Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family
Summary
Interleukin-33 (IL-33) is a tissue-derived nuclear cytokine from the IL-1 family abundantly expressed in endothelial cells, epithelial cells and fibroblast-like cells, both during homeostasis and inflammation. It functions as an alarm signal (alarmin) released upon cell injury or tissue damage to alert immune cells expressing the ST2 receptor (IL-1RL1). The major targets of IL-33 in vivo are tissue-resident immune cells such as mast cells, group 2 innate lymphoid cells (ILC2s) and regulatory T cells (Tregs). Other cellular targets include T helper 2 (Th2) cells, eosinophils, basophils, dendritic cells, Th1 cells, CD8+ T cells, NK cells, iNKT cells, B cells, neutrophils and macrophages. IL-33 is thus emerging as a crucial immune modulator with pleiotropic activities in type-2, type-1 and regulatory immune responses, and important roles in allergic, fibrotic, infectious, and chronic inflammatory diseases. The critical function of IL-33/ST2 signaling in allergic inflammation is illustrated by the fact that IL33 and IL1RL1 are among the most highly replicated susceptibility loci for asthma. In this review, we highlight 15 years of discoveries on IL-33 protein, including its molecular characteristics, nuclear localization, bioactive forms, cellular sources, mechanisms of release and regulation by proteases. Importantly, we emphasize data that have been validated using IL-33-deficient cells.
This article is part of a series of reviews covering The IL-1 cytokine and receptor family appearing in Volume 281 of Immunological Reviews.
1 INTRODUCTION
IL-33 activates many immune cell types involved in type-2 immunity and allergic inflammation, including ILC2s, mast cells, Th2 cells, eosinophils, basophils, dendritic cells and alternatively activated macrophages (AAM).1-3 ILC2s (also designated natural helper cells, nuocytes or innate helper 2 cells) are GATA3-dependent lineage negative innate lymphoid cells, which produce extremely high amounts of the Th2 cytokines IL-5 and IL-13 in response to IL-334-7 and play critical roles in type-2 immunity, allergic inflammation and eosinophil homeostasis.1-3, 8-10 Analysis of patient samples and studies in murine models support a crucial role of the IL-33/ILC2 axis in allergic inflammation in different tissues (lung, nasopharynx, skin) and diseases (asthma, atopic dermatitis, allergic rhinitis, chronic rhinosinusitis).1-3 Interestingly, genetic studies suggest a major role of this axis in asthma susceptibility.1, 11, 12 Indeed, the genes IL-33 and IL1RL1 (encoding ST2) were reproducibly identified in all major genome-wide association studies of asthma,13-17 and several other genes important for ILC2 biology were also identified as susceptibility loci in some of these studies.1, 14-16
Studies performed over the past several years have revealed that the action of IL-33 is not limited to the activation of immune cells involved in type-2 immune responses. Indeed, these studies have uncovered important roles of IL-33 in the activation of immune cells involved in type-1 immunity, infection and chronic inflammation, such as Th1 cells, NK cells, CD8+ T cells, neutrophils, macrophages, B cells and NKT cells.1-3, 18-21 This pleiotropic nature of IL-33 is likely to explain why IL-33 has been implicated in a wide variety of non-allergic diseases, including infectious diseases (fungal, helminth, protozoa, bacterial, and viral infection), cardiovascular diseases, chronic obstructive pulmonary disease (COPD), fibrotic diseases, musculoskeletal diseases, inflammatory bowel diseases, diseases of the central nervous system (Alzheimer), cancer, graft versus host disease (GVHD), obesity, and diabetes (reviewed in 3).
Recently, it has been discovered that IL-33 plays important roles in regulatory immune responses.2, 3, 22 Highly suppressive ST2+FoxP3+ Tregs subsets regulated by IL-33 were identified in several tissues, including lymphoid organs,23, 24 gut,25 lung,26 and adipose tissues.27-29 These recent observations with tissue Tregs,26 together with previous studies on ILC2s,30 suggest important roles of IL-33 in the maintenance of tissue homeostasis, resolution of inflammation, and repair of tissue damage.2, 3
There are comprehensive reviews about the role of IL-33 in various diseases and activation of immune cells,2, 3 to which the reader is referred. In this review, we rather focus on IL-33 biology and we summarize the important advances that have been made over the past 15 years. The reader will found specific answers to important questions such as:
- How IL-33 was discovered?
- What are its molecular characteristics and bioactive forms?
- What is its subcellular localization and what are its cellular sources in vivo?
- How is it released from producing cells and how its activity is regulated?
Specificity of reagents has turned out to be an important issue in the IL-33 field. Therefore, whenever possible, we emphasize data that have been validated using IL-33 knockout (mouse) or knockdown (human) cells.
2 THE DISCOVERY OF IL-33
We visualized endogenous IL-33 protein and mRNA for the first time in human tissues in 2003.31 We initially designated the protein ‘nuclear factor from high endothelial venules (NF-HEV)’,31 because we discovered it as a nuclear protein abundantly expressed in HEVs, specialized blood vessels that mediate the entry of lymphocytes into lymph nodes and other lymphoid organs.32, 33 We detected high levels of IL-33/NF-HEV mRNA in HEVs from diverse human lymphoid organs, including tonsils, Peyer's patches and lymph nodes by in situ hybridization.31 We also reported the location of the human IL-33 gene on the short arm of chromosome 9 at 9p24.1 and the identification of the murine IL-33/NF-HEV orthologue.31
Although the primary sequence of NF-HEV did not exhibit significant homology to known proteins, we found a strong similarity with a predicted canine protein encoded by an unknown mRNA (DVS27), identified by Onda et al34 in 1999 (Figure 1) as an mRNA highly upregulated in vasospastic cerebral arteries after subarachnoid hemorrhage. Alignment of the human and mouse IL-33/NF-HEV protein sequences with the canine DVS27 sequence suggested that DVS27 protein is the canine orthologue of IL-33/NF-HEV.31
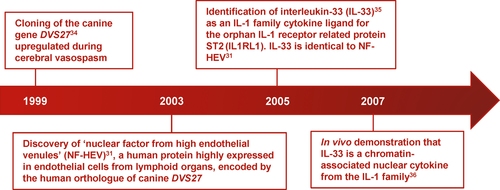
In 2005, Schmitz et al35 reported that the carboxy-terminal part of the human NF-HEV protein (amino-acids 112 to 270) exhibits a three-dimensional fold similar to IL-1 family cytokines, and functions as a cytokine that induces type 2 immune responses through activation of ST2 (IL-1 receptor-like-1, IL1RL1), an orphan receptor of the IL-1 receptor superfamily. Based on these observations, they proposed the name of interleukin-33 (IL-33) for this novel member of the IL-1 family. Schmitz et al did not mention that the protein was not novel and that it was in fact 100% identical to the human NF-HEV protein sequence identified 2 years earlier.31 One year later, we reported the identity between IL-33 and NF-HEV, and demonstrated that IL-33 is a chromatin-associated nuclear factor in vivo.36
3 MOLECULAR CHARACTERISTICS OF IL-33 GENE AND PROTEIN
3.1 IL-33 gene
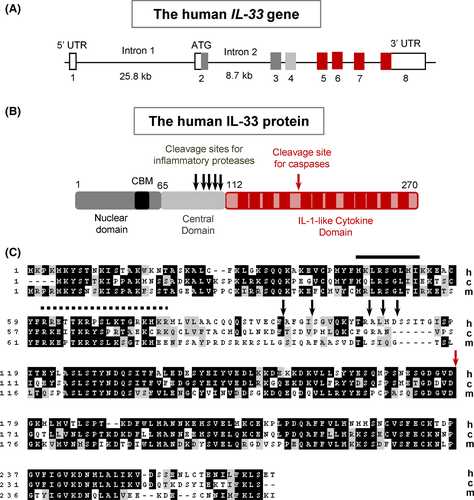
The gene encoding IL-33 (Figure 2A) comprises eight exons both in humans and mice.3 The promoter for the major human IL-33 mRNA form (NM_033439; 2718 nucleotides) is located upstream of an untranslated exon (exon 1 or 1a), more than 20 kb upstream of exon 2 that contains the AUG initiation codon.3, 37 It is active in normal human keratinocytes, endothelial cells and fibroblasts, and it contains an interferon stimulated response element (ISRE) and several gamma interferon activation sites (GAS).37 An alternative exon 1 sequence (exon 1b), located 4.6 kb upstream of exon 2, corresponding to the use of an alternative promoter has been described both in humans and mice.37, 38 Transcription start sites are located downstream of consensus TATA box sequences in both promoters.38 Interestingly, many of the single nucleotide polymorphisms (SNPs) in the human IL-33 gene associated with asthma are located in the promoter and intron 1.11 They could thus influence the activity of the two promoters of the IL-33 gene. SNPs in IL-33 are generally associated with increased asthma susceptibility.1, 11-17 However, a rare loss of function mutation in IL-33 that disrupts a canonical splice acceptor site before exon 8 and is predicted to result in the production of a truncated IL-33 protein, has been found to cause reduced numbers of eosinophils in blood and to protect against asthma.39 Eosinophils counts are also reduced in IL-33-deficient mice.39 Splice variants lacking exons 3, 4 and/or 5 have been described.40-42 However, they have only been detected after amplification by qPCR and they appear to be very minor components in primary cells. At this point, it is therefore unclear whether alternative splicing is a relevant mechanism of IL-33 regulation.
3.2 IL-33 protein
IL-33 protein is composed of two evolutionary conserved domains, the N-terminal nuclear domain and the C-terminal IL-1-like cytokine domain, separated by a divergent central part (Figure 2B). Human IL-33 exhibits 58% and 52% identity over 270 residues (Figure 2C) with its canine and murine orthologues, respectively.31 The theoretical molecular weight (MW) of the human IL-33 precursor is 30 759 Da and its isoelectric point (pI) is 8.89. The cytokine domain is highly acidic (aa 112-270, MW = 17 994 Da, pI = 4.80) similar to mature IL-1α (pI = 5.3), whereas the N-terminal part is highly basic (aa 1-111, MW = 12783 Da, pI = 10.28).
3.3 N-terminal nuclear domain
The N-terminal part of IL-33 contains a chromatin-binding motif (CBM, aa 40-58)43 and a predicted bipartite nuclear localization sequence (NLS, aa 61-78).31 The N-terminal domain (aa 1-65), that contains the IL-33 CBM, has been shown to be necessary and sufficient for nuclear targeting of epitope-tagged (GFP-fused) IL-33 in human cells,36 but the possibility that the bipartite NLS may be required for nuclear targeting of untagged IL-33 has not been excluded. The N-terminal part of IL-33 containing the CBM and NLS (aa 1-112) has been shown to be necessary and sufficient for targeting of a dsRed reporter to the nuclei of producing cells in vivo in a genetically engineered mouse model.44, 45 Based on structural predictions, the N-terminal domain of IL-33 was initially believed to contain a putative homeodomain-like helix-turn-helix DNA-binding domain.31 However, this was not confirmed in later studies which revealed that IL-33 associates with chromatin through CBM-mediated protein-protein interactions rather than through protein-DNA interactions.43
Characterization of the IL-33 CBM uncovered a unique example of molecular mimicry between a chromatin-associated cytokine and a DNA tumor virus.43 Indeed, the IL-33 CBM shares striking structural similarities with the CBM of Kaposi sarcoma herpes virus LANA (latency-associated nuclear antigen), which mediates attachment of viral genomes to chromosomes of infected host cells.46 Similar to LANA CBM, IL-33 CBM adopts a tight hairpin structure stabilized by an array of intramolecular hydrogen bonds, and uses a conserved MXLRSG hexapeptide to bind to the acidic pocket formed by histones H2A and H2B at the surface of chromatin.43 Strikingly, a single-point mutation of IL-33 Arg48 (or mutations of H2A acidic patch residues) has been shown to abrogate the interaction between IL-33 and the H2A-H2B heterodimer.43
3.4 C-terminal IL-1-like cytokine domain
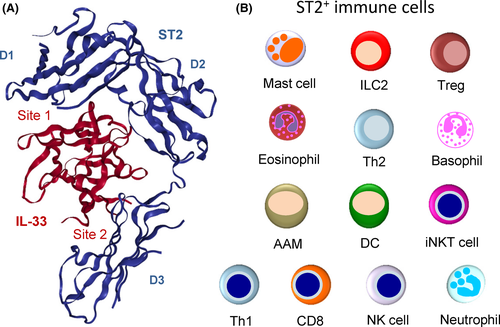
The three-dimensional structure of the IL-1-like cytokine domain of human IL-33 has been solved both by nuclear magnetic resonance47 and X-ray crystallography.48 The domain adopts a β-trefoil fold similar to that described for IL-1α, IL-1β and IL-18.47, 48 The 12 β strands of the β-trefoil (β1-β12) are arrayed in three pseudo-repeats of four β strand units.47 Crystal structure of the IL-33/ST2 complex (Figure 3A) revealed that IL-33 interacts with all three Ig-like domains of the ST2 ectodomain through a large binding interface composed of two distinct binding sites.48 The surface of IL-33 at site 1 exhibits strong negative charge, and the corresponding surface of ST2 is dominated by positive charge.48 IL-33 acidic residues Glu144, Glu148, Asp149 and Asp244 make critical salt-bridge interactions with ST2 basic residues Arg38, Lys22, Arg198 and Arg35. At site 2, IL-33 acidic residue Glu165 has salt-bridge interaction with Arg313 of ST2, and a significant hydrophobic cluster is formed by Tyr163 and Leu182 of IL-33 and Leu246, Leu306 and Leu311 of ST2. IL-33 binds to ST2 with an affinity of 0.45-0.74 nM.47, 48 Single-point mutations of IL-33 acidic residues at sites 1 and 2 strongly decreased the ST2 binding affinity, indicating that charge complementarity plays a critical role in specific recognition of IL-33 by ST2.48
4 ACTIVATION OF ST2-EXPRESSING CELLS
Binding of IL-33 allows membrane ST2 (also designated ST2L) to interact with IL-1RAcP, a co-receptor shared with other IL-1 family members (IL1α, IL1β, IL-36). The IL-33/ST2/IL1RAcP complex then induces signaling through MyD88 adaptor, IRAK1 and IRAK4 kinases, and TRAF6, that culminates in the activation of MAP kinases and NFκB transcription factors.3, 35 The signaling pathways activated by IL-33 in target cells are very similar to those activated by IL-1 and IL-18. Differential expression of ST2, IL-1R, and IL-18R on target cells is thus likely to be critical to explain the unique biological effects of IL-33.2, 3
Tissue-resident immune cells that express ST2 constitutively are major targets of IL-33.1-3 These include mast cells,49-51 group 2 innate lymphoid cells (ILC2s)4-7 and tissue regulatory T cells (Tregs)23-29 (Figure 3B). Many (but not all) mast cells, ILC2s and tissue Tregs express high levels of ST2, and convincing evidence that IL-33 is a critical regulator of these tissue-resident immune cells has been provided in many studies.1-3 Modulation of ST2 expression on immune cells (constitutive vs inducible) in different conditions could explain why IL-33 has been reported to play crucial roles in both type-2 and type-1 immune responses and associated diseases. For instance, in a murine model of COPD, ST2 was shown to be downregulated on ILC2s, and induced on NK cells and macrophages that don't express it at steady state.21 Induction of ST2 expression has also been observed on Th1 cells and CD8+ T cells after activation.20, 22
Although they are less well characterized, non-immune cells may also constitute important cellular targets of IL-33 in vivo. These include endothelial cells, epithelial cells, fibroblasts, astrocytes, and neurons.52-56
5 IL-33, A NUCLEAR CYTOKINE
5.1 Nuclear localization
We were surprised in 2005 when Schmitz et al35 reported that the carboxy-terminal part of nuclear factor NF-HEV corresponds to a cytokine ligand for a membrane receptor. Therefore, we confirmed the strict nuclear localization of IL-33/NF-HEV in vivo31 using three distinct antisera directed against the N-terminal nuclear and C-terminal IL-1-like cytokine domains.36 We stained more than 50 distinct human tissues and found that endogenous IL-33 accumulates in the nuclei of endothelial cells in almost all tissues analyzed.57 We also observed strict nuclear localization of IL-33 in other producing cells, such as epithelial cells and fibroblasts.57 We made similar observations in mice.58 For instance, we found strong nuclear expression of IL-33 in epithelial cells from barrier tissues and fibroblastic reticular cells (FRCs) from lymphoid organs.58 Importantly, we validated the specificity of IL-33 staining using IL-33−/− mice.58 Nuclear localization of IL-33 in vivo has been confirmed by many other groups and it was validated in most studies using IL-33-deficient cells or mice.29, 53, 59-62 In contrast, although cytoplasmic localization of IL-33 has been reported in some studies, it has not been validated using IL-33-deficient cells. Validation is essential because we have observed that even the best IL-33 antibodies available can give specific nuclear staining of IL-33 producing cells (staining lost in IL-33−/− mice) and non-specific cytoplasmic staining of other cells (staining still observed in IL-33−/− mice, despite absence of IL-33).58 We believe that, in the absence of validation (knockout/knockdown data), the cytoplasmic staining reported with IL-33 antibodies in some studies should be interpreted with caution. The presence of IL-33 in cytoplasmic vesicles has been reported by Kakkar et al63 who used murine NIH3T3 immortalized cell lines with ectopic expression of a tetracysteine-tagged form of human IL-33 under the control of the strong EF1 promoter. Ectopic expression of tagged-IL-33 in immortalized cells may result in artefactual localization. Moreover, cysteine residues have been shown to control IL-33 conformation and activity,64 and the four extra-cysteine residues could alter correct folding of the protein (bioactivity of the tetracysteine-tagged form was not demonstrated). The presence of endogenous IL-33 in cytoplasmic vesicles was not shown in the study, and although cytoplasmic staining was observed, it was not validated using IL-33-deficient cells.63
5.2 Role of IL-33 nuclear localization
IL-33 accumulates in the nucleus of producing cells,31, 36, 57 binds to histones and chromatin.36, 43 In addition, evolutionary conservation of the N-terminal nuclear domain (Figure 2C) supports a critical role of nuclear localization and chromatin-association of IL-33. Initial studies indicated that IL-33 exhibits some transcriptional repressor properties when overexpressed in mammalian cells.36, 65 We thus proposed that IL-33 could be a dual function protein, acting intracellularly as a nuclear factor regulating transcription, and extracellularly as a potent cytokine.36 However, after more than 10 years of research efforts, convincing evidence that endogenous nuclear IL-33 regulates gene or protein expression is still lacking. Using a global proteomic approach, we recently showed that extracellular IL-33 cytokine, and not endogenous nuclear IL-33, regulates protein expression in primary human endothelial cells.55 High-resolution mass spectrometry analyses revealed that knockdown of endogenous nuclear IL-33 using two independent RNA silencing strategies had no reproducible effect on the endothelial cell proteome.55 A single protein was modulated on a global scale and it was IL-33 itself. Although it will be important to confirm these results in other cell types producing IL-33, particularly in mucosal epithelial cells, these proteome-wide analyses do not support the previous view of IL-33 as a dual function protein.55
Nuclear localization and chromatin association of IL-33 may thus have been selected during evolution for purposes different from regulation of gene expression. As discussed below, an essential role of nuclear localization appears to be the regulation of IL-33 cytokine activity though nuclear sequestration.44
6 BIOACTIVE FORMS OF IL-33
6.1 IL-33 full length precursor (IL-33FL)
Schmitz et al35 initially proposed that processing of IL-33 full length (IL-33FL) precursor was required for biological activity. During several years, IL-33 was thus believed to be activated by caspase 1 and inflammasomes,35 similar to IL-1β and IL-18.66 However, in 2009, we67 and others68, 69 discovered that maturation of IL-33 is not essential for activation of ST2, and that IL-33FL is a bona fide bioactive form of IL-33 that can induce ST2-dependent NFκB activity and cytokine production in target cells. Since shorter forms of IL-33 were observed during the bioassays used in some of these studies,69 it was questioned whether IL-33FL itself represented the bioactive species.69 To address this issue, we performed additional experiments and demonstrated that recombinant IL-33FL precursor produced in three different systems had biological activity and, importantly, that it was still in a full length form at the end of the bioassays.70 These later data provided definitive evidence that IL-33FL precursor is a bioactive form of the cytokine. Thus, unlike IL-1β and IL-18, and similar to IL-1α,66 IL-33 has biological activity as a full length molecule.
6.2 IL-33 mature forms
The observation that IL-33FL precursor is a bioactive form of the protein did not exclude the possibility that shorter mature forms encompassing the IL-1-like cytokine domain may also be produced during inflammation. Indeed, using a combination of in vitro and in vivo approaches, we demonstrated that inflammatory serine proteases from neutrophils70 and mast cells71 can cleave IL-33FL precursor to release shorter mature forms of 18-21 kDa. The sites of cleavage of IL-33FL by inflammatory proteases were precisely identified by mass spectrometry, within the central part of IL-33, upstream of the IL-1-like cytokine domain.70, 71 Six distinct inflammatory serine proteases were shown to cleave human IL-33FL precursor, including neutrophil cathepsin G, elastase and proteinase 3 (PR3), as well as mast cell chymase, tryptase and granzyme B. Human IL-3395-270 and IL-33109-270 are likely to be major mature forms of IL-33 in vivo because they can be generated by both activated neutrophils and IgE-activated mast cells. In mouse, mature forms of IL-33 of 19-20 kDa have been detected in BAL fluids after acute lung injury70 or exposure to fungal aeroallergen Alternaria alternata,64, 72 and in the lungs in response to chitin or migratory helminths.73
Interestingly, we observed that IL-33 mature forms generated by inflammatory proteases had significantly increased biological activity compared to IL-33FL precursor.70, 71 Indeed, at least 10 to 30 fold higher concentrations of IL-33FL were required to obtain similar levels of IL-6 secretion by MC/9 mast cells, and IL-5 or IL-13 secretion by ILC2s, respectively.70, 71 Processing of IL-33FL into the highly active mature forms could play a crucial role in vivo, particularly when IL-33FL is present at low concentrations and has no or very little activity. The observation that multiple proteases are able to cleave and activate the IL-33FL precursor71 suggests that proteolytic maturation is an important step in the regulation of IL-33 bioactivity. Neutrophil proteases could be critical for IL-33 activation during infection and type-1 inflammatory responses70 and mast cell proteases during allergic type-2 inflammation.71
7 CELLULAR SOURCES OF IL-33
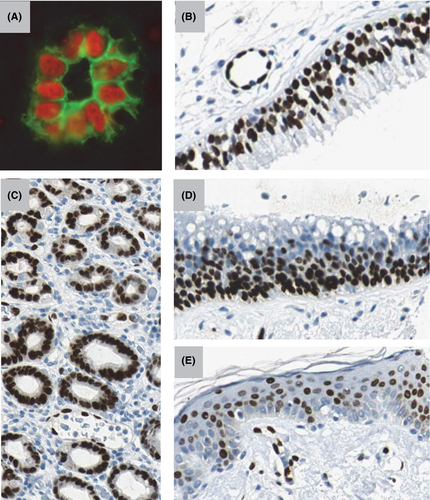
Analyses of the cellular sources of IL-33 in human and mouse tissues have revealed that IL-33 is a tissue-derived nuclear cytokine principally produced by endothelial cells (Figure 4A and B), epithelial cells (Figure 4B–E), fibroblast-like cells and myofibroblasts, rather than CD45+ hematopoietic cells, both during steady state and inflammation.36, 57, 58, 74
7.1 Endothelial cells
The endothelium is likely to constitute the major cellular source of IL-33 in the human body during steady state.36, 57, 74 We observed constitutive and abundant expression of IL-33 along the vascular tree (>50 tissues analyzed), in the nuclei of endothelial cells from large and small blood vessels of healthy tissues.57 Primary human endothelial cells in culture, particularly quiescent endothelial cells, were found to express nuclear IL-33, and specificity of the nuclear staining and identity of IL-33FL band were validated using knockdown cells.55, 67, 74, 75 Notch signaling was shown to play a key role in the induction of nuclear IL-33 in quiescent endothelial cells.75
Endothelial cells are also major sources of IL-33 during human disease. For instance, IL-33 was abundantly expressed in blood vessels from chronically inflamed tissues of patients with rheumatoid arthritis and Crohn's disease,36 and in peritumoral blood vessels from various human tumor tissues,57 although intratumor angiogenic blood vessels were negative.74
Surprisingly, although IL-33 expression was observed in a few murine vascular beds (adipose tissue, liver, ovaries),29, 58, 76, 77 the protein is generally not constitutively expressed along the vascular tree in mouse during steady state,58 revealing important species differences between humans and mice. Particularly striking is the absence of IL-33 in HEVs from murine lymphoid organs58 because we discovered IL-33/NF-HEV initially based on its abundant expression in human HEVs31, 36 (Figure 4A).
Although not constitutive, IL-33 expression in murine endothelial cells can be induced during inflammation. For instance, we observed strong upregulation of nuclear IL-33 in endothelial cells of the colon during colitis.58, 78 Others have shown that IL-33 is induced in cardiac endothelial cells after myocardial pressure overload, and that endothelium-derived IL-33 induces systemic inflammation, and plays a critical role in the heart's response to pressure overload.59
7.2 Epithelial cells
Although IL-33 expression was restricted to endothelial cells in many human tissues during homeostasis, we also observed high levels of nuclear IL-33 in the nuclei of epithelial cells in barrier tissues that are exposed to the environment.57 Indeed, mucosal epithelial cells constitute major sources of IL-33 in the respiratory and digestive tracts (Figure 4B–D), the skin (Figure 4E) and the female reproductive system, both in humans and mice.57, 58, 60, 79, 80 In mouse, we observed a very good correlation between expression of endogenous IL-33 protein in the nuclei of barrier epithelial cells and activity of the endogenous IL-33 promoter, visualized using an IL-33-LacZ reporter strain.58 Nuclear IL-33 staining was specific because it was not observed in IL-33-deficient mice.
The nasal epithelium constitutes an important source of IL-33 in the respiratory tract, both in humans (Figure 4D) and mice, with critical implications for allergic rhinitis and allergic airway inflammation.60, 81 In the lungs, the major sources of epithelial IL-33 may differ according to species. In humans, bronchial epithelial cells appear to be the major producers (Figure 4B), particularly in patients with severe asthma82, 83 or COPD.21, 84 In COPD, IL-33 expression was traceable to a subset of airway basal cells with progenitor characteristics.84 IL-33 is generally not expressed in murine airway epithelium but it can be induced after viral infection84 or exposure to cigarette smoke.21 In the murine lung, alveolar type II (ATII) pneumocytes expressing prosurfactant protein C constitute the major cellular source of IL-33 during homeostasis.21, 61, 73, 85 IL-33 expression is further upregulated during inflammation and increased numbers of IL-33+ ATII cells were found after induction of allergic airway inflammation,85-87 exposure to cigarette smoke,21 infection with nematode Strongyloides venezuelensis or intranasal administration of chitin.61 ATII cells thus remain the major IL-33-expressing cells in the murine lungs during inflammation. It is not yet clear whether alveolar epithelial cells produce IL-33 in human alveoli. Endothelial cells could represent an alternative source.
Squamous epithelial cells in the vagina58 and uterus79 produce high levels of nuclear IL-33 in female reproductive tissues. In the digestive tract, IL-33 is expressed by epithelial cells from salivary glands, mucosal surface of the stomach and gastric glands (Figure 4C), both in humans57 and mice.58 In the skin, IL-33 expression is constitutive in murine keratinocytes but inducible in human keratinocytes,80 particularly during atopic dermatitis88 where it may be regulated by p53 family member p63 (specifically ΔNp63).89 In our studies, we consistently observed nuclear IL-33 staining in human skin keratinocytes and epithelial cells of the stomach, but we noticed significant variability between different cells, different part of the tissues and different individuals, suggesting local regulation by environmental cues.57 IFNγ appears to be a critical inducer of IL-33 in human skin keratinocytes.80, 90 Inducible and heterogeneous expression of IL-33 has also been seen in epithelial cells of the colon and the intestine, during colitis and intestinal polyposis, respectively.52, 78, 91
7.3 Fibroblast-like cells and myofibroblasts
We discovered that IL-33 is abundantly expressed in the nuclei of FRCs in lymphoid organs of humans57 and mice.58 These fibroblast-like cells, which are abundant in the T-cell areas of spleen, lymph nodes, Peyer's patches, tonsil and appendix, express α-smooth muscle cell (SMC) actin (α-SMA) and have characteristics of myofibroblasts.57 The strong expression of IL-33 in α-SMA+ FRCs could be related to their contractile myofibroblastic phenotype.57 FRCs are known targets of viral infection and IL-33 produced by FRCs could play important roles in antiviral immune responses.20 Similar to FRCs from lymphoid organs, fibroblast-like cells in adipose tissue27 and α-SMA+ subepithelial myofibroblasts (SEMFs, also called pericryptal fibroblasts) in the intestine and colon52, 54, 78 express high levels of nuclear IL-33 during steady state.
In contrast to FRCs and SEMFs, fibroblasts from other normal human tissues generally do not express IL-33 constitutively.57 However, during inflammation, activated fibroblasts, fibroblast-like cells and myofibroblasts, constitute important sources of IL-33 protein, particularly in human diseases associated with tissue fibrosis, such as hepatic fibrosis,92 chronic pancreatitis,93, 94 systemic sclerosis,95 obstructive renal injury,96 or diseases associated with mucosal ulceration, such as ulcerative colitis and gastric ulcers.97 Cardiac fibroblasts,59 synovial fibroblasts,50, 98 tenocytes,99 dermal fibroblasts,80, 100 uterine stromal cells79 and FALC (fat-associated lymphoid clusters) stromal cells,101 have been shown to produce nuclear IL-33 after activation. Inflammatory cytokines such as IL-1β and TNFα are potent inducers of IL-33 in fibroblasts.93, 94, 97-99, 102
7.4 Other cell types
In addition to epithelial barrier tissues and lymphoid organs, we detected abundant expression of IL-33 in the brain and eye of wild-type mice during steady state.58 Strong activity of the IL-33 promoter was observed in the corpus callosum, hippocampus (dentate gyrus), thalamus and the cerebellum (granular layer and white matter).58 In the healthy brain, IL-33 is produced by glial cells and astrocytes, but not by microglia and neurons.53, 103, 104 Post-mitotic OLIG2+ oligodendrocytes are the major sources of nuclear IL-33, and they rapidly release IL-33 following brain injury to promote recovery.53 Strikingly, about one-third of isolated murine brain cells are IL-33+.53 It is not yet known whether IL-33 is expressed at such high levels in the human brain. In the eye, in addition to the optic nerve, we identified Sox2+ Muller glial cells from the retinal inner nuclear layer as major producers of IL-33.58 Strong nuclear expression of IL-33 in Sox2+ Muller glial cells was also observed by others in the human eye,45 and IL-33 was shown to mediate increased inflammatory responses following retinal injury in mouse.45 In addition to Muller glial cells, nuclear IL-33 is also produced by epithelial cells of the ciliary body58 and the conjunctiva105 in the murine eye.
We have observed nuclear expression of IL-33 in visceral SMCs of the gastrointestinal and urogenital tracts in healthy human tissues but it was significantly lower than in endothelial cells or epithelial cells.57 In addition, vascular SMCs did not express IL-33 in vivo during steady state,57 despite the fact IL-33 mRNA was previously detected in vascular SMCs in culture.35 Airways SMCs have been shown to express nuclear IL-33 in severe asthma, but again expression levels were low, compared to epithelial cells and endothelial cells in the same lung tissue.83 Although IL-33 is not expressed in liver hepatocytes during homeostasis, high levels have been detected in the nuclei of murine hepatocytes during acute hepatitis.76, 106
CD45+ hematopoietic cells, such as macrophages and mast cells, have been claimed to represent major cellular sources of IL-33 in several reports.107-113 However, convincing evidence to support these claims has not yet been provided. Bona fide cellular sources of IL-33 (i.e. endothelial cells, epithelial cells, fibroblast-like cells and oligodendrocytes) have been established by demonstrating IL-33 production using different techniques, including immunofluorescence/immunohistochemistry (detection of nuclear IL-33), western blot (detection of IL-33FL and/or cleaved IL-33), flow cytometry (detection of intracellular IL-33) and/or ELISA (detection of secreted IL-33), and by including the appropriate controls in the experiments (IL-33 knockout/knockdown cells). These later controls which are absolutely essential because of the problems with specificity of IL-33 reagents have been provided for structural cells20, 29, 53, 55, 58-61, 67, 74, 80, 81 but are lacking for CD45+ hematopoietic cells. In most studies that reported IL-33 protein expression in CD45+ leukocytes, intracellular flow cytometry was used,107-109, 111-113 but specificity of the assay was never validated in IL-33-deficient mice. Strong expression of IL-33 protein in CD45+ cells and/or F4/80+ alveolar macrophages has not been confirmed in studies that included appropriate controls.61, 62, 84
IL-33 mRNA is clearly detectable in activated CD45+ hematopoietic cells. However, since very little mRNA is expressed during steady-state, 10 to 100 fold induction in qPCR experiments does not mean that IL-33 mRNA is abundantly expressed in leukocytes. Accordingly, the use of an IL-33-citrine reporter revealed that IL-33 promoter activity is not detectable in lung macrophages, dendritic cells, T cells, B cells, eosinophils and granulocytes, even after induction of allergic airway inflammation.87 In addition, IL-33 mRNA levels observed in macrophages or dendritic cells after stimulation are considerably lower than those found in structural cells and tissues,38 and the protein levels are below the detection limit of western blot even after immunoprecipitation with anti-IL-33 antibodies.114 Some functional differences between bone marrow-derived dendritic cells115 and mast cells113 isolated from wild-type and IL-33-deficient mice have been described, but, since IL-33 is produced by bone marrow stroma,116 these could be due to indirect effects resulting from modification of leukocyte progenitors in the bone marrow of IL-33−/− mice. However, the possibility that specific leukocyte subsets could produce low levels of functional IL-33 under certain circumstances115 cannot be excluded. The use of cell-specific knock out approaches in future studies will resolve this issue.
8 MECHANISMS OF IL-33 RELEASE
IL-33 does not possess a signal sequence and is therefore unlikely to be secreted from cells via the classical ER-Golgi secretory pathway. Based on the constitutive and abundant expression of IL-33 in endothelial and epithelial cells from various human tissues, and its nuclear localization, we proposed already in 2008 and provided the first experimental evidence in 2009, that IL-33 may function as an alarm signal (alarmin) released in the extracellular space after cellular damage or mechanical injury (Figure 5), to alert the immune system of cell or tissue damage.57, 67 We showed that endogenous IL-33FL is released from endothelial cells after mechanical wounding of monolayers by scratching or induction of cell necrosis by several cycles of freezing and thawing.67 Similar to our findings, Luthi et al68 observed IL-33 release after cell necrosis induced by hydrogen peroxide or streptolysin O, a bacterial pore-forming toxin.
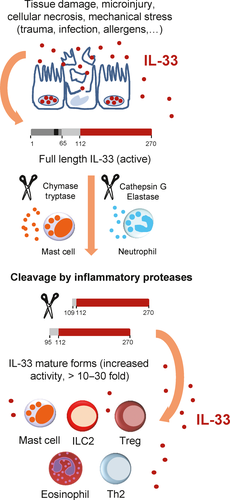
Although the mechanisms of IL-33 release in vivo remain incompletely defined, cellular damage and tissue injury appear to be critical. Indeed, in a murine model of acute lung injury associated with alveolar epithelium damage and neutrophil accumulation in the alveolar wall, we showed that IL-33FL and cleaved IL-33 forms were released in BAL fluids 2 hours after the injury.70 Viral infection can also induce lung epithelium damage, and infection with influenza virus, rhinovirus or respiratory syncytial virus has been shown to trigger IL-33 release from primary bronchial epithelial cells.21 In subjects with asthma, rhinovirus infection resulted in increased levels of IL-33 in nasal fluids.117 In mice, specific loss of IL-33+ epithelial cells in infected lung areas was observed following influenza virus-induced cell damage in vivo.21 Disappearance of IL-33 producing cells (oligodendrocytes) from the lesion site have also been observed in the brain after spinal cord physical injury.53 This was associated with rapid release of endogenous IL-33 in cerebrospinal fluid 1 hours postinjury53 followed by activation of meningeal ILC2s.118 IL-33 release was very transient since the cytokine was not detected at 3 hours or at 24 hours postinjury.53
Transient release of endogenous IL-33 has also been reported after mechanical skin injury.119 Serum IL-33 levels increased significantly 1 hour after injury (tape stripping) and returned to normal by 6 hours and at 24 hours.119 Loss of nuclear IL-33 staining in keratinocytes was observed 6 hours after hapten-induced immediate-type contact hypersensitivity,120 skin wounding (ear punch model), or intradermal injection of live Staphyloccus aureus.80 Although the epidermis became overtly necrotic 24 hours after S. aureus injection, there were no signs of epidermal necrosis at 6 hours supporting the possibility of prenecrotic secretion of IL-33.80 In primary cultures of human endothelial cells, we found that IL-33FL was released extracellularly after mechanical stress induced by cell scraping, a process that mimics the transient sub-lethal membrane disruptions that are observed in cells subjected to mechanical forces in vivo.67 Therefore, in certain circumstances, IL-33 could be released after a ‘near cell death experience’ followed by cell recovery or death at later time points.
In one study, IL-33 has been proposed to be secreted from living cells upon biomechanical stress in the absence of cell death.63 However, this was essentially demonstrated using an NIH3T3 fibroblast cell line stably transfected with a lentiviral construct coding for an exogenous form of human IL-33 fused to a tetracysteine tag, and under the control of the strong EF1 promoter.63 Transfection, exogenous overexpression in a cell line, and epitope-tagging may have modified the normal behavior of the protein, particularly the tetracysteine tag, since cysteine residues are critical regulators of IL-33 folding and activity.64 Therefore, although mechanical stress is likely to be an important inducer of IL-33 release in vivo,59, 67, 121 definitive evidence that this can occur in the absence of cell death or cell damage remains to be provided.
Other agents that cause cellular damage or necrotic cell death were found to induce rapid release of IL-33 in extracellular fluids. For instance, bee venom phospholipase A2, a major allergen that is known to cause membrane damage,122 and alum, a potent adjuvant that induces cellular necrosis,123 were found to induce IL-33 release in peritoneal fluids 30 minutes after intraperitoneal injection.122, 123 In addition, IL-33 has been shown to be released in plasma after necroptosis, a specialized pathway of programmed necrosis.124
Fungal allergen A. alternata has been proposed to induce IL-33 release in the absence of cell death, via cellular stress, through extracellular secretion of ATP, increases in intracellular calcium concentration, and generation of reactive oxygen species (ROS).72, 125, 126 A. alternata is a potent inducer of IL-33 release in vivo.64, 125, 127, 128 Indeed, a single intranasal exposure of naïve mice to A. alternata extract resulted in a very rapid increase in IL-33 levels (peak level between 15 minutes and 1 hours after exposure) in BAL fluids,64, 125, 127, 128 that correlated with a significant reduction in IL-33+ ATII cells at 1 hours.127 At the doses used, A. alternata has been shown to cause epithelial damage in vivo and to induce exfoliation of necrotic airway epithelial cells in BAL fluids within 30 minutes.129 Therefore, A. alternata-induced cell death or damage could be the major mechanism of IL-33 release in vivo. This would not exclude an important role of extracellular ATP125 or oxidative stress72, 126 in the regulation of epithelial cell death and IL-33 release. Indeed, administration of P2 purinergic receptor antagonists125 or antioxidant glutathione72 has been shown to attenuate IL-33 release in BAL fluids after intranasal exposure to A. alternata. Protease allergens, such as cysteine protease papain, have also been found to induce IL-33 release in BAL fluids shortly (1-3 hours) after intranasal exposure.130 Papain, which is known to cause lung tissue injury when used at high dose,130 was proposed to act through release of endogenous uric acid into the airway lumen.131
The capacity of A. alternata to generate oxidative stress72, 126 is a property shared with other allergens, particularly plant pollens.132 In humans, IL-33 has been shown to be released very rapidly (10 minutes) in BAL fluids from asthma patients after segmental lung challenge with plant pollens and house dust mite.133 In mice, nasal challenge with ragweed pollen has been shown to result in a prompt (1-4 hours) increase in IL-33 levels in nasal fluids.60 IL-33 release was associated with a decrease in IL-33+ nasal epithelial cells in the nasal mucosa 1 hours after challenge.60 In addition, nuclear IL-33 staining in the nasal epithelium was considerably lower in mice after continuous challenge with ragweed pollen60 or house dust mite,81 and in human patients with allergic rhinitis,60 suggesting that exposure to allergens reduced IL-33 expression by causing epithelial cells to secrete IL-33.
It has been suggested in several studies that IL-33-producing primary cells release IL-33 extracellularly after exposure to extracellular ATP,84, 103, 125, 126, 134 uric acid131 or thapsigargin (an inducer of intracellular calcium).125 However, the specificity of IL-33 detection was not validated with IL-33-deficient cells in most cases, and the possibility that IL-33 release may have occurred through cell death was not excluded in some of these studies. Indeed, it is difficult to exclude some level of cell death/necrosis in cell culture experiments. Moreover, the capacity of ATP or thapsigargin to induce cytoplasmic translocation and extracellular release of IL-33 has not been confirmed in other studies.80 Therefore, additional studies are needed to firmly establish the capacity of extracellular ATP and other agents to induce IL-33 release through cellular stress, in the absence of cell death. One possibility is that cellular and/or mechanical stress may induce a low level of IL-33 release, and that high levels of IL-33 release may only occur upon cell death and/or extensive cell or tissue damage.
9 REGULATION OF IL-33 ACTIVITY
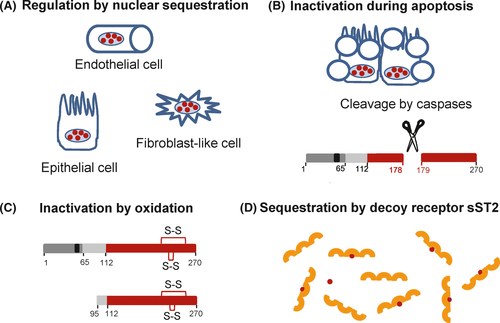
IL-33 is a very potent cytokine constitutively expressed in healthy tissues. Therefore, its activity needs to be tightly regulated at multiple levels. Several distinct mechanisms appear to be involved (Figure 6): nuclear localization or sequestration, regulation by proteases, inactivation by oxidation of cysteine residues, or sequestration by soluble decoy receptor sST2.
9.1 Nuclear sequestration
A major mechanism of regulation appears to be through nuclear localization/sequestration of the protein. Indeed, deletion of the N-terminal nuclear domain containing the CBM has been shown to result in early lethality in a knock-in mouse model.44 Heterozygous mice bearing the mutation displayed marked multi-organ inflammation with dense infiltrates of eosinophils, inflammatory monocytes, neutrophils, and macrophages. The mice died 3 months after birth. Death was associated with constitutive release of IL-33 in serum and was prevented in ST2-deficient mice, indicating that it was caused by the potent pro-inflammatory effects of extracellular IL-33.44 Overexpression of mature IL-33 in skin keratinocytes in transgenic mice has been shown to cause an inflammatory skin phenotype, whereas overexpression of full length IL-33 in the same system did not cause spontaneous cutaneous inflammation.135 Keeping IL-33 in the nucleus of producing cells is thus essential for avoiding unwanted cytokine activity and maintaining survival of the organism.
9.2 Regulation by proteases
We67 and others68, 136 have shown that IL-33 is cleaved and inactivated by caspases during apoptosis, a process that does not trigger inflammation in vivo. In cells undergoing apoptosis, endogenous caspases were found to cleave IL-33 after residue Asp178 (Asp175 in mouse),67, 68, 136 at a consensus site of cleavage for caspase-3 (DGVDG) (Figure 2). This cleavage site, which is located in a flexible loop between β4 and β5 strands in the middle of the IL-1 cytokine domain, is not found in other IL-1 family members. This suggests specific regulation of IL-33 during apoptosis, probably related to the high levels of constitutive expression of the cytokine in healthy tissues. Recombinant caspase-3 and caspase-7, another caspase activated during apoptosis, processed IL-33 at the DGVDG site in vitro.67, 68 Recombinant caspase-1 was also able to induce the cleavage of IL-33 after Asp178 when added to cell extracts,67 although the cleavage may be indirect and mediated by caspase-7.137 Interestingly, caspase-7, which is a substrate for caspase-1,138 translocates to the nucleus following activation by caspase-1.139 It could thus play an important role in processing and inactivation of IL-33 in the nucleus. Activation of caspase-1 and caspase-7 has been proposed to be involved in resolution of type-2 allergic inflammation, through the inactivation of IL-33.137, 140
Inflammatory proteases are other critical regulators of IL-33 bioactivity. As discussed above, they process IL-33FL in the central domain, upstream of the IL-1 cytokine domain, and generate mature forms with increased biological activity.70, 71 Calpain has also been reported to cleave IL-33141 but since the processing site has not been mapped, it is not known whether this cleavage results in IL-33 activation or inactivation.
Inflammatory proteases, when present in large amounts, could also be involved in IL-33 inactivation, through protein degradation. Indeed, mast cell chymase has been shown to play a role in IL-33 degradation in mouse,142 although this was not observed in human,71 suggesting the existence of species differences. Proteinase 3, another protease of neutrophils, has a dual role in IL-33 activation and inactivation.143 It has been shown in several models that IL-33 accumulates during a couple of hours after release and that it is generally no longer detectable after 6 hours.53, 60, 64, 70, 72, 119, 122, 125, 130 IL-33 degradation by inflammatory or other proteases is thus likely to be one of the important mechanisms that limit IL-33 activity in vivo.
9.3 Inactivation by oxidation
Biological activity of IL-33 in the extracellular microenvironment has been found to be rapidly terminated by the formation of two disulfide bridges.64 It was shown that IL-33 is oxidized on critical cysteine residues shortly after extracellular release in a reduced form.64 Cysteine oxidation then drives a conformational switch to a biologically inactive disulfide-bonded form of IL-33. Bioactive reduced IL-33 was no longer detectable in BAL fluids 2 hours after exposure to A. alternata.64 The four critical cysteine residues were identified as Cys208, Cys227, Cys232, and Cys259, and mutation of these four cysteines resulted in prolonged activity of IL-33 in the lung microenvironment.64 Oxidation of cysteines, shortly after extracellular release, is likely to be an important mechanism of IL-33 inactivation in vivo. It could be even more rapid than protein degradation. In any case, this means that the first few hours or even the first few minutes after IL-33 release are likely to be critical for activation of target cells.
9.4 Sequestration by soluble ST2 receptor
A third mechanism that may limit IL-33 activity after extracellular release is its possible sequestration by soluble (decoy) receptor sST2.144 The mRNA encoding sST2 is generated by alternative splicing and lacks the sequence encoding the transmembrane domain of full length ST2 (ST2L). sST2 is produced by various cell types, including mast cells and Th2 cells.145, 146 High amounts of sST2 are present in the serum of patients with various inflammatory diseases, and often correlate with disease severity.144, 145 Soluble sST2 can neutralize IL-33 in biological fluids121, 144, 147 and high levels of sST2 may thus significantly inhibit IL-33 activity in vivo.
10 CONCLUDING REMARKS
More than 2000 publications have been published on IL-33 since the first visualization of the protein in human tissues 15 years ago.31 IL-33 has emerged as a major regulator of tissue Tregs and ILC2s, with important roles in type-2, type-1, and regulatory immune responses. Given the multiple roles of IL-33 in the regulation of immune cells and tissue responses, and its implication in an ever growing number of diseases, there are no doubts that the number of publications will continue to expand in the coming years. Human genetics provide a strong rationale to develop IL-33 inhibitors for therapy of asthma and other allergic airway diseases such as allergic rhinitis and chronic rhinosinusitis. Indeed, clinical trials with antibodies targeting IL-33 have already been initiated. Delivering recombinant IL-33 could be useful in other conditions. For instance, it could favor tissue repair and restore tissue homeostasis in infectious and chronic inflammatory diseases. However, many fundamental questions remain to be addressed regarding IL-33 biology. The mechanisms of IL-33 release remain incompletely characterized. In addition, very little is known about the bioactive forms of IL-33 in vivo, particularly in human tissues and diseases, and how they access target cells (local effects or diffusion). The relative importance of the different cellular sources of IL-33 will need to be defined, for instance, using tissue- or cell-specific knock-out approaches. It will be also crucial to determine how the observations made in mouse models are applicable to humans, because important species differences have already been identified. It is likely that new questions will arise as we get closer to fully understanding the modes of action, mechanisms of regulation and functions of IL-33 in health and disease.
ACKNOWLEDGEMENTS
We thank actual and past members of our team and host institute for constructive discussions and essential contributions to the discoveries we have made on the IL-33 topic in the past 15 years. Work in the Girard laboratory was supported by the Agence Nationale de la Recherche (ANR-12-BSV3-0005-01, ANR-16-CE15-0009-01), Fondation ARC (SL220110603471, PGA120150202411), INCa (2013-098), LABEX TOUCAN and IDEX (ATS 2014).
CONFLICTS OF INTEREST
The authors declare no conflict of interest.




