Does measurement of ankle-brachial index contribute to prediction of adverse health outcomes in older Chinese people?
Abstract
Background/Aims
This study examined whether ankle-brachial index (ABI) is predictive of all-cause mortality, cardiovascular mortality, hospital admission for stroke, ischaemic heart disease or myocardial infarction among older people aged 65 years and above, and whether the inclusion of ABI in prediction models adds any incremental value to traditional cardiovascular risk factors.
Methods
Four thousand men and women living in the community aged 65 years and over were recruited. ABI was measured, and information regarding comorbidity, smoking habit, physical activity and physical limitation was obtained at baseline. Hospital admissions for stroke and ischaemic heart disease/myocardial infarction were documented after a mean period of 6.0 years and mortality after a mean of 9.1 years.
Results
ABI <0.9 alone was predictive of all outcomes with the exception of hospital admission for stroke. Inclusion of ABI in a model that includes other ‘traditional’ cardiovascular risk factors such as age; physical activity scale of the elderly; history of hypertension and other cardiovascular diseases, diabetes and smoking; and systolic blood pressure >140/90 reduced the hazard ratios but did not alter the overall results. Comparison of prediction models with and without ABI showed little difference. When different values of ABI were examined for all outcomes, values between 0.9 and 1.0 had high specificity but low sensitivity.
Conclusion
ABI measurement (<0.9) predicted adverse outcomes with high specificity but low sensitivity. However, it added little incremental value to prediction of adverse outcomes using traditional cardiovascular risk factors.
Introduction
Measurement of ankle-brachial index (ABI) is a clinic-based procedure that was first developed for detection of peripheral artery disease.1 Subsequently, the ABI has been suggested to represent generalised atherosclerosis because it is likely that the atherosclerotic process is unlikely to be confirmed to leg arteries alone.2 This view is supported by several studies that ABI is a good predictor of subsequent cardiovascular event among the general adult population age 55–74 years,3 among hypertensive subjects,4 among those with a history of stroke,5 and also among older population aged 80 years and above.6 Two systematic reviews concluded that ABI <0.9 in the general population is predictive of all-cause mortality, cardiovascular mortality, and occurrence of coronary heart disease and stroke,2, 7 and that the majority of subjects with low ABI is symptomatic. Among older patients aged 80+ years in the primary care setting, the prevalence is as high as 40% and 80% of these patients did not have symptoms.8 These observations led to suggestions that ABI should be included as part of cardiovascular risk factor screening in addition to traditional cardiovascular risk factors in order to improve prediction of outcomes.2 A meta-analysis based on 16 predominantly white cohorts with >480 000 person years of follow up showed that ABI improves on prediction of cardiovascular events and mortality using Framingham risk score.9 Ethnic and gender differences have also been documented.10 There are few large-scale population prospective studies among older Chinese to examine whether ABI predicts all-cause mortality, cardiovascular mortality, incident coronary heart disease and stroke. It is uncertain whether the use of the cut point of <0.9 widely used among the white people population is applicable to the Chinese. Finally, if ABI is predictive of outcomes, then the question is whether it provides any incremental value over and above conventional risk factors before it can be recommended to be included as part of cardiovascular assessment.
In a community-based study of 4000 Chinese men and women aged 65 years and over living in the community, we examined whether ABI is predictive of 7-year all-cause mortality, cardiovascular mortality, hospital admission for stroke, ischaemic heart disease or myocardial infarction. In addition, the cut-off value is examined, and calculations were made to determine whether the inclusion of ABI in prediction models adds any incremental value to traditional cardiovascular risk factors.
Methods
Two thousand men and two thousand women aged 65 years and over living in the community were invited to attend a health check carried out in the School of Public Health of the Chinese University of Hong Kong by placing recruitment notices in community centres for the elderly and housing estates. Several talks were also given at these centres explaining the purpose, procedures and investigations to be carried out. Subjects were volunteers and the aim was to recruit a stratified sample so that approximately 33% were in each of these age groups: 65–69, 70–74 and >75. Participants who were unable to walk without assistance were excluded. The study was approved by the Clinical Research Ethics Committee of the Chinese University of Hong Kong, which requires informed consent to be obtained.
A questionnaire containing information regarding demographics, socioeconomic status, medical history, smoking habit and physical activity level was administered by an interviewer. The presence or absence of disease was based on subjects' report of diagnosis by their doctors. Physical activity level was assessed using the physical activity scale of the elderly (PASE). This is a 12-item scale measuring the average number of hours per day spent in leisure, household and occupational physical activities over the previous 7-day period. Activity weights for each item were determined based on the amount of energy expended, and each item score was calculated by multiplying the activity weight by activity daily frequency. A summary score of all the items reflects the daily physical activity level.11
Measurement of blood pressure ABI
Resting pulse rate was measured twice with subjects in sitting position for 30 s, after at least 5 min of rest in a quiet room. The average pulse rate was obtained. Duplicate measures of supine blood pressure in right arm and both ankles were performed using a standard mercury sphygmomanometer and an 8 MHz Doppler probe. The study coordinator placed an appropriately sized cuff over the right brachial artery and around each ankle, proximal to the malleolus. The blood pressure cuff was rapidly inflated to 30 mmHg above the audible systolic pressure and deflated over each artery slowly. Using a hand-held Doppler (Pocket Doppler Model 841-A, Parks Medical Electronics, Inc., Aloha, OR, USA) with an 8 MHz probe, the pressure in each artery was recorded as the first audible systolic pressure. For each subject, systolic pressure was measured in the right brachial artery first, then in the right lower extremity and left lower extremity. Two measurements were performed at each site. The ABI was calculated for each leg by dividing the posterior tibial systolic pressure in each lower extremity by the upper extremity pressure. The current standard for diagnosis peripheral artery disease is defined as an ABI of less than 0.90.12 An ABI of less than 0.90 is 95% sensitive and 99% specific for angiographically diagnosed peripheral artery disease.1 The lowest ankle-arm index of the two was used to determine the extent of ischaemic disease.
The study commenced in 2001, and the participants were followed up for a mean of 9.1 years (Fig. 1). Hospital admission data were recorded after an average follow-up time of 6.0 years, with a cut-off date of 30 September 2008. After a mean follow-up period of 9.1 year, mortality was documented through a search of the Hong Kong Death Registry. The cut-off date for determining mortality was 31 March 2012.
Recruitment flow chart.
Cause of death from cardiovascular diseases (CVD) was noted by including those with International Classification of Diseases (ICD; version 10) codes 100–199 Diseases of the circulatory system. Hospital admission into hospitals under the hospital authority and the principal diagnosis of stroke or ischaemic heart disease/myocardial infarction was identified from the hospital authority's computerised patient information system using ICD (9 cm) codes, v.17.1, 431 432.9433.434 for stroke; 410–414, v.17.3, v.81.0, for ischaemic heart disease; and 410.0 acute myocardial infarction. The hospital authority covers 95% of hospital admissions in Hong Kong. The study population as a whole was not representative of the general population of Hong Kong, in that the education level was higher.
Statistical analysis
Statistical analyses were performed using the statistical package SAS, version 9.1.3 (SAS Institute, Inc., Cary, NC, USA). Two sample independent t-tests were used for continuous variables, while Chi-squared tests for categorical variables. Four outcomes including mortality, cardiovascular mortality, hospital admission for stroke and ischaemic heart disease/myocardial infarction were examined. With respect to hospital admission modelling, the outcome was death from ischaemic heart disease (or stroke) or hospital admission, whichever came first. To assess the risk prediction ability of ABI for different outcomes in addition to the other ‘traditional’ cardiovascular risk factors treated as covariates, M1 and M2 were compared. M1 included age, education level, PASE, hypertension, diabetes, smoking, CVD and systolic blood pressure (SBP), while M2 contained covariates in M1 and ABI. All models used Cox proportional hazard regression. The area under the curve (AUC) estimated by Harrell C statistic was used to measure the concordance of predictive values with actual outcomes. AUC was compared using Wilcoxon tests between them. Reclassification improvement was calculated using the net reclassification improvement (NRI) index and the integrated discrimination improvement (IDI) index.13 For the assessment of reclassification improvement, we defined four risk categories (low, intermediate-low, intermediate-high and high) by using 0–4.9%, 5–9.9%, 10–19.9%, and 20% or above. All statistical tests were two-sided. A P-value of <0.05 was considered statistically significant.
Results
The characteristics of the participants at baseline, all-cause mortality, cardiovascular mortality and hospital admissions for CVD at year 6 are shown in Table 1. ABI <0.9 occurred more frequently among women, those who were older, less physically active, with higher SBP, and a history of hypertension and diabetes. Mortality, cardiovascular mortality and hospital admission for ischaemic heart disease/myocardial infarction were higher among the ABI <0.9 group. ABI alone was predictive of all-cause mortality, cardiovascular mortality and hospital admission for ischaemic heart disease/myocardial infarction (Table 2, Fig. 2). When other ‘traditional’ cardiovascular risk factors such as age; PASE; history of hypertension and other CVD, diabetes and smoking; and SBP >140/90 were added to the model (M2), the results were unchanged, although the hazard ratios became lower. Comparison of the two models with and without ABI (M2 vs M1) using AUC, IDI and NRI shows that inclusion of ABI improved prediction of mortality at 9 years by 0.5% (P = 0.002) using IDI, for hospital admission for ischaemic heart disease/myocardial infarction by 0.3% (P = 0.046) using IDI and 5.7% (P = 0.029) using NRI. No significant differences were observed using AUC for any of the outcomes. When different values of ABI were examined for all outcomes, values between 0.9 and 1.0 had high specificity but low sensitivity (Table 3).
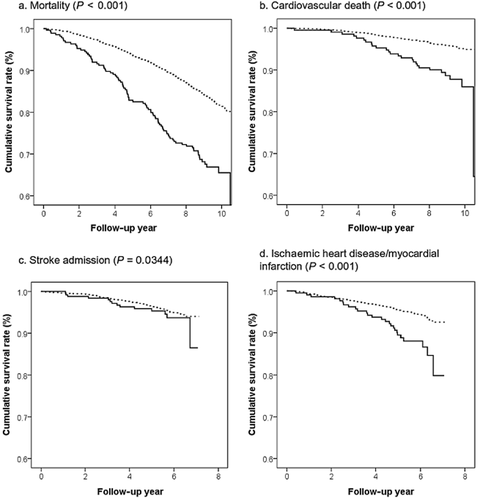
Kaplan–Meier curves (P-value of log-rank test). ( ) ≥0.9; (
) ≥0.9; ( ) <0.9.
) <0.9.
| Mean (SD)/Freq (%) | |||
|---|---|---|---|
| ABI ≥0.9 (n = 3724) | ABI <0.9 (n = 274) | P-value | |
| Female | 1820 (48.9%) | 179 (65.3%) | <0.0001 |
| Age | 72.24 (5.01) | 75.78 (6.33) | <0.0001 |
| Primary education or below | 2543 (71.0%) | 218 (79.6%) | 0.0024 |
| PASE | 92.26 (43.22) | 79.04 (37.85) | <0.0001 |
| Systolic blood pressure | 142.13 (19.00) | 149.31 (19.40) | <0.0001 |
| Hypertension | 1554 (41.7%) | 151 (55.1%) | <0.0001 |
| Diabetes | 512 (13.8%) | 67 (24.5%) | <0.0001 |
| Smoking | 251 (6.7%) | 23 (8.4%) | 0.2956 |
| CVD | 760 (20.4%) | 71 (25.9%) | 0.0302 |
| Mortality | 621 (16.7%) | 89 (32.5%) | <0.0001 |
| CVD mortality | 143 (4.4%) | 26 (12.3%) | <0.0001 |
| Hospital admission for stroke | 163 (4.57%) | 14 (5.47%) | 0.5084 |
| Hospital admission for ischaemic heart disease/myocardial infarction | 175 (5.49%) | 26 (11.93%) | <0.0001 |
- ABI, ankle-brachial index; CVD, cardiovascular disease; PASE, physical activity scale of the elderly; SD, standard deviation.
| Mortality | CVD mortality | |||||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) of ABI | AUC | IDI | NRI | HR (95% CI) of ABI | AUC | IDI | NRI | |
| ABI <0.9 only | 2.23 (1.79, 2.78) | — | — | — | 2.91 (1.92, 4.43) | — | — | — |
| M1: Covariatea only | — | 0.705 | — | — | — | 0.762 | — | — |
| M2: ABI <0.9 + covariatea | 1.64 (1.29, 2.07) | 0.709 | — | — | 1.82 (1.16, 2.87) | 0.764 | — | — |
| M2 versus M1b | — | 0.004 | 0.005 | 0.011 | — | 0.002 | 0.004 | 0.026 |
| P-value of M2 versus M1 | — | 0.795 | 0.002 | 0.267 | — | 0.930 | 0.110 | 0.338 |
| Hospital admission for stroke | Hospital admission for ischemic heart disease/myocardial infarction | |||||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) of ABI | AUC | IDI | NRI | HR (95% CI) of ABI | AUC | IDI | NRI | |
| ABI <0.9 only | 1.30 (0.75, 2.25) | — | — | — | 2.37 (1.57, 3.57) | — | — | — |
| M1: Covariatea only | — | 0.667 | — | — | — | 0.674 | — | — |
| M2: ABI <0.9 + covariatea | 0.93 (0.53, 1.63) | 0.668 | — | — | 1.99 (1.29, 3.05) | 0.677 | — | — |
| M2 versus M1b | — | 0.001 | 0 | 0.001 | — | 0.003 | 0.003 | 0.044 |
| P-value of M2 versus M1 | — | 0.980 | — | 0.947 | — | 0.909 | 0.045 | 0.126 |
- a Covariate: sex, age, education level, PASE, hypertension, diabetes, smoking, CVD and systolic BP.
- b Denotes improvement in prediction using AUC, IDI or NRI in comparing M2 versus M1: positive value × 100 = percentage increase. ABI, ankle-brachial index; AUC, area under curve for proportional hazards regression by Harrell C statistic; BP, blood pressure; CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; IDI, integrated discrimination improvement; NRI, net reclassification improvement; PASE, physical activity scale of the elderly; —, not applicable.
| Cut-off | Percentage at high-risk group (%) | Sensitivity (%) | Specificity (%) | Σ (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| Mortality | ||||||
| 0–1.00 versus ≥1.01 | 25.0 | 30.8 | 76.3 | 107.1 | 20.8 | 84.5 |
| 0–0.95 versus ≥0.96 | 12.9 | 20.0 | 88.7 | 108.7 | 26.2 | 84.6 |
| 0–0.90 versus ≥0.91 | 6.9 | 12.5 | 94.3 | 106.9 | 30.9 | 84.2 |
| Cardiovascular death | ||||||
| 0–1.00 versus ≥1.01 | 24.1 | 32.0 | 76.3 | 108.3 | 5.5 | 96.3 |
| 0–0.95 versus ≥0.96 | 12.1 | 26.0 | 88.7 | 114.7 | 9.0 | 96.5 |
| 0–0.90 versus ≥0.91 | 6.1 | 15.4 | 94.3 | 109.7 | 10.5 | 96.3 |
| Stroke admission | ||||||
| 0–1.00 versus ≥1.01 | 24.7 | 26.0 | 75.4 | 101.4 | 4.9 | 95.5 |
| 0–0.95 versus ≥0.96 | 12.6 | 15.8 | 87.5 | 103.4 | 5.8 | 95.5 |
| 0–0.90 versus ≥0.91 | 6.7 | 7.9 | 93.3 | 101.2 | 5.4 | 95.4 |
| Ischaemic heart disease/myocardial infarction | ||||||
| 0–1.00 versus ≥1.01 | 24.4 | 32.3 | 76.1 | 108.5 | 7.9 | 94.7 |
| 0–0.95 versus ≥0.96 | 12.3 | 18.4 | 88.1 | 106.5 | 8.9 | 94.5 |
| 0–0.90 versus ≥0.91 | 6.4 | 12.9 | 94.0 | 106.9 | 11.9 | 94.5 |
- Σ, sum of sensitivity and specificity; PPV, positive predictive value; NPV, negative predictive value.
Discussion
This study shows that among ambulant community-dwelling older Chinese without a history of CVD, a low ABI value (<0.9) is predictive of all-cause mortality, cardiovascular mortality and hospital admissions for ischaemic heart disease/myocardial infarction. Although the findings confirm previous observations that ABI <0.9 is predictive of future cardiovascular outcomes, with high specificity but low sensitivity, the new contribution to existing knowledge is that while ABI <0.9 may predict adverse outcomes, it conferred little incremental predictive value in addition to the more traditional risk factors.
ABI was suggested by Leng et al.3 to be included in the routine screening of cardiovascular status in 1592 men and women age 55–74 years attending general practice clinics, as it improved on predictions by conventional risk factors. In older people with a mean age of 80 years in the Framingham study, low ABI only predicted incident stroke or transient ischaemic attacks but not coronary heart disease or mortality.6 Two systematic reviews confirm the predictive value of ABI among asymptomatic individuals suggesting that it is added to traditional cardiovascular risk factors in predicting adverse outcome.2, 7 It was proposed to use ABI to screen out individuals at risk because it had high specificity but low sensitivity.2 It has also been proposed as a useful predictive tool for those with existing hypertension4 or a history of stroke.5
A meta-analysis among white people showed that while adding Framingham risk score to the prediction of total and cardiovascular mortality using an ABI value of <0.9 reduced the hazard ratio conferred by ABI alone, the ratio remained significantly elevated, suggesting that ABI measurement improves predictive accuracy.9
As a result of these observations and also the high prevalence of reduced ABI among patients aged 80 years and over among general practice clinics,8 the inclusion of ABI measurement in the clinic may be considered. While this study among the older Chinese population shows similar findings with respect to hazard ratios, we carried out additional analysis to specifically address the question of predictive power using models with and without ABI. The findings that while both models (ABI alone and in combination with ‘traditional’ cardiovascular risk factors) predicted adverse outcomes, ABI has little incremental predictive value over traditional cardiovascular risk factors. Furthermore, with respect to detection of silent peripheral vascular diseases in the primary care setting, it had been shown that palpation compared with ABI measurement was better at predicting absence of disease.8 However, one may argue that a single ABI measurement in the absence of other cardiovascular risk factor data may have some utility; in clinical practice, it is unlikely that other cardiovascular risk factors will not be documented.
It is possible that among patients at high cardiovascular risk (estimated using Framingham risk scores), the prevalence of ABI <0.9 would be high and vice versa. In these scenarios, measurement of ABI may add little predictive value compared with those at intermediate risk. Using available data from this study to stratify participants into low-, medium- and high-risk groups (modified SBP prediction omitting cholesterol and electrocardiogram14) and repeating the analysis comparing predictive power with and without ABI for each of these groups, the findings were essentially unchanged. There was no difference in predictive power in the low and intermediate risk groups, while prediction was marginally improved in the high-risk group by 0.8% (P = 0.008) for all-cause mortality (IDI) and 0.7% (P = 0.049) for hospital admission for ischaemic heart disease/myocardial infarction (IDI).
There are limitations to this study. Baseline medical diagnoses were based on history reported by participants and some disease may be silent. Some participants may have been admitted to hospitals in the private sector, although the hospital authority covers 95% of admission. There may be some degree of inaccuracies in death certification among older patients. The strengths of the study include a relatively large community-dwelling population, the longitudinal design and the inclusion of multiple adverse outcomes.
Conclusion
In spite of the limitations, we conclude that among older Chinese with a mean age of 72 years, ABI measurement (<0.9) is able to predict all-cause mortality, cardiovascular mortality and hospital admission for ischaemic heart disease/myocardial infarction with high specificity but low sensitivity. However, ABI measurement provided little incremental value to prediction of adverse outcomes using traditional cardiovascular risk factors.
Acknowledgements
This cohort was established in 2001 with support from the Hong Kong Jockey Club Charities Trust led by Dr EMC Lau.




