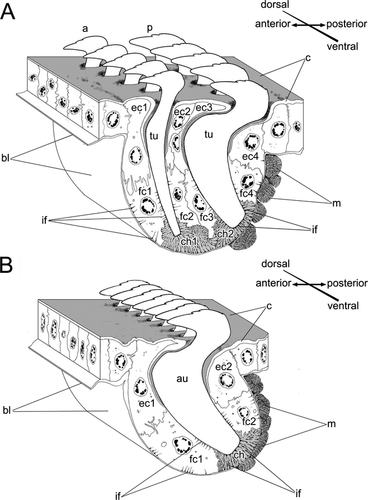
Each abdominal parapodium had a notopodial and a neuropodial chaetal sac. The notopodial chaetal sac was located on the lateral body wall and gave rise to a row of uncini and a row of inner chaetae (Figs. 1B,D, 4B). The inner chaetae were small (up to 15 μm long and 2 μm in diameter), and were completely embedded in the epithelium, never extending to the surface; they were positioned in a regular fashion between uncini along the uncinial row (Figs. 1D,5A–D). Both uncini and inner chaetae had ventral formative sites that were clearly visible as dark cells filled with electron-dense stained vesicles (Fig. 5B). Degenerative sites were not found in the notopodial chaetal sac; cells at the ventral edge of a chaetal sac were similar to the other cells in the chaetal sac, except for formative site cells (Fig. 5A). The neuropodial sac of capillary chaetae was located on the ventrolateral body wall (Fig. 1B,D). The formative sites of these chaetal rows were dorsal; we found no degenerative sites in the neuropodial chaetal sac (Fig. 1B,D).
Organization of abdominal notopodia chaetal sacs
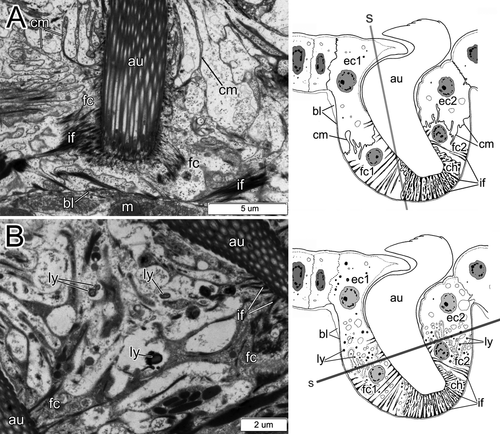
Each intact uncinal chaetal sac was organized as an invagination of the epithelial basal lamina, forming a groove where uncinal follicles were situated (Figs. 4B, 5A,B, 6). Uncini were formed in the formative site situated in the cavity on one end of the chaetal sac. Each uncinus was surrounded by its own follicle. Five follicular cells—the chaetoblast, two follicle cells, and two associated epidermal cells—were visible in transverse sections of the follicle of a fully differentiated uncinus (Figs. 4B, 6). The chaetoblast, which was found in the basal part of the uncinal follicle, was extremely ramified and interdigitated with folds of adjacent follicle cells, and its cytoplasm stained intensely owing to numerous intermediate filaments attaching the uncinus to the basal lamina.
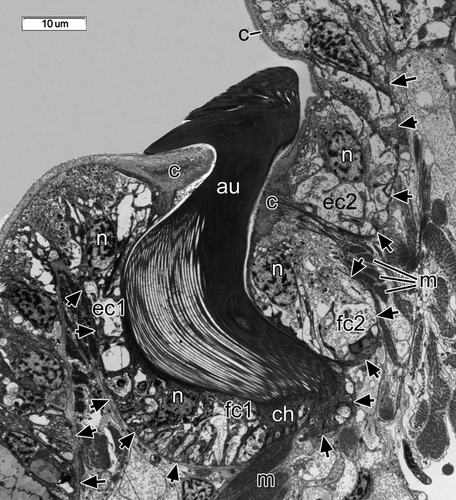
Two follicle cells were adjacent to the apical part of chaetoblast in the middle part of the follicle; together, these covered the shaft of the uncinus (Figs. 4B, 6). These follicle cells formed distinctive folds (Figs. 4B, 6), and their cytoplasm was paler than that of the chaetoblast. Intermediate filaments inside the follicle cells attached the uncinus both to the basal lamina and to the musculature of the chaetal sac (Figs. 4B, 6). The apical part of the follicle consisted of two specialized epidermal cells surrounding the neck-like region of the uncinus (Figs. 4B, 6). Unlike other epidermal cells, these had a thick cuticle forming a ring-like region around the uncinal shaft (Figs. 4B, 6).
Each inner chaeta had its own follicle that consisted of two cells, a ramified darkly stained chaetoblast and a large follicle cell (Fig. 5C,D).
Chaetal replacement during regeneration
Chaetal replacement in a notopodium
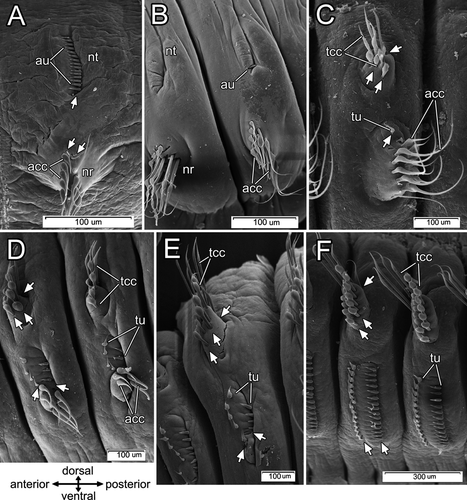
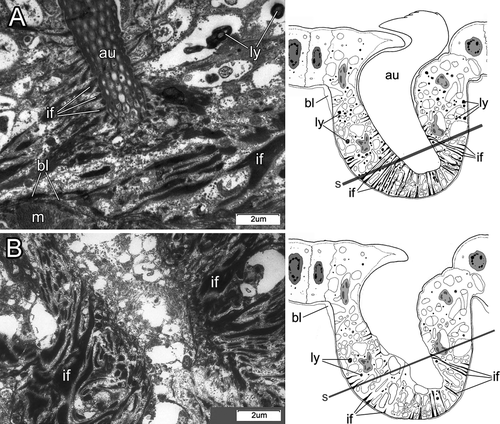
One to five days after fragmentation (n=6 individuals), there were no external signs of chaetal reorganization (Fig. 3A). During the 7th–10th days (n=8), the notopodial uncini of transforming segments fell out very quickly, usually within 18–20 h (Fig. 3B). The ventralmost uncini from the main notopodial tori were lost first, but the most recently formed uncini, still located near the formative site in the dorsal part of the row, remained in the notopodia several hours longer (Fig. 3B). The spot where the uncini fell out was marked by a deep groove (Fig. 3B). During this period, a new sac of thoracic capillary chaetae started to form. It emerged near the ventral side of the notopodial sac as a small invagination of the basal lamina filled with the darkly stained cells characteristic of new formative sites. During the 11th–15th days (n=5), a number of large electron-transparent vesicles appeared in chaetal sac cells, the cells membranes disappeared, and the nuclei became pale and disintegrated (Fig. 7A–C). The intermediate filaments were easily seen, and the muscular system of the chaetal sac remained present in the parapodial tissue (Fig. 7C).
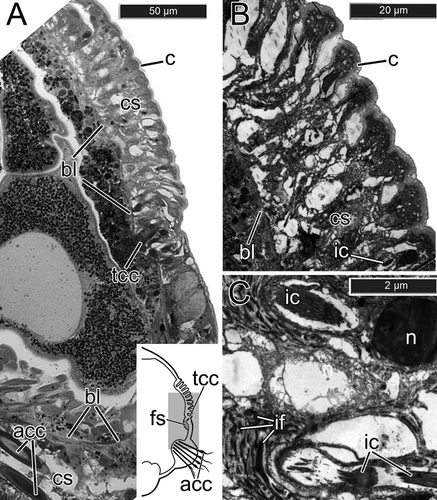
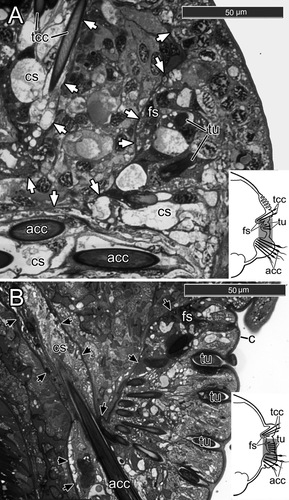
The new sac of capillary chaetae was much deeper in the parapodium than the old uncinal sac, because capillary chaetae are much longer than uncini. Three formative sites of three new chaetal rows were localized under the old uncinal sac, and as a result, the new thoracic chaetae grew through the degenerating uncinal chaetal sac (Fig. 7A). First, one or two narrowly hooded capillary chaetae from the superior row appeared. A day later, one paleate capillary chaeta from the anterio-inferior row and one paleate capillary chaeta from the posterio-inferior row emerged on the notopodial surface. On the 16th–20th day after the fragmentation (n=6), the old uncinal sac disappeared completely. The new chaetal sac produced 4–5 narrowly hooded capillary chaetae in the superior row, 1–2 paleate capillary chaetae in the anterio-inferior row, and 3–4 paleate capillary chaetae in the posterio-inferior one (Fig. 3C, Fig. 8A).
The podial bulb and the musculature of the new chaetal sac appeared by the 21st–30th day post-fragmentation (n=4). During this period, notopodia already bore 5–6 narrowly hooded chaetae in the superior row, 2–3 paleate capillary chaetae in the anterio-inferior row, and 3–4 paleate capillary chaetae in the posterio-inferior row (Fig. 3D). At the 50th day (n=2) post-fragmentation, there were 6–8 narrowly hooded chaetae in the superior row, 3–4 paleate capillary chaetae in the anterior row, and 4–5 paleate capillary chaetae in the posterior one. The musculature of the notopodia continued to develop, and the podial bulb enlarged significantly (Fig. 3E). Subsequently, the number of chaetae in notopodia gradually increased; completely reorganized notopodia were present by the 90th–120th day post-fragmentation (n=5) (Fig. 3F).
Chaetal replacement in a neuropodium
Formation of new neuropodia took longer than that of notopodia. On the 10th–15th days after fragmentation (n=5), when the first new notopodial capillary chaetae appeared on the body surface, the neuropodium showed no external changes (Fig. 3B). During this period, a new neuropodial chaetal sac with formative sites of two uncinal rows appeared between the new notopodial chaetal sac and the old neuropodial one. In semi-thin sections, the newly forming uncini become visible 15–18 d after fragmentation (n=3) (Fig. 8A). At this point, the new thoracic uncinal sac started to elongate toward the old abdominal chaetal sac (Fig. 8A).
During the 16th–20th days post-fragmentation (n=6), new uncini appeared at the body surface (Fig. 3C). Avicular uncini appeared 1–3 d later than acicular ones. During the 25th–30th days (n=8), both types of uncini formed two more or less parallel rows, each consisting of 4–7 uncini (Fig. 3D). As the uncinal rows became longer, a new uncinal sac extended ventrally and fused with the old neuropodial sac (Fig. 8B). At the same time, the old neuropodial sac became smaller, and its muscular system began to be resorbed (Fig. 8B). This process occurred around the 50th day post-fragmentation (n=2).
The last remaining capillary chaetae were shed from the neuropodium during the 60th–80th day after fragmentation (n=8). Finally, after 90 d (n=5), the old neuropodial chaetal sac disappeared completely and the former abdominal parapodium (both noto- and neuropodium) reached the adult thoracic condition (Fig. 3F).
Follicle cell degeneration during chaetal regeneration
During the 1st–3rd day after fragmentation (n=3), there were no signs of degeneration in follicle cell cytoplasm; their membranes remained distinct and easily visible (Fig. 9A). The first changes in the follicle cell cytoplasm occurred during the 4th–5th day (n=2); during this time, numerous lysosomes appeared in the cytoplasm, and cell membranes began to disintegrate (Fig. 9B). Simultaneously, small electron-transparent vesicles appeared in follicle cells. Two days later, at the 6th or 7th day post-fragmentation (n=2), follicle cell membranes were indistinct (as observed by TEM), but the cytoplasm was still distinct. The number of lysosomes increased (Fig. 10A). Although cell membranes disintegrated, the uncinal intermediate filament system was still present in the cytoplasm. On the 7th–10th day post-fragmentation (n=1), uncini fell out, electron-transparent vesicles enlarged considerably, and the follicle cell cytoplasm looked torn and porous (Fig. 10B). The intermediate filament system disappeared around the 10th–12th day post-fragmentation (n=3).
Discussion
We have shown that parapodial reorganization in Pseudopotamilla reniformis proceeds via disintegration of the old chaetal sacs and formation of new chaetal sacs. In both old abdominal-type and new thoracic-type parapodia, notopodial formative sites are ventral and neuropodial formative sites are dorsal. New thoracic chaetae (or uncini) follow the abdominal ones in their course of formation (the row elongates toward the dorsal body side in a notopodium and toward the ventral body side in a neuropodium). It is unclear whether the notopodial formative site persists, triples, and begins to produce a new type of chaetae during the parapodial reorganization, or if it is replaced by three new formative sites. It is also unclear if two old neuropodial formative sites are replaced by two new uncinal formative sites, or they switch to formation of two uncinal rows. Because of significant morphological and topological differences between the uncinal and capillary chaetal sacs, we suspect that all formative sites disappear along with the old abdominal chaetal sac and the new formative sites form in a new thoracic sac (Fig. 2).
During uncinal sac disintegration, all uncini in the row can fall out simultaneously and thus chaetal loss is not associated with a degenerative site located in a particular area of the sac. Our investigation allows subdivision of the process of chaetal degeneration into three main periods. Initially, numerous lysosomes and small electron-transparent vesicles appear in the cytoplasm of follicle cells, indicating the beginning of membrane disintegration. The next step involves disintegration of the follicular cell membranes. The final stage is complete dissolution of follicle cells, resulting in chaetae being shed from the parapodium.
However, a totally different type of chaetal degeneration was described in rows of acicular uncini of the maldanid polychaete Clymenella torquata (Pilgrim 1977). In this species, chaetae are not shed, but chaetal degradation occurs by formation of “waste sacs” at degenerative sites where old and broken chaetae are deposited into the coelom and later completely destroyed by coelomocytes. Such a mode of chaetal loss seems unusual because it means that the old chaetae have to pass through the basal lamina and musculature of the chaetal sac. It is possible that similar chaetal resorption takes place during the degeneration of inner chaetae in P. reniformis (Figs. 7B,C). Although we have not specifically examined the process of chaetal replacement in intraepithelial chaetae, it seems that they are resorbed inside chaetal sacs, along with follicle cells, at the same time as parapodia get reorganized.
The presence of numerous large lysosomes indicating the breakdown of old chaetae was also reported for degenerative sites in rows of hooked chaetae of Capitella capitata by Schweigkofler et al. (1998) and Owenia fusiformis by Meyer & Bartolomaeus (1996). It suggests that in all three species (the capitellid C. capitata, the oweniid O. fusiformis, and the sabellid P. reniformis), at the cellular level, chaetal degeneration takes place in the same manner regardless of the presence or absence of functioning degenerative sites in parapodia. Therefore, the presence of numerous lysosomes is a universal marker of chaetal loss 2–3 d before it occurs, and so may serve as a histological indicator of chaetal degeneration in a wide range of annelids. Such a marker makes it possible to detect signs of chaetal degeneration whether they are restricted to specialized degenerative sites, or occurring anywhere along the chaetal row.
In the parapodia of intact (non-regenerating) individuals of P. reniformis, TEM shows no evidence of chaetal degeneration, such as numerous lysosomes, electron-transparent vesicles, or empty follicles. Scanning electron microscopy of the parapodia of intact adults of P. reniformis shows that both smaller juvenile-type and larger adult-type chaetae and uncini are present. The adult uncini are positioned close to the formative sites, whereas the juvenile-type chaetae are situated at the opposite end of the uncinal row. This situation is a result of a row formation, when new uncini develop in the formative site and are gradually displaced toward the opposite end of the row as the parapodium grows (Figs. 2, 3). During parapodial reorganization in P. reniformis, capillary chaeta formation takes about 3–7 d, while uncini form in 2–5 d (considering that capillary chaetae continue their formation 1–2 d after they project above the body surface, unlike uncini, which complete their formation below the body surface). The presence of juvenile-type uncini in the parapodia of intact P. reniformis, as well as the absence of any histological signs of chaetal degeneration, may suggest that even if such uncini get replaced as the animal grows, this replacement occurs very slowly. This would make it difficult to pinpoint the moment of chaetal replacement using SEM and histological methods. On the other hand, the life span of P. reniformis is unknown, and the rate of formation of both capillary chaetae and uncini is not uniform, as during regeneration 2–3 new chaetae from a row appear simultaneously, after which chaetal formation decelerates. Therefore, to assess the rate of chaetal replacement, one also needs to compare the relation between the juvenile and adult type of chaetae (or uncini) in worms of different ages.
Having juvenile and adult chaetae in one chaetal row has been described in the terebellids Eupolymnia nebulosa (Montagu 1818), Lanice conchilega (Pallas 1766), Neoleprea streptochaeta (Ehlers 1897), Nicolea zostericola Örsted 1844, Pista cretacea (Grube 1860), Pista cristata (Müller 1776), and Thelepus setosus (Quatrefages 1866) (Bhaud 1988; Duchene & Bhaud 1988). In these studies, the capillary chaetae and uncini varied in size and shape with age (Bhaud 1988; Duchene & Bhaud 1988), but the observed slight variation was only noticeable in comparison with chaetae within the same row. In all these cases, slow and gradual chaetal replacement most likely involves a degenerative site, as described for C. capitata and O. fusiformis (Meyer & Bartolomaeus 1996; Schweigkofler et al. 1998).
More complicated types of age-related chaetal replacement are found in some Lumbrineridae and Spionidae. In the chaetigers of Lumbrinereis fragilis (Lumbrineridae), hooks appear more posteriorly in older than in younger specimens. With age, hooks of anterior chaetigers are replaced by capillary chaetae; thus, the position of hook-bearing chaetigers depends on worm size (Tzetlin 1990). Similarly, in the spionid Dipolydora concharum, capillaries develop between hooks in new segments, and are lost as the worm grows. Therefore, capillary chaetae are always present only in a few newly developed posterior-most chaetigers (Radashevsky & Fauchald 2000). In another spionid, Scolelepis squamata, the abdominal neuropodia initially bear a row of capillary chaetae and a row of hooks, but the capillary row degenerates before its formation is complete, leading to a single transverse row of hooded hooks in anterior abdominal neuropodia (Hausen & Bartolomaeus 1998). Thus, in Lumbrineridae and Spionidae, one chaetal row replaces another row inside the common chaetal sac. Such a replacement requires reorganization of one formative site type into the other, because a formative site produces only one type of chaetae. Usually, when a chaetal row consists of two alternating types of chaetae, it suggests presence of two closely spaced chaetal rows with two different formative sites. Therefore, we propose that in abdominal notopodia of P. reniformis, there are two separate formative sites that produce chaetae in rotation and give rise to two different rows.
For the examples of Lumbrineridae and Spionidae mentioned above, it is not clear whether the replacement of an old chaetal row occurs through a degenerative site, or if all chaetae in the row fall out simultaneously. Chaetal replacement clearly takes place without involvement of degenerative sites during rapid parapodial reorganization, when chaetae are shed by entire chaetal sacs, as described here for P. reniformis during regeneration. Similarly, rapid chaetal replacement was also observed in nereidids during epitokal transformation as described for Perinereis cultrifera (Grube 1840) by Clark (1961), and in P. reniformis during asexual reproduction (Kolbasova et al. 2013). Parapodial reorganization takes place as a result of induced post-traumatic regeneration in the sabellids Branchiomma nigromaculata (Baird 1865), Sabella melanostigma (Schmarda 1861), Sabella pavonina (Savigny 1822), Bispira volutacornis (Montagu 1804), Megalomma vesiculosum (Montagu 1815), Branchiomma bombyx (Dalyell 1853), and Potamilla torelli (Malmgren 1866) as described by Berrill (1931, 1977, 1978), Berrill & Mees (1936a,b), and Licciano et al. (2012). The same happens during spontaneous asexual reproduction in serpulids Filograna Berkeley 1835 and Salmacina Claparède 1870 (ten Hove 1979; Nishi & Nishihira 1994; Pernet 2001; ten Hove & Kupriyanova 2009). In the sabellids B. nigromaculata, Potamethus elongatus (Treadwell 1906), and several other unnamed “dwarf” sabellids, such parapodial reorganization was observed during juvenile development (Berrill 1977). Although none of the above studies addressed the type of chaetal loss, we hypothesize that during parapodial reorganization in Sabellida in general, chaetal replacement proceeds without involvement of degenerative sites.
In conclusion, we suggest that chaetal degeneration and loss in polychaetes can occur either through degenerative sites, or without them, depending on the type of chaetal replacement. Loss through degenerative sites is associated with slow chaetal replacement observed during gradual compensation of worn-out chaetae and in age-related chaetal change. Chaetal loss without degenerative sites is observed when rapid chaetal degeneration is important. It occurs in parapodial reorganization during such crucial ontogenetic events such as asexual reproduction and post-trauma regeneration, sexual reproduction with epitoky, and larval metamorphosis and juvenile growth.
Acknowledgments
Thanks to A.G. Bogdanov (Laboratory of Electron Microscopy, MSU) and S. I. Metelev (Laboratory of Electron Microscopy, I. Papanin Institute of Biology of Internal Waters) for their help with SEM and TEM, respectively. We also thank A.A. Prudkovsky and I.A. Kosevich (Department of Invertebrate Zoology, MSU) for their help with laboratory experiments, and three anonymous reviewers for their valuable comments. This study was funded by grant 11.G34.31.0008 from the Russian Ministry of Science and Education, and by grant 13-04-00078-a from the Russian Fund for Basic Research.




