Antigen 43/Fcε3 chimeric protein expressed by a novel bacterial surface expression system as an effective asthma vaccine
Summary
The IgE Fcε3 domain is an active immunotherapeutic target for asthma and other allergic diseases. However, previous methods for preparing IgE fusion protein vaccines are complex. Antigen 43 (Ag43) is a surface protein found in Escherichia coli that contains α and β subunits (the α subunit contains multiple T epitopes). Here we constructed a novel Ag43 surface display system (Ag43 system) to express Ag43 chimeric proteins to disrupt immune tolerance against IgE. The Ag43 system was constructed from the E. coli strain Tan109, in which the Ag43 gene was deleted and a recombinant plasmid (pETAg43) expressing a partial Ag43 gene was introduced. The Fcε3 domain of the IgE gene was then subcloned into plasmid pETAg43, resulting in a recombinant plasmid pETAg43/Fcε3, which was used to transform Tan109 for Ag43/Fcε3 surface expression. Thereafter, Ag43/Fcε3 was investigated as an asthma vaccine in a mouse model. Ag43/Fcε3 was expressed on and could be separated from the bacterial surface by heating to 60° while retaining activity. Ag43/Fcε3, as a protein vaccine, produced neutralizing autoantibodies to murine IgE, induced significant anti-asthma effects, and regulated IgE and T helper cytokines in a murine asthma model. Data show that Ag43/Fcε3 chimeric protein is a potential model vaccine for asthma treatment, and that the Ag43 system may be an effective tool for novel vaccine preparation to break immune tolerance to other self-molecules.
Abbreviations
-
- Ag43
-
- antigen 43
-
- Ag43/Fcε3
-
- antigen 43 and IgE Fcε3 chimeric protein
-
- AHR
-
- airway hyper-responsiveness
-
- BALF
-
- bronchoalveolar lavage fluid
-
- ELISPOT
-
- enzyme-linked immunospot assay
-
- IL-2
-
- interleukin-2
-
- mAb
-
- monoclonal antibody
-
- OVA
-
- ovalbumin
-
- aPBS
-
- vaccine vehicle, the mixture of Alum adjuvant and PBS
-
- rIgE
-
- recombinant IgE
Introduction
More than 20% of the world's population suffers from one or more of the clinical manifestations of an allergy, and these are increasing in developing countries.1, 2 However, most currently available allergy treatments focus on symptom relief, not prevention of an allergic response.3 Allergen-specific IgE antibodies have important roles in the development and regulation of allergic responses,4-6 and the constant domain, Fcε3, of IgE antibodies is a ligand that can bind to high-affinity IgE receptors (FcεRI) on the surfaces of mast cells, basophils and eosinophils.5 Then, upon allergen re-exposure, the allergen binds to cell-bound IgE, precipitating an allergic response by triggering the release of histamine and other inflammatory mediators.5 Hence, the IgE Fcε3 domain may be a potential therapeutic target for allergy control.
Until now, a humanized anti-Fcε3 monoclonal antibody (mAb) omalizumab has been used clinically for severe asthma.7-9 Also, active vaccine strategies with hepatitis B surface antigen, a rice-based edible vaccine expressing multiple T-cell epitopes, or xenogeneic recombinant fusion proteins (peptides) to induce immune responses against IgE Fcε3 have been examined as anti-allergy treatments in several pre-clinical studies.10-15 However, these strategies have significant disadvantages that limit their use. Anti-IgE mAb has a short half-life, requires frequent injections, and is cost-prohibitive. Recombinant fusion proteins expressed in Escherichia coli typically have inclusion bodies,16 so complex protocols are needed to isolate and purify their use as protein vaccines. Hence, we need better methods to create fusion proteins that can serve as active therapeutic vaccines.
Antigen 43 (Ag43) is a surface protein found in bacteria such as E. coli and is abundantly expressed (~ 50 000 copies per cell).17 Transportation of Ag43 to the outer membrane does not require chaperones because transportation information is contained in the protein.18 Ag43 is initially synthesized as a 1039-amino-acid precursor molecule that matures after removal of a signal peptide that is comprised of an α-subunit at the N-terminus and a β-subunit at the C-terminus.19, 20 The β-subunit forms a pore-like barrel structure on the outer membrane, through which the α-subunit passes to reach the bacterial surface (Fig. 1a).21, 22 Because the α-subunit associates with the β-subunit non-covalently, it can be detached by heating.23 These characteristics suggest that Ag43 fusion proteins may be easier to isolate and may be potential vaccines for breaking immune tolerance against self-molecules.
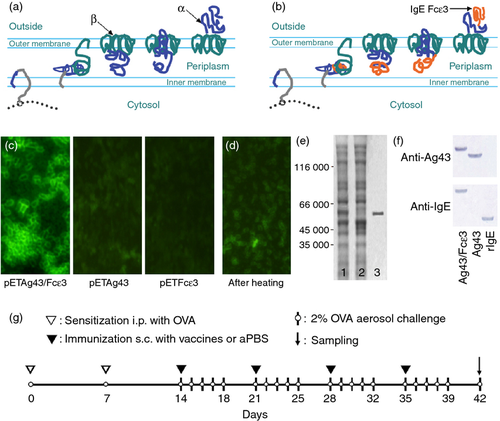
We constructed a novel Ag43 chimeric protein expression system (Ag43 system) and used the IgE Fcε3 domain as a model self-antigen to test the efficacy of our Ag43 system. We confirm that the Ag43/Fcε3 chimeric protein could be displayed on the bacterial surface and easily removed by heating to 60°. More importantly, vaccination of mice with Ag43/Fcε3 induces neutralizing autoantibodies to murine IgE, which protected animals against asthma without significant adverse effects.
Materials and methods
Construction of the Ag43 surface expression system
The Ag43 system consists of an E. coli strain with an Ag43-gene-knockout (Tan109), and a recombinant Ag43 plasmid, pETAg43. Deletion of the Ag43 gene from JM109 was performed with a TargeTron™ Gene Knockout System (Sigma-Aldrich, St Louis, MO) by insertion of a group II intron into the Ag43 gene at site 1812/1813 as described by the manufacturer. Bacterial strain knockout was confirmed by PCR and sequencing. To construct the recombinant plasmid, pETAg43, the Ag43 gene (598–3210, GenBank Accession no. CP000948) was amplified by PCR using primers 5′-CCAAAGCTTAACGGTGATACCGG-3′ and 5′-CCACTCGAGTCAGAAGGTCACAT-3′. The product was then digested and cloned into the backbone of plasmid pET-22b(+) (Novagen®, Merck KGaA, Darmstadt, Germany). Plasmid pETAg43 was then verified by restriction enzyme digestion and sequencing.
Induction of Ag43 chimeric protein expression and vaccine preparation
The Fcε3 domain of the IgE gene (517-804, GenBank Accession no. J00476) was amplified with reverse transcription PCR using primers 5′-CCGGATCCTACCTACCTGATCCCACCC-3′ and 5′-CCACTCGAGGGTGATGGAACGCTC-3′. The PCR product was then subcloned into the pETAg43 plasmid. The resultant plasmid – pETAg43/Fcε3 – was verified by restriction enzyme digestion and sequencing. Surface expression of Ag43 chimeric proteins was performed in Tan109 by growing the transformed bacteria in 1 mm isopropyl-β-d-thiogalactoside, and surface expression was measured with anti-IgE (Santa Cruz Biotechnology, Santa Cruz, CA) immunofluorescence. Bacteria were then suspended in purified water, heated to 60° for 5 min and centrifuged at 2300 g for 3 min. Supernatants were separated by SDS–PAGE and subsequent Western blotting was used to confirm the presence of chimeric proteins. The murine recombinant IgE protein (rIgE) was prepared as previously reported.24 Supernatant proteins were directly lyophilized using a lyophilizer (FreeZone 4.5; Labconco, Kansas City, MO) and stored at 4°. Protein endotoxin isolated from bacterial expression was measured with a Limulus amoebocyte lysate assay and endotoxin units were reduced to less than 0·5/μg with a Sartobind Q filter. Before vaccination, proteins were dissolved at 200 μg/ml in PBS and Imject® Alum adjuvant (Pierce Biotechnology, Rockford, IL) was added, which contained an aqueous solution of aluminium hydroxide (40 mg/ml) and magnesium hydroxide (40 mg/ml) plus inactive stabilizers, dropwise with constant mixing for 30–60 min of each protein solution to a final volume ratio of Imject® Alum to protein of 1 : 1 as previously described.25
SDS–PAGE and Western blot analysis
SDS–PAGE and Western blot analysis was performed as described previously.24 Briefly, sample proteins were separated with 12% SDS–PAGE and stained with Coomassie Brilliant Blue (Bio-Rad, Hercules, CA). Proteins were then transferred onto a PVDF membrane (Bio-Rad) using a mini Trans-Blot system (Bio-Rad). Membranes were blocked in 5% non-fat dry milk at 4°, washed, and probed with IgE antibodies (Santa Cruz) at 1 : 500, or with sera. Membranes were washed and incubated with a biotinylated secondary antibody (biotinylated rabbit anti-mouse IgG) and stained with the Vectastain ABC Kit (Vector Laboratories, Burlingame, CA).
Animals
Animal care and use were carried out in accordance with the guidelines of the Dutch Committee of Animal Experiments. All experiments were conducted under a protocol approved by the Animal Care and Use Committee (approval ID: HNMCE10012-5). Specific pathogen-free male BALB/c mice between 6 and 8 weeks old were purchased from Guangzhou Animal Centre, China. Mice were housed in makrolon cages under a laminar flow cabinet and provided with ovalbumin (OVA)-free food and water ad libitum.
Mouse asthma model and vaccine immunization
To establish a mouse allergic asthma model, mice were sensitized to OVA (Sigma-Aldrich) using two intraperitoneal injections (7 days apart) of 100 μl alum-precipitated antigen composed of 10 μg OVA absorbed onto 2·25 mg Imject® Alum (Pierce Biotechnology). Thereafter, mice were exposed to an aerosol challenge containing 2% OVA (2 mg/ml saline) for 60 min/day. The challenge was performed in a Plexiglas exposure chamber coupled to a Pari LC Star nebulizer (particle size 2·5–3·1 μm; PARI Respiratory Equipment, Midlothian, VA) driven by compressed air at a flow rate of 6 l/min. Details are given in Fig. 1(g).
To test anti-asthma effects, sensitized mice were randomly divided into four groups (n = 10). Group 1 (Ag43/Fcε3) mice were first sensitized with OVA at days 0 and 7, challenged with 2% OVA aerosol, and immunized subcutaneously with Ag43/Fcε3 (10 μg/mouse in 100 μl). The other three were control groups (Ag43, rIgE and aPBS) and were sensitized, challenged and immunized with the corresponding proteins (aPBS contained no protein).
Lung histology
For histological evaluation of lung tissues, lungs were removed and fixed in 10% PBS. Left lung lobes were embedded in paraffin, sectioned (3–5 μm), and stained with haematoxylin and eosin. Cellular infiltrates were measured in five randomly selected fields under a microscope (400 ×). Groups were analysed in a blind fashion, as previously described.24
Bronchoalveolar lavage
On day 42, after 2% OVA challenge, mice were killed by CO2 asphyxiation, and samples of bronchoalveolar lavage fluid (BALF) and lung tissue were collected as previously described.26 Briefly, 10 mouse lungs were perfused through the pulmonary artery and lavaged through the trachea three times with 1 ml PBS. Total number of BALF cells were measured using a haemocytometer. BALF cells were then spun onto slides by cytocentrifugation, fixed and visualized using Hansel stain. Differential cell counts were made on at least 400 cells as previously described.24
Quantification of eosinophils
Peripheral blood samples, lung tissues and BALF were collected at the end of the experiment. Carbol's chromotrope–haematoxylin stain was used to identify eosinophils using morphological criteria, and these were quantified as previously described.24
Measurement of airway hyper-responsiveness
Airway hyper-responsiveness (AHR) was assessed using methacholine-induced airflow obstruction in conscious mice placed in a whole body Plethysmography (model PLY 3211; Buxco Electronics, Wilmington, NC). Measurement of dimensionless parameters such as enhanced pause (Penh) was performed as previously described.24, 27 Briefly, mice were placed in the plethysmograph chamber and exposed to an aerosol of normal saline water (baseline readings) and then to increasing concentrations of β-methacholine (3 mg/ml). The aerosol was generated by an ultrasonic nebulizer and drawn through the chamber for 2 min. The inlet was then closed, and Penh readings were taken for 3 min and averaged. Values are reported as the per cent increase over baseline.
Enzyme-linked immunosorbent assay
The autoantibodies against self-IgE, OVA-specific IgE, immunoglobulin subclasses, T helper type 1 (Th1) and and type 2 (Th2) cytokines were measured by ELISA using kits or antibodies sets according to the manufacturer's protocol (BD Biosciences, San Jose, CA). Enzyme activity was measured with an ELISA reader (Bio-Tek, Winooski, VT) as described previously.28
Enzyme-linked immunospot assay (ELISPOT)
ELISPOT was used to quantify spleen B cells secreting anti-IgE, OVA-specific IgE antibody or histamine as reported previously.24 Briefly, PVDF-bottomed 96-well filtration plates (Millipore, Billerica, MA) were coated with 25 μg/ml of murine rIgE protein, OVA, or anti-histamine antibody. Mononuclear cells prepared from spleens or lymph nodes were incubated in plates at 37° in 5% CO2 for 5 hr. IgG, IgE or histamine (after a 20 μg/ml OVA challenge) bound to the membrane was stained with alkaline phosphatase-conjugated anti-mouse IgG, IgE, or anti-histamine antibodies.
Purification and adoptive transfer of immunoglobulin
Antibodies were purified using affinity chromatography (CM Affi-Gel blue gel kit; Bio-Rad) from pooled sera derived from mice at day 7 after the fourth immunization as published elsewhere.24 To assess antibody efficacy for anti-asthma activity in vivo, purified antibodies (50 mg/kg) were intravenously transferred 1 day before mice were challenged with 2% OVA aerosols (see Materials and methods; Fig. 1g). Animals were treated twice weekly until they were killed. Thereafter, lung histology, inflammatory cells, eosinophils and AHR were evaluated as described.
Histamine assay
To measure histamine release, lymph nodes were separated and cultured with OVA (10 μg/ml) for 2 hr. Then, supernatants were collected and histamine was measured with ELISA (Neogen, Lexington, KY).
Flow cytometry
The mast cell marker FcεRIα and intracellular expression of histamine were measured by flow cytometry after surface staining with an FITC-conjugated anti-mouse FcεRIα antibody (BD Biosciences) and intracellular staining with anti-histamine antibodies conjugated with phycoerythrin (BD Biosciences). For intracellular histamine staining, mononuclear cells prepared from spleens were incubated on six-well plates with OVA (20 μg/ml) at 37° and 5% CO2 for 5 hr, then fixed, permeabilized and stained with phycoerythrin-conjugated anti-mouse histamine antibodies (BD Biosciences). Flow cytometry was performed with a FACS Canto II (BD Biosciences) and analysed using bd fcsdiva software and fcs epress 4 software (De Novo Software, Los Angeles, CA).
Statistical analysis
Significant differences between experimental groups were analysed with analysis of variance and unpaired Student's t-test. Values were calculated as mean ± SEM. Differences in means were considered to be significant at P < 0·05.
Results
Surface expression of Ag43/Fcε3 and Ag43 proteins and their antigenicity
The Ag43 system consists of an Ag43-deleted strain of E. coli Tan109 and a recombinant plasmid, pETAg43. The Fcε3 domains of the mouse IgE gene were inserted into recombinant plasmid pETAg43, producing a new plasmid, pETAg43/Fcε3. This pETAg43/Fcε3 was then transformed into Tan109 to induce surface expression of Ag43/Fcε3 chimeric protein (Fig. 1b). To confirm Ag43/Fcε3 surface expression, Tan109 cells were fixed on slides and incubated with anti-IgE mAb followed by FITC-conjugated secondary antibody. Green signals were observed only in bacteria transformed with plasmid pETAg43/Fcε3, not in those transformed with plasmids pETAg43 or pETFcε3 (Fig. 1c). Heating Tan109 cells expressing Ag43/Fcε3 and Ag43 proteins to 60° followed by SDS–PAGE confirmed a green signal on pETAg43/Fcε3-expressing Tan109 cells that almost disappeared after heating (Fig 1d). The SDS–PAGE results indicated that bacterial cells expressed Ag43/Fcε3 protein (Fig. 1e, lane 1) and there was loss of a protein band in heat-exposed cells (Fig. 1e, lane 2). However, a single band of a molecular weight similar to the lost band was observed in cell supernatant expressing Ag43/Fcε3 (Fig. 1e, lane 3). Ag43 was similar with respect to SDS–PAGE (data not shown). Hence, heating detached the Ag43/Fcε3 and Ag43 proteins expressed on the surface of Tan109. Figure 1(f) depicts specificity of anti-IgE monoclonal antibody to Ag43/Fcε3 and rIgE protein and binding of Ag43 sera to Ag43/Fcε3 and Ag43, respectively, suggesting that Ag43/Fcε3, Ag43 and rIgE have excellent antigenicity.
Decreased lung inflammation
Haematoxylin and eosin staining of lung sections from mice immunized with Ag43/Fcε3 revealed almost normal lung histology, with only marginal perivascular and peribronchiolar lymphocytic infiltrates (Fig. 2a). In contrast, lung sections from mice immunized with Ag43, rIgE or aPBS vaccine had significant mucus hypersecretion and airway inflammation with peribronchiolar and perivascular infiltrates, consisting of lymphocytes, eosinophils and some neutrophils (Fig. 2a). These results suggest that Ag43/Fcε3 immunization decreased mucus hyperproduction and airway inflammation.
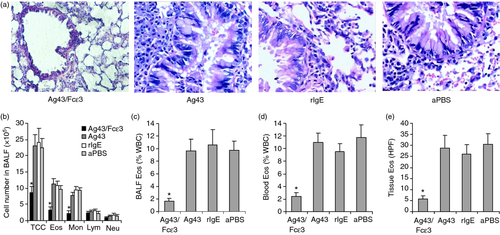
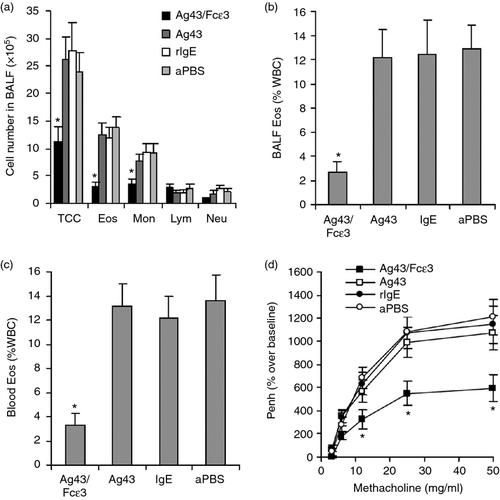
Inflammatory cells (including eosinophils) were observed in BALF, blood and lung tissues. Inflammatory cells in BALF of mice immunized with Ag43/Fcε3 were fewer than in control groups (Fig. 2b). In addition, eosinophils as measured with Carbol's chromotrope–haematoxylin stain were significantly attenuated in BALF, blood and lung tissue of Ag43/Fcε3-immunized mice compared with control mice (Fig. 2c–e). These results indicate that Ag43/Fcε3 can significantly reduce inflammation in an asthmatic mouse model.
Inhibition of airway hyper-responsiveness
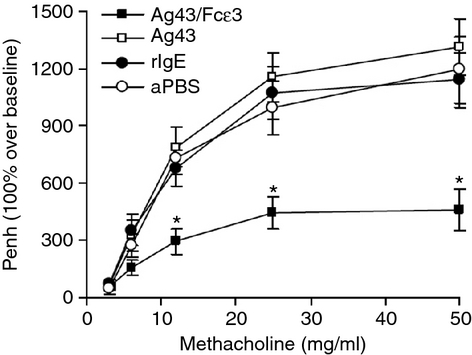
Airway hyper-responsiveness to various bronchoconstrictive stimuli is a pathological characteristic of asthma.6, 29, 30 In the present study, AHR was measured by challenging animals with increasing concentrations of methacholine at different time-points (see AHR development in Fig. 3). Mice immunized with Ag43 and rIgE, or aPBS vaccine developed significant AHR. However, mice immunized with Ag43/Fcε3 had less AHR (Fig. 3), indicating that Ag43/Fcε3 can significantly inhibit the development of AHR in an allergic asthma mouse model.
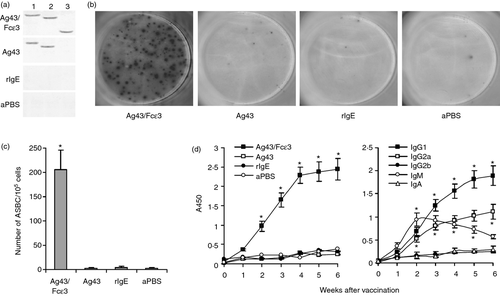
Production of autoantibodies against mouse self-IgE
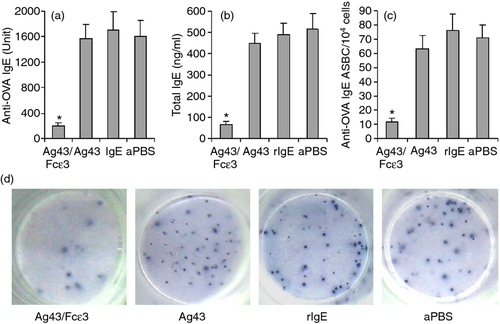
To investigate autoantibodies against mouse self-IgE, sera were isolated from experimental mice. As shown with Western blot, sera from Ag43/Fcε3-immunized mice recognized not only Ag43/Fcε3, but also Ag43 and rIgE proteins (Fig. 4a), whereas sera from Ag43-immunized mice recognized Ag43/Fcε3 and Ag43, but not rIgE protein (Fig. 4a). In contrast, sera from both rIgE-immunized and aPBS-immunized mice did not recognize Ag43/Fcε3, Ag43, and rIgE (Fig. 4a) as shown with ELISA, sera from mice immunized with Ag43/Fcε3 contained IgG antibodies against self-IgE (rIgE), and major immunoglobulin subclasses responding to Ag43/Fcε3 were significantly elevated in IgG1 and IgG2a (Fig. 4d). However, antibodies against self-IgE were not observed in sera from control mice (Fig. 4d, left). Mouse spleens were collected and anti-IgE antibody-secreting B cells were measured with ELISPOT. Compared with control groups, antibody-secreting B cells secreting antibodies against self-IgE were significantly increased in spleens of mice immunized with Ag43/Fcε3 (Fig. 4b and c). Hence, Ag43/Fcε3 immunotherapy may bypass immune tolerance and induce the production of autoantibodies against mouse self-IgE.
Anti-asthma effects by passive adoptive transfer of antibodies
Passive adoptive transfer of purified antibodies isolated from Ag43/Fcε3-immunized and control mice was performed to investigate anti-asthma effects. Inflammatory cells (including eosinophils) in BALF and peripheral blood and AHR were assayed as described above. Data show that passive adoptive transfer of purified antibodies from mice immunized with Ag43/Fcε3 also reduced inflammatory cells in BALF (Fig. 5a), significantly decreased eosinophils in both BALF (Fig. 5b) and peripheral blood (Fig. 5c) and inhibited AHR development (Fig. 5d). These results agree with those from mice immunized with Ag43/Fcε3, indicating that anti-asthma activity of the Ag43/Fcε3 vaccine is related to autoantibodies against self-IgE.
Down-regulation of IgE production
Total IgE and OVA-specific IgE were measured with ELISA. Sensitization and challenge with OVA elicited significant OVA-specific and total IgE responses in control mice, but not in mice immunized with Ag43/Fcε3 (Fig. 6a,b). Also, ELISPOT was used to measure OVA-specific IgE-secreted B cells. The antibody-secreting B cells in mice immunized with Ag43/Fcε3 significantly decreased compared with control mice (Fig. 6c,d). Hence, vaccination with Ag43/Fcε3 inhibits production of IgE antibody.
Reversion of Th2 cytokine production
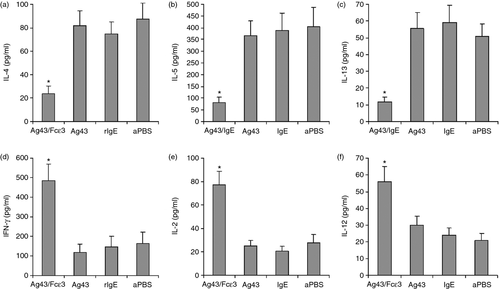
To determine whether reduced inflammation and AHR in OVA-induced allergic asthma mice immunized with Ag43/Fcε3 correlated with alterations in T helper cytokines, we measured interleukin-4 (IL-4), IL-5 and IL-13 to represent the Th2 profile, and interferon-γ, IL-2 and IL-12 to represent the Th1 profile. Lymph node cells were stimulated with OVA in vitro and cytokines were detected with ELISA. High IL-4 (Fig. 7a), IL-5 (Fig. 7b) and IL-13 (Fig. 7c) and low interferon-γ (Fig. 7d), IL-2 (Fig. 7e) and IL-12 (Fig. 7f) were measured in the lymph nodes from control mice. However, vaccination with Ag43/Fcε3 significantly decreased Th2 cytokine production and greatly increased Th1 cytokine synthesis (Fig. 7).
Decreased histamine release
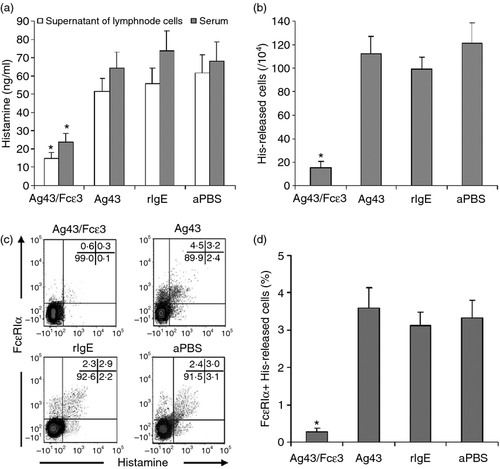
Compare with the control mice, histamine released in the serum and culture medium (Fig. 8a) was significantly reduced in mice immunized with Ag43/Fcε3. Also, histamine-secreting cells detected by ELISPOT were significantly decreased in mice immunized with Ag43/Fcε3 (Fig. 8b). Moreover, spleen monocytes double stained with ant-FcεRIα and anti-histamine antibodies and then examined with flow cytometry revealed that FcεRIα-positive and histamine-expressing monocytes were significantly decreased in mice immunized with Ag43/Fcε3 compared with control mice (Fig. 8c,d). As we know, FcεRIa is the major cell marker of mast cells and basophils. However, mast cells are mainly in the organs and basophils in the peripheral blood. Hence, the FcεRIα-positive and histamine-expressing monocytes from spleens in our current study are possibly mast cells. These data indicate that decreased histamine release is strongly related to fewer histamine-releasing mast cells.
Discussion
In this study, we expressed an Ag43 and IgE chimeric protein (Ag43/Fcε3) on a bacterial surface using a novel Ag43 surface expression system (Ag43 system). The Ag43/Fcε3 chimeric protein was then used as a vaccine against asthma in a murine asthma model. Sera from mice immunized with Ag43/Fcε3 contained autoantibodies against murine IgE and anti-IgE antibodies were secreted in spleen monocytes, suggesting that Ag43/Fcε3 chimeric protein can disrupt immune tolerance against self-Fcε3 and induce production of anti-Fcε3 autoantibodies.
Immunoglobulin E is a proven target molecule for active allergy immunotherapy.31, 32 At present, a humanized anti-IgE mAb7-9 and several recombinant fusion proteins have been reported as vaccine strategies10-15 for asthma treatment. However, anti-IgE mAb has a short half-life, requires frequent injections, and is expensive, which decreases its clinical utility. Moreover, recombinant fusion proteins usually expressed in E. coli have inclusion bodies and require complex protocols to isolate and purify them for use as protein vaccines.16 Hence, IgE mAbs and recombinant fusion protein are limited for clinical patients.
Here we constructed a novel Ag43 system in an Ag43-knockout E. coli strain, Tan109, in which a recombinant Ag43 plasmid (pETAg43) and an exogenous gene (Fcε3) could be inserted. We observed that the Ag43/Fcε3 chimeric protein can be easily expressed on the surface of E. coli Tan109, and it can be detached from the bacterial surface by heating to 60° so that it can be used as an anti-asthma vaccine. Our methods offer an easier approach for preparing recombinant fusion (chimeric) proteins. Also, because most of the multiple cloning sites from the pET-22b(+) backbone are preserved in our plasmid, pETAg43, our Ag43 surface expression system could be a universal tool for expressing other Ag43 chimeric proteins.
The amino acid regions involved in receptor binding are mainly on the Fcε3 domain.33-35 Site-targeted mutagenesis studies to identify amino acid residues directly involved in this interaction have been performed for both IgE and FcεRI.36, 37 Moreover, Garman and colleagues unveiled the crystal structure of the human IgE–Fcε–FcεRIα complex.38 Therefore, antibodies against the Fcε3 domain, such as omalizumab, can clear IgE from the circulation without causing receptor cross-linking and mediator release. Vernersson and colleagues investigated an active vaccine strategy that can reduce IgE to a clinically significant extent.13 The active vaccine component is a chimeric IgE molecule, Fcε2–Fcε3–Fcε4, in which the receptor-binding target domain, Fcε3, is derived from the recipient species, whereas the flanking domains, Fcε2 and Fcε4, are derived from a xenogeneically distant mammal, which provides foreign T-cell epitopes to break T-cell tolerance against self-Fcε3. The Fcε3 domain is the main region for the interaction of the IgE molecule with its high-affinity receptors for IgE (FcεRIα) on the target cell surface, triggering IgE-binding cell degranulation and release of mediators responsible for allergic symptoms.39, 40 Also, studies indicate that serum IgE is correlated with AHR both in adults and children,41, 42 hence IgE may represent a potential therapeutic target for allergy and asthma.
In our current study, vaccination with the Ag43/Fcε3 chimeric protein significantly alleviated asthmatic airway inflammation and hyper-reactivity, decreased production of anti-IgE autoantibodies, and decreased serum IgE. In addition, passive transfer of purified immunoglobulin from mice immunized with Ag43/Fcε3 to non-immunized mice caused similar anti-asthma effects. These data strongly suggest that Ag43/Fcε3 protein made in our Ag43 system may be an effective anti-asthma vaccine.
The Ag43/Fcε3 vaccine may break down immune tolerance against self-IgE, chiefly the Fcε3 domain and production of anti-IgE autoantibodies. Anti-IgE autoantibodies can bind to IgE antibodies responsible for the allergic cascade. This specific binding functionally blocks and eliminates IgE antibodies. Decreased serum-free IgE can not only directly reduce allergic symptoms induced by mast cell degranulation but also can indirectly reduce allergic inflammation by decreasing FcεRIα expression on the surface of mast cells and even dendritic cells.10, 43 The latter effect may be more significant than direct actions on IgE. In addition, previous studies suggest that suppression of Th2 or reversion of Th2 to a Th1 immune response can decrease allergic airway inflammation and airway hyper-reactivity.4, 7, 10, 44
We found that the Ag43/IgE chimeric protein vaccine could reverse the Th2 to Th1 response and that suppression of airway inflammation and airway hyper-reactivity is related to this. We have no direct data, so further study is needed to unveil this mechanism.
In summary, we confirm that the Ag43/Fcε3 chimeric protein expressed by our Ag43 system can significantly induce anti-asthma activity in an asthma model. Immune tolerance against self-Fcε3 was disrupted by the Ag43/Fcε3 vaccine and significant inhibition of lung inflammation and AHR was also observed in asthmatic mice immunized with Ag43/Fcε3. In addition, reversal of Th2 cytokines to Th1 cytokines and decreased total IgE and antigen (OVA) -specific IgE were observed in asthmatic mice immunized with Ag43/Fcε3. Moreover, vaccination with Ag43/Fcε3 decreased histamine release in asthmatic mice. Collectively, anti-asthma activity induced by Ag43/Fcε3 vaccination is related to a break in immune tolerance against self-Fcε3 and production of autoantibodies against Fcε3. In addition, our data suggest that Ag43/Fcε3 is a potentially safe asthma vaccine for humans. Because many self-molecules, such as cytokines, angiogenesis-related molecules and tumour antigens, are targets for active immunotherapy in various diseases, our Ag43 surface expression system may have diverse applications for future vaccine design.
Acknowledgements
This study was supported by grants from the National Basic Research Programme of China (2010CB534909), the National Natural Science Foundation of China (81160288, 81260262, 81360482), and Hainan Provincial Natural Science Foundation (812198, 061009). We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Authors' contributions
GHT conceptualized, designed and performed the experiments, collected and analysed the data and contributed to writing the manuscript; FYH, CCW and YHH performed experiments, collected and analysed data and contributed to writing the manuscript. HGZ, JLG, SLZ, HW and YYL performed significant components of the experiments. All authors read and approved the manuscript.
Disclosures
The authors declare no financial or commercial conflict of interest.




