Chromosome social distancing and crowd control: the dual role of Ki67
Graphical Abstract
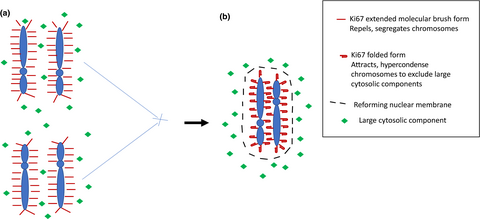
The nuclear protein Ki67 has long been a useful tool for the examination of cell division, but only recently has its role in the mechanics of chromosome organization been appreciated. The study of Cuylen-Haering et al.1 has identified dual (and apposing) roles in both the mechanics of chromosome separation and then clustering of chromosomes during cell division, resulting in exclusion of contaminating high-molecular weight cytosolic proteins from the re-forming nuclei (Figure 1).
From an immunological perspective, the central role of clonal selection in the generation of biologically significant numbers of antigen-specific lymphocytes has cemented the assessment of cell division as a key parameter in the study of immunity. Although cell division is such a critical component of immune responses, immunologists have largely ignored its mechanical complexities and have primarily been concerned with quantification of division.
Over several decades, there have been multiple techniques developed to measure cell proliferation but no one method has proved to be the universal solution for every circumstance. The milestone dates for some of the more widely used techniques are listed below.
1958: Tritiated Thymidine—Yoffey et al.2 Thoracic duct lymph of guinea pig—in vitro labeling of replicating DNA
1971: 5-bromo-2′-deoxyuridine uptake—Siskind and Thorbecke.3 Lymph node cells of immunized rabbits—in vitro labeling of replicating DNA
1983: MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay—Mosmann.4 Assessment of in vitro cell viability as surrogate of division
1983: Ki67—Gerdes et al.5 Monoclonal antibody binding a division-related nuclear protein
1994: PKH26—Ashley et al.6 Dilution of a membrane-inserting fluorescent dye
1994: Carboxyfluorescein diacetate succinimidyl ester—Lyons and Parish.7 Dilution of a protein-binding fluorescent dye
These techniques are incredibly useful and have helped to shape our understanding of the regulation of cell division. However, cell division is increasingly being revealed to be an incredibly convoluted process. For cell division to occur, it is necessary to dissolve the nuclear membrane to allow the mechanics of spindle formation and segregation of chromosomes to proceed. But as nuclear and cytosolic contents are strictly segregated in these compartments, it is necessary to ensure this remains so after division. BCR-ABL, the causative agent of chronic myeloid leukemia, is one example of how expression of a normal nuclear kinase in the cytosol can have profound effects on cell behavior.
The subject of this commentary, Ki67, differs from the other techniques above in that it is a division-related marker, rather than a direct measurement of a physical aspect of cell division. Interestingly, the name Ki67 refers to the city of origin (Kiel, Germany) and the position of the hybridoma in the 96-well plate; such is the inventiveness of scientists! For a long time, the role of the target protein bound by the monoclonal antibody Ki67 was unknown. With the passage of time, a finer examination of its synthesis and spatial position identified it as being involved in the mechanics of the cell division process itself. In particular, Ki67 was shown to be associated with condensed chromosomes as the nuclear membrane dissolves and then reconstituted as mitosis proceeds, and later shown to be involved in ensuring the segregation of individual chromosomes.
In the current paper by Cuylen-Haering et al.1 evidence is presented to support the idea that Ki67 has a dual role in cell division Figure 1. As wellas having a “molecular brush” conformation, which provides an electrostatic means to keep condensed chromosomes spatially distant, Ki67 also has the role in facilitating a form of hypercondensation of the newly formed chromosomes prior to re-formation of the nuclear membrane. This serves to exclude larger cytosolic components from the newly formed nuclei, which is necessary as the active transport mechanisms rebalancing the nuclear contents are limited to molecules less than 39 nm in size. This property of collapse of the molecular brush structure coincided with a change to the distance between the C and N termini of Ki67, imaged using two spectrally distinct fluorophores. To support the concept that clustering was an active result of Ki67 confirmation change, a Ki67 knockout line was used, with the addition of overexpression of core histones to promote chromosome individualization. In the absence of Ki67, chromosomes failed to exhibit the typical condensation seen after mitotic exit in Ki67 wild-type cells. To exclude an effect as a result of histone overexpression, the drug Trichostatin A was used to promote chromosome individualization using global DNA hypermethylation, with the same result. Together, these data support the dual role of Ki67 in both segregation of chromosomes in division and exclusion of large cytosolic molecules from the nucleus after mitotic exit and re-formation of the nuclear membrane.
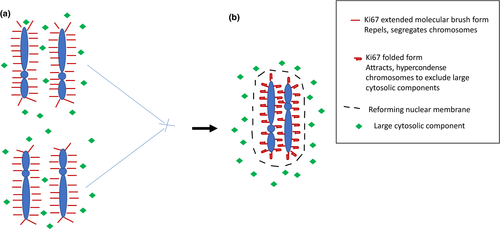
However, there remain significant unknowns. Firstly, is there an as yet undescribed mechanism to regulate the binary nature of Ki67 activity as a chromosomal segregator or aggregator? It is perhaps easy to imagine how the extended conformation of Ki67 can act as a molecular brush to form a protective space around the chromosome during prometaphase. However, the way the different conformation promotes chromosome clustering remains to be uncovered. The lack of exclusion of larger cytosolic components in the absence of Ki67 suggests this is an active process, rather than a passive result of absence of the molecular brush. Secondly and even more importantly, although Ki67 is conserved across species, its function does not appear essential, as a murine Ki67 knockout exhibits normal cellular development and function (Sobecki et al.8). Although a lack of Ki67 does not affect proliferation of cell lines either, it may be required for maintenance of the cancer stem cell phenotype (Cidado et al.9). Thus, intriguing questions about Ki67 function remains: if it has a central role in the re-establishment of nuclear/cytosolic compartmentalization, why does its absence not result in aberrant cell division or some other physiological consequences?
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Author Contribution
Alan Bruce Lyons: Conceptualization; Writing-original draft; Writing-review & editing. Susanne Heinzel: Conceptualization; Writing-original draft; Writing-review & editing.