Fc receptor is not required for inducing antibodies but plays a critical role in conferring protection after influenza M2 vaccination
Summary
The ectodomain of matrix protein 2 (M2e) of influenza virus is considered a rational target for a universal influenza A vaccine. To better understand M2e immune-mediated protection, Fc receptor common γ chain deficient (FcRγ−/−) and wild-type mice were immunized with a tandem repeat of M2e presented on virus-like particles (M2e5x VLP). Levels of M2e-specific antibodies that were induced in FcRγ−/− mice after immunization with M2e5x VLP were similar to those in wild-type mice. In addition, M2e antibodies induced in FcRγ−/− mice were found to be equally protective as those induced in wild-type mice. However, M2e5x VLP-immunized FcRγ−/− mice were not well protected, as shown by severe weight loss, higher lung viral titres and interleukin-6 inflammatory cytokine production upon influenza virus challenge compared with M2e5x VLP-immunized wild-type mice. Importantly, FcRγ−/− mice that were immunized with inactivated influenza virus induced haemagglutination inhibition activity and were well protected without a significant weight loss. Interestingly, interferon-γ-producing CD4 T and CD8 T cells were found to be prevalent in lungs from M2e5x VLP-immunized FcRγ−/− mice, which appeared to be correlated with a faster recovery after infection. These results indicate that Fc receptors play a primary role in conferring M2e-specific antibody-mediated protection whereas T cells may contribute to the recovery at later stages of infection.
Introduction
Infection with influenza A virus is a significant cause of morbidity and mortality worldwide. To evade the immune response, influenza A viruses continue to evolve through antigenic drift and shift. Current influenza virus vaccine strategies stimulate immune responses to two major viral surface glycoproteins haemagglutinin and neuraminidase, and can help to protect against the prevalent subtypes of influenza virus. However, continuous emergence of antigenically distinct new strains can represent a global pandemic threat. Therefore, influenza vaccines capable of inducing cross-protection against different influenza variants or strains need to be developed.
M2 is a membrane-anchored tetrameric protein incorporated into influenza virions and expressed on infected cell surfaces.1 The extracellular domain of M2 (M2e) is highly conserved across influenza A subtypes and has hardly changed over the last 90 years, probably because its genetic code is shared with the M1 matrix core protein.2 Therefore, M2e-based vaccines may be promising candidates for a universal influenza vaccine with a broad spectrum of prevention.3 Several studies have shown that mice immunized with M2e-carrier constructs and subsequently challenged with either a homosubtypic or heterosubtypic influenza virus infection were protected from virus-induced death.4-6 It was reported that anti-M2e antibodies did not have virus neutralizing and haemagglutination inhibiting activities.7, 8
It was suggested that alveolar macrophage and Fc receptor (FcR)-dependent mechanisms might play a role in conferring protection by anti-M2e antibodies, as evidenced by passive immunization experiments.9 Contradictory findings have been reported regarding the involvement of complement or antibody-dependent cell-mediated cytotoxicity by natural killer cells.7, 10, 11 In addition, it was reported that anti-M2e antibodies reduced the release of viruses into the extracellular fluid or stimulated the uptake by phagocytic cells via the FcRs.12 However, the mechanism of protection by M2e active immunization has not yet been investigated.
Kim et al.13 reported a construct with tandem repeat of heterologous M2e sequences (M2e5x), which was expressed in a membrane-anchored form and presented on enveloped virus-like particles (M2e5x VLP). In the present study, we investigated the in vivo protective mechanism by active immunization of FcRγ−/− and wild-type (WT) BALB/c mouse strains with M2e5x VLP in comparison with inactivated A/PR/8/1934 (H1N1, A/PR8) virus.
Materials and methods
Preparation of influenza virus and M2e5x VLPs
Influenza A/PR/8/1934 (H1N1, A/PR8) virus was grown in the allantoic sacs of 11-day-old chicken embryos at 37° for 2 days. The allantoic fluid was harvested and stored at −70° until used. The influenza virus was inactivated by mixing the virus with formalin at a final concentration of 1 : 4000 (volume/volume) as described previously.14 M2e5x VLP containing a tandem repeat of influenza virus M2e was prepared using the recombinant baculovirus expression system as described previously.13 In brief, Sf9 insect cells were infected with recombinant baculoviruses expressing influenza virus M1 matrix core protein and tandem repeat of heterologous M2e (M2e5x). Insect cell culture supernatants containing released M2e5x VLP were harvested, cell debris was removed by low-speed centrifugation, and finally M2e5x VLP vaccine was purified using sucrose-gradient ultracentrifugation. Human immunodeficiency virus VLP containing the full-length HIV-1 Env protein was prepared using the recombinant baculovirus expression system as described previously.15
Immunization and challenge
FcRγ-deficient mice (FcRγ−/− encoded by Fcer1g on the BALB/c genetic background) were purchased in a breeding pair (Taconic Farms, Hudson, NY) and maintained in the animal facility at Georgia State University (GSU). For animal experiments, 6- to 8-week-old female WT BALB/c mice (n = 8 WT mice per group) and FcRγ−/− mice (n = 8 knockout mice per group) were intramuscularly immunized with 10 μg of M2e5x VLP (total protein) or HIV VLP or inactivated A/PR8 virus at weeks 0 and 4. Blood samples were collected at 3 weeks after prime, and at 3 and 7 weeks after boost. Immunized mice were then challenged with a lethal dose (50% mouse lethal dose, 1 × LD50, approximately 50–100 plaque-forming units) or a sublethal dose (0·5 × LD50) of A/PR8 influenza virus at 8 weeks after boost immunization. All animal experiments presented in this study were approved by the GSU IACUC review board (IACUC A11026).
In vivo protection assay
Heat-inactivated immunized or naive sera were mixed with a lethal dose (4 × LD50) of A/PR8 influenza virus and incubated at room temperature for 30 min as described elsewhere.13 Naive BALB/c mice were infected with a mixture of virus and sera, and were monitored for their survival rates and weight loss for 14 days post-infection.
Determination of antibody responses
Influenza virus-specific or M2e-specific antibody titres were determined by ELISA as previously described.16 Briefly, synthetic M2e peptide (SLLTEVETPIRNEWGSRSN) or inactivated influenza virus was used as a coating antigen (4 μg/ml) on the ELISA plates with overnight incubation at 4°. The wells were washed with PBS containing 0·05% Tween-20 (PBST) and blocked with PBST containing 3% BSA for 2 hr at 37°. Serially diluted serum samples were added and incubated for 1·5 hr at 37° then horseradish peroxidase-conjugated goat anti-mouse IgG, IgG1 and IgG2a (Southern Biotechnology, Birmingham, AL) were used as secondary antibodies. The tetramethylbenzidine peroxidase substrate (Sigma-Aldrich, St Louis, MO) was used to develop colour and the optical density was read at 450 nm. Receptor destroying enzyme (RDE; Denka Seiken, Tokyo, Japan) treated serum samples were used for haemagglutination inhibition (HI) assay as previously described.17 Briefly, the serum samples were incubated with RDE (one part serum/three parts RDE) for 16 hr at 37° before heat inactivation for 60 min at 56°. The HI assay was performed as described previously.18 HI titres were determined using four haemagglutination units of inactivated A/PR8 virus and 0·5% chicken erythrocyte suspension with twofold diluted serum samples after RDE treatment.
Preparation of bronchoalveolar lavage fluid and lung extracts
For bronchoalveolar lavage fluids (BALF), the lungs were lavaged with 1 ml PBS via a 25-gauge catheter inserted in the trachea. Each mouse lung was homogenized and centrifuged at 1400 g at 4° for 20 min. The infectivity of the virus in the supernatants was determined from the 50% egg infective dose in embryonated chicken eggs. Cytokine levels in BALF samples were determined using ELISA kits for interleukin-6 (IL-6) as described above.
Flow cytometric analysis
For cell phenotype analysis, the cells were stained with fluorophore-labelled surface markers. Anti-mouse CD16/32 was used as an FcR blocker and then, an antibody cocktail that contained anti-mouse CD45-peridinin chlorophyll protein complex (PerCP), CD11b-allophycocyanin (APC), MHCII-r-phycoerythrin (PE), MHCII-PerCP, CD11c-PE-Cy7, F4/80-fluorescein isothiocyanate, CD40-PE, CD80-pacific blue was used to treat the cells. For intracellular cytokine staining, stimulated cells were surface stained for anti-CD45-PerCP, anti-CD4-APC and anti-CD8α-PE antibodies and then permeabilized using the Cytofix/Cytoperm kit (BD Biosciences, San Jose, CA). Intracellular cytokines were revealed by staining the cells with anti-interferon-γ (IFN-γ)-APC-Cy7 antibodies. All antibodies were purchased from eBioscience (San Diego, CA) or BD Bioscience. Stained BALF or lung cells were analysed using LSRFortessa (BD Biosciences) and flowjo software (Tree Star Inc., Ashland, OR).
Determination of T-cell responses
Interferon-γ-secreting or IL-4-secreting cell spots were determined on Multi-screen 96-well plates (Millipore, Billerica, MA) coated with cytokine-specific capture antibodies as described elsewhere.19 Briefly, 0·2 × 106 lung cells per well or 0·5 × 106 spleen cells per well were cultured with M2e peptide (2 μg/ml) as an antigenic stimulator. After 36 hr of incubation, the number of IFN-γ-secreting or IL-4-secreting T cells was counted using an ELISpot reader (BioSys, Miami, FL).
Preparation and in vitro stimulation of bone marrow-derived dendritic cells
Bone marrow-derived dendritic cells (BMDCs) were prepared from bone marrow cells of FcRγ−/− or WT BALB/c mice treated with 10 ng/ml of mouse granulocyte–macrophage colony-stimulating factor for 6 days. BMDCs were stimulated with various concentrations of M2e5x VLP at 2 × 105 cells/ml in 96-well plates for 2 days. IL-6 and tumour necrosis factor-α (TNF-α) cytokines were determined in the BMDC culture supernatants using ELISA according to the manufacturers’ instructions in duplicate (eBioscience). For mixed lymphocyte reactions, BMDCs were first treated with 5 μg/ml of M2e5x VLPs. After washing, BMDCs were co-cultured with carboxyfluorescein succinimidyl ester (CFSE)-labelled allogeneic C57BL/6 splenocytes with a BMDC to splenocyte ratio of 1 : 10. After 5 days, the cells were washed and the proliferation and activation of the T cells were assessed by flow cytometry.
Statistical analysis
All results are expressed as the mean ± standard error of the mean (SEM). Significant differences among treatments were evaluated by one-way or two-way analysis of variance where appropriate. P-values of ≤ 0·05 were considered statistically significant.
Results
Immune responses to vaccination with M2e5x VLP in FcRγ−/− mice
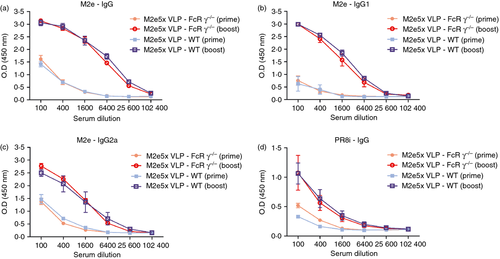
To evaluate the possible roles of FcRs in inducing antibody responses and conferring protection, groups of WT and FcRγ−/− mice were intramuscularly immunized and antibody responses in sera were determined at 3 weeks after prime, and at 3 and 7 weeks after boost. Similar levels of antibodies were observed between 3 and 7 weeks after boost (data not shown). As shown in Fig. 1, immunization of FcRγ−/− mice with M2e5x VLP vaccine induced similar levels of M2e-specific and virus-specific antibodies to those in WT mice at 3 weeks after prime and 7 weeks after boost. After boost immunization, antibodies specific for M2e were observed at significantly increased levels, over 60-fold higher compared with those observed after priming (Fig. 1a). When we determined IgG isotypes (IgG1 and IgG2a) specific for M2e peptide antigen, the level of IgG1 was found to be similar to that of IgG2a in boost immune sera in both groups (Fig. 1b,c). Hence, the mice with a deficiency of the FcRγ chain did not show any defect in inducing IgG1 as well as IgG2a antibody responses to vaccination with M2e5x VLPs. In other words, these results suggest that the FcRγ chain is not required for inducing primary and secondary antibody responses to vaccination.
M2e5x VLP immunized FcRγ−/− mice show a significant defect in protection
To determine the possible roles of FcRγ that mediate protection by M2e-specific antibodies, groups of immunized or control mice were challenged with a lethal dose (1 × LD50) of A/PR8 virus at 8 weeks after boosting. WT mice that were vaccinated with M2e5x VLPs showed a slight loss in body weight post challenge and then recovered to normal body weight, resulting in 100% protection (Fig. 2a,b). In contrast, FcRγ−/− mice vaccinated with M2e5x VLPs showed a significant loss (P < 0·001) in body weight and received only partial protection compared with WT mice. Nonetheless, M2e5x VLP immunized FcRγ−/− mice recovered faster from influenza-induced weight loss than unimmunized naive FcRγ−/− mice after challenge (P < 0·05). El Bakkouri et al.9 reported that WT and FcRγ−/− mouse strains were equally susceptible to influenza A virus challenge. Concordantly, infected naive WT and FcRγ−/− mice also showed a severe weight loss (approximately 23%) and a significant delay in recovery of body weight (Fig. 2a,b).
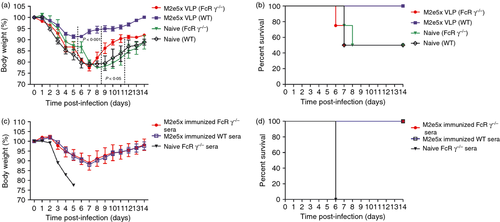
To further determine the protective quality of anti-M2 antibodies induced in WT and FcRγ−/− mice, we carried out an in vivo protection assay. Naive WT mice were infected with a mixture of sera and four lethal doses (4 × LD50) of A/PR/8/1934 influenza virus (Fig. 2c,d). Naive WT mice infected with a mixture of A/PR8 virus with immune sera from either M2e5x VLP vaccinated WT or FcRγ−/− mice showed a similar 12% loss in body weight at day 7 but recovered to a normal weight. As negative controls, all mice that received naive sera mixed with A/PR8 virus were not protected and reached 25% weight loss at endpoint. In contrast, after active immunization, despite the fact that similar levels of antibodies specific for M2e were induced in both FcRγ−/− and WT mice, there was a significant difference in severity of weight loss and survival rates between WT and FcRγ−/− mice (Fig. 2a,b), indicating a defect in conferring M2e-antibody-mediated protection without FcRs. These results (Fig. 2c,d) suggest that the protective quality of M2e antibodies themselves induced in FcRγ−/− mice was not inferior to that of WT mice.
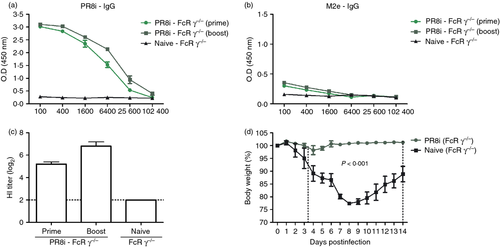
To evaluate the possible roles of FcRγ in conferring protection by virus neutralizing antibodies, groups of FcRγ−/− mice were intramuscularly immunized with 2 μg of inactivated whole A/PR8 virus. As shown in Fig. 3, immunization of FcRγ−/− mice with inactivated A/PR8 induced high levels of virus-specific antibodies but low levels of M2e-specific antibodies (Fig. 3a,b). As an indicator of virus neutralizing activity, immune sera of inactivated A/PR8 virus vaccination showed high titres of HI activity up to seven of log2 (Fig. 3c). More importantly, FcRγ−/− mice that were vaccinated with inactivated A/PR8 virus showed good protection without showing any substantial weight loss, resulting in 100% protection. Taken together, these results demonstrate that FcRs are not essential for neutralizing (HI active) antibody-mediated protection; this is different from non-neutralizing M2e antibody-mediated protection, which is dependent on the presence of the FcRγ chain (Fig. 2).
FcRγ is important for effective clearance of viral load by anti-M2e antibodies
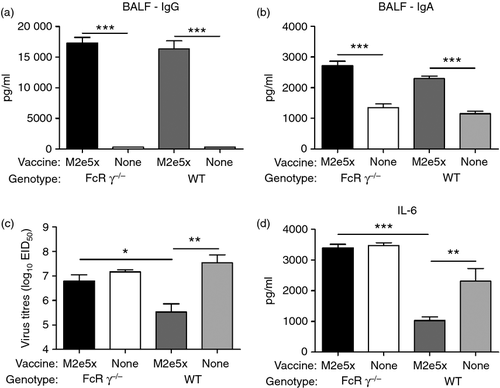
M2-specific IgG (Fig. 4a) and IgA (Fig. 4b) antibody concentrations in BALF from WT and FcRγ−/− mice vaccinated with M2e5x VLP were significantly higher than those in infected unimmunized mice on day 5 after infection. The group of WT mice vaccinated with M2e5x VLP showed significantly lower lung viral titres compared with those in the naive WT control group (P < 0·001, Fig. 4c). Furthermore, the levels of IL-6 in lung homogenates from WT mice vaccinated with M2e5x VLP were also significantly lower than those in homogenates from the naive WT control group on day 5 after infection (P < 0·01, Fig. 3d). However, viral titres (P < 0·05) as well as IL-6 (P < 0·001) levels in the lungs of FcRγ−/− mice vaccinated with M2e5x VLP were significantly higher than those in WT mice vaccinated with M2e5x VLP. Therefore, despite the similar levels of M2e antibodies between FcRγ−/− and WT mice, effective lung viral clearance was dependent on the presence of FcRs (Fig. 4c).
Enhanced effector function of M2e-specific CD8+ T cells in lungs in the absence of FcRγ
Next, we studied the induction of M2e-specific T cells in lungs and spleens. After in vitro stimulation of lung and spleen cells with M2e peptide, IFN-γ or IL-4 cytokine-producing cell spots were measured as an indicator of T-cell responses (Fig. 5). The spot numbers of IFN-γ- or IL-4-secreting lung cells were detected at threefold to fivefold higher levels in WT mice vaccinated with M2e5x VLP than in naive WT mice, respectively (P < 0·001, Fig. 5a,b). Interestingly, over twofold higher levels of IFN-γ- or IL-4-secreting lung cells were observed in FcRγ−/− mice vaccinated with M2e5x VLP compared with those in WT mice vaccinated with M2e5x VLP (Fig. 5a,b). In contrast, M2e5x VLP immunized WT mice showed higher levels of IFN-γ- or IL-4-secreting spleen cells than those detected in immunized FcRγ−/− mice, which is a reverse pattern compared with that observed with lung cells.
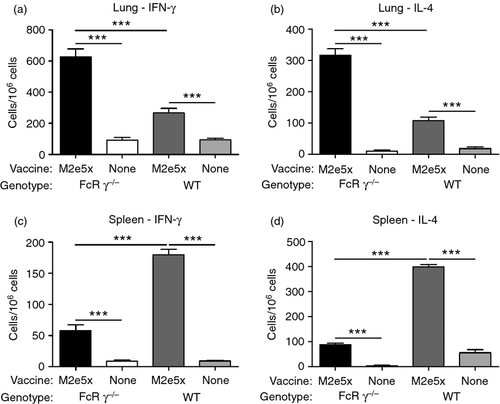
Intracellular staining would be informative in identifying the phenotypes of cells secreting IFN-γ antiviral cytokine (Fig. 6). The percentages of M2e-specific IFN-γ-producing CD4+ T cells were elevated over sixfold in WT and FcRγ−/− mice vaccinated with M2e5x VLP compared with the naive mice (Fig. 6a). The frequency of M2e-specific IFN-γ-producing CD8+ T cells was significantly increased over twofold or sixfold in FcRγ−/− mice vaccinated with M2e5x VLP compared with WT mice vaccinated with M2e5x VLP or naive FcRγ−/− mice, respectively (Fig. 6b). It is likely that M2e-specific IFN-γ-producing CD8+ T cells contributed to better recovery and protection.

FcRγ does not influence the antigen-presenting cell populations
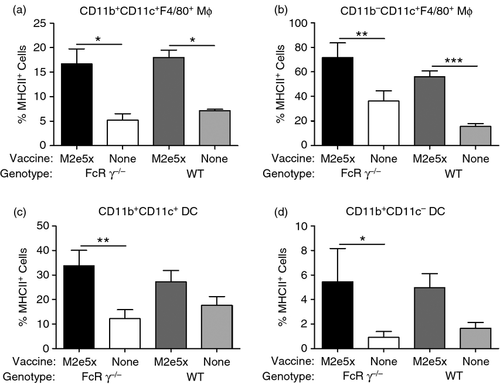
To determine whether the deletion of FcRγ affects the population of lung antigen-presenting cells and expression of MHC class II or co-stimulatory molecules after M2e5x VLP vaccination, BAL cells and lung cells from mice were harvested before and 7 days after M2e5x VLP vaccination. There were no significant differences between the WT and FcRγ−/− mice before and 7 days after M2e5x VLP vaccination, respectively (data not shown). However, expression of MHC class II antigen on CD11b+ CD11c+ macrophages (Fig. 7a), CD11b− CD11c+ macrophages (Fig 7b), CD11b+ CD11c+ phenotype dendritic cells (Fig. 7c), and CD11b+ CD11c− phenotype dendritic cells (Fig. 7d) were significantly increased in BAL cells from WT and FcRγ−/− mice vaccinated with M2e5x VLP compared with naive mice after challenge with A/PR8. There was no significant difference in the population of APCs between WT and FcRγ−/− mice.
To determine the possible roles of FcRs in antigen presentation and T-cell activation, cytokine levels were quantified in the supernatants of WT and FcRγ−/− BMDCs in vitro stimulated with M2e5x VLP. The levels of TNF-α (see Supporting information, Fig. S1a) and IL-6 (Fig. S1b) in the supernatants from BMDCs treated with M2e5x VLP were increased in a dose-dependent manner. The expression of MHCII, and co-stimulatory molecules CD40 and CD80 provides essential signals to naive T cells to initiate an optimal adaptive response. FcRγ−/− BMDCs treated with M2e5x VLP showed similar levels of MHCII, CD40 and CD80 activation markers compared with those of WT BMDCs (data not shown). There were no significant differences between the WT and FcRγ−/− BMDCs.
To investigate whether BMDCs pulsed with M2e5x VLP would translate into activating T cells, CFSE-labelled allogeneic splenocytes were incubated for 5 days with BMDCs that had been pre-treated with M2e5x VLP. T-cell proliferation was evaluated by measuring the decrease in CFSE labelling in proliferating cells. WT and FcRγ−/− BMDCs that were pulsed with M2e5x VLP similarly induced the proliferation of CD4+ and CD8+ T cells (Fig. S1c). Furthermore, WT and FcRγ−/− BMDCs when stimulated with M2e5x VLP induced similar levels of in vitro IFN-γ-secreting CD4+ and CD8+ T cells. Hence, these results suggest that FcRγ−/− mouse-derived BMDCs do not have defects in stimulating antigen-presenting cells and T cells.
M2e5x VLP can confer protection against a low-dose infection even in the absence of Fcγ receptors
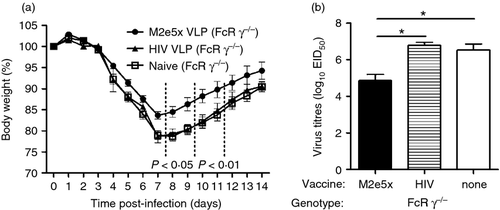
To determine the possible roles of M2e-specific T cells that contribute to protection against influenza infection, groups of M2e5x VLP or irrelevant antigen HIV VLP-immunized or naive FcRγ−/− mice were challenged with a sublethal dose (0·5 × LD50) of A/PR8 virus at 8 weeks after boost (Fig. 8). The naive and HIV VLP-immunized FcRγ−/− mice showed > 22% loss in body weight and a significant delay in recovering from weight loss, but M2e5x VLP-immunized FcRγ−/− mice exhibited a transient loss (approximately 15%) in body weight. Furthermore, viral titres in the lungs of FcRγ−/− mice vaccinated with M2e5x VLP were significantly lower than those in naive and HIV VLP immunized FcRγ−/− mice (P < 0·05) on day 7 after infection. These results may correlate with the induction of cellular responses (Figs 5 and 6), suggesting that the induction of IFN-γ+ CD4+ and CD8+ T cells are probably contributing to preventing severe weight loss and a faster recovery even in the absence of FcRs.
Discussion
Previous studies have shown that anti-M2e antibodies transferred passively could mediate protection against influenza infection in vivo.20, 21 However, protective immune mechanisms of non-neutralizing antibodies such as M2e antibodies are not yet well understood. In this study, we have attempted to better understand the protective immune mechanisms and the roles of FcRs by using two different types of influenza vaccines, the M2e-based vaccine (M2e5x VLP) and haemagglutinin-based vaccine (whole inactivated A/PR8 virus). The whole inactivated influenza vaccines were found to induce protective haemagglutinin immune responses such as HI activity similar to haemagglutinin-based VLP vaccines.22 To better address the contribution of FcRs to immunogenicity and protection after vaccination, we compared immune responses and protection in WT and FcRγ−/− mice immunized with M2e5x VLP or inactivated A/PR8 virus. Anti-M2e IgG antibody titres and IgG isotype distributions in sera were found to be similar in WT and FcRγ−/− mice vaccinated with M2e5x VLP. In addition, both immune sera of WT or FcRγ−/− mice vaccinated with M2e5x VLP were equally protective against influenza A virus. Therefore, this study suggests that FcRs were not required for inducing antibodies in terms of their quantity and protective quality after prime and boost vaccination with M2e5x VLP. That is, it is possible that the FcRγ chain may not be involved in a pathway of up-taking immune complexes of M2e5x VLP vaccine antigen and antibodies. Alternatively, these immune complexes may not be formed in vivo, even during the boost immune responses.
Interestingly, we found that immunized FcRγ−/− mice showed a significant defect in inducing protection by M2e antibodies induced in vivo after M2e5x VLP vaccination, but not by antibodies induced after vaccination with inactivated A/PR8 virus. FcRγ−/− BALB/c mice vaccinated with inactivated A/PR8 and WT mice vaccinated with M2e5x VLPs were well protected after challenge with A/PR8 virus. Huber et al.23 reported that FcRγ−/− mice were susceptible to influenza infection upon intranasal immunization with an influenza subunit vaccine. This discrepancy might be partially due to the fact that whole inactivated virus is typically more immunogenic and induces higher HI titres than subunit vaccines.22, 24-26 Also, differences in experimental protocols including route of immunization may result in differential outcomes of protection in haemagglutinin-based vaccine immunized FcRγ−/− mice between this study and others. In contrast, FcRγ−/− mice vaccinated with M2e5x VLPs showed severe symptoms of illness despite the presence of anti-M2e antibodies at similar levels. Furthermore, on day 5 after infection, viral titres as well as IL-6 in the lungs of the FcRγ−/− mice vaccinated with M2e5x VLP were significantly higher than that in WT mice vaccinated with M2e5x VLP despite the similar levels of IgG and IgA antibodies in BALF. These findings provide direct evidence that FcRs play a crucial role for non-neutralizing M2e-antibody-mediated protection whereas the induction of HI active virus-neutralizing antibodies can confer good protection in the absence of FcRγ-containing receptors. M2 tetramers are expressed at high density in the plasma membrane of infected cells and are well accessible to M2e-specific antibodies, but M2 is incorporated into the envelope of mature infectious virus particles at low levels.27, 28 Binding of M2e antibodies to virus-infected cells would facilitate their opsonophagocytosis by macrophages or complement-mediated lysis. The FcRs could be involved in antibody-dependent cell-mediated cytolysis by natural killer cells. Therefore, this study suggests that FcRs might contribute to protection through the elimination of infected cells by anti-M2e IgG antibody-dependent cell-mediated cytotoxicity or phagocytosis.
Optimum protection against influenza A virus requires antibody production by B cells as well as cytotoxic and soluble mechanisms mediated by T cells.29 Activated influenza-specific T cells have been shown to be associated with protection against influenza in human studies.30, 31 In this study, FcRγ−/− mice that were vaccinated with M2e5x VLPs recovered from severe weight loss significantly faster than naive FcRγ−/− mice. Moreover, the frequency of M2e-specific IFN-γ-producing T cells was significantly increased and the viral titres were significantly decreased in the lungs of FcRγ−/− mice vaccinated with M2e5x VLP compared with those of naive FcRγ−/− mice after influenza challenge as evidenced by ELISpot and intracellular cytokine analysis. Also, BMDCs from FcRγ−/− mice were found to have capabilities similar to those in WT mice in proliferating CD4+ and CD8+ T cells as well as stimulating IFN-γ-producing CD4+ and CD8+ T cells in response to M2e5x VLPs (Fig. S1). Eliasson et al.32 have demonstrated that an M2e-specific CD4+ T-cell response, but not CD8+ T-cell response, was induced in BALB/c mice that were intranasally immunized with protein fusion vaccine (CTA1-M2e-DD) using cholera toxin as a carrier. The results in this study suggest the induction of both CD4+ and CD8+ T-cell responses. Anti-influenza CD8+ cytotoxic T-lymphocyte activity was known to contribute to recovery from influenza infection in mice.33 Other studies have demonstrated the presence of MHC class I or II restricted epitope in M2e in mice or humans.32, 34-36 Although it is not clear why there is a discrepancy in T-cell responses among different studies, differences in immunization protocols such as types of vaccines (soluble versus particulate forms), routes of immunization (mucosal versus systemic) and use or lack of adjuvants may affect differential outcomes of immune responses. It is likely that M2e-specific CD4+ and CD8+ T-cell immunity plays an important role during recovery from illness caused by influenza infection. Inflammatory responses in the airways must be tightly regulated to ensure rapid virus clearance while avoiding excessive or chronic inflammation that may damage the delicate tissue–air interface. The spot numbers of IFN-γ-secreting or IL-4-secreting cells were increased in FcRγ−/− mice vaccinated with M2e5x VLP compared with those in WT mice vaccinated with M2e5x VLP, whereas the reverse pattern was observed with spleen cells. Considering the fact that IL-6 levels positively correlate with inflammation, high viral loads in lungs from FcRγ−/− mice vaccinated with M2e5x VLP might contribute to recruiting activated lymphocytes into the infected sites of lungs locally. Collectively, we have shown that anti-M2e antibodies, probably in cooperation with M2e-specific CD8+ T cells, are able to provide protection against influenza virus infection. The ability of M2e5x VLP vaccines to induce both M2e-specific antibodies and IFN-γ-producing T cells should help confer a broader range of cross-protection.
Acknowledgements
This work was supported by NIH/NIAID grants AI105170 (S.M.K.) and AI093772 (S.M.K.), and partially by Animal and Plant Quarantine Agency grant (S.M.K.).
Disclosures
The authors declare no competing conflicts of interest.




