Immunometabolism of Leishmania granulomas
Abstract
Leishmania are parasitic protists that cause a spectrum of diseases in humans characterized by the formation of granulomatous lesions in the skin or other tissues, such as liver and spleen. The extent to which Leishmania granulomas constrain or promote parasite growth is critically dependent on the host T-helper type 1/T-helper type 2 immune response and the localized functional polarization of infected and noninfected macrophages toward a classically (M1) or alternatively (M2) activated phenotype. Recent studies have shown that metabolic reprograming of M1 and M2 macrophages underpins the capacity of these cells to act as permissive or nonpermissive host reservoirs, respectively. In this review, we highlight the metabolic requirements of Leishmania amastigotes and the evidence that these parasites induce and/or exploit metabolic reprogramming of macrophage metabolism. We also focus on recent studies highlighting the role of key macrophage metabolic signaling pathways, such as mechanistic target of rapamycin, adenosine monophosphate-activated protein kinase and peroxisome proliferator receptor gamma in regulating the pathological progression of Leishmania granulomas. These studies highlight the intimate connectivity between Leishmania and host cell metabolism, the need to investigate these interactions in vivo and the potential to exploit host cell metabolic signaling pathways in developing new host-directed therapies.
Introduction
The parasitic protist Leishmania spp. is the causative agent of human leishmaniasis, a spectrum of diseases that includes both localized and disseminating cutaneous and mucocutaneous infections, as well as infections of the liver/spleen/intestine (visceral leishmaniasis), which is fatal in over 95% of untreated cases.1 Despite some success in reducing the incidence of visceral leishmaniasis, human leishmaniasis remains a significant global health burden with a minimum global case load of 12 million, 0.7–1 million new cases per annum and 30 000 deaths/year.2, 3 A further 120 million people are thought to be asymptomatically infected,4 contributing to transmission and the reactivation of disease in immunocompromised individuals. A safe vaccine has yet to be developed and current drug treatments are suboptimal because of toxicity, expense, low efficacy in generating sterile cure, and emerging resistance.1
Leishmania develop as flagellated promastigotes in the midgut and mouthparts of the sandfly vector, before being injected into the skin of vertebrate hosts during the sandfly blood meal. Nondividing metacyclic promastigotes are initially phagocytosed by neutrophils, which rapidly infiltrate the site of tissue damage.5 Infected apoptotic neutrophils and/or released parasites are subsequently taken up by tissue-resident macrophages (dermal macrophages in the skin and Kupffer cells in liver) and dendritic cells, which triggers the differentiation of internalized parasites into obligate intracellular amastigotes within the parasitophorous vacuole (PV) of these host cells.5 Further recruitment of monocytes and other immune cells leads to the development of granulomatous lesions in the skin composed of infected and uninfected macrophages, monocytes and dendritic cells, as well as a significant number of neutrophils (that can also be infected) and eosinophils6-8 (Figure 1a). Expansion of the granuloma occurs through the interferon-γ (IFNγ)-mediated recruitment of inflammatory monocytes that rapidly outnumber tissue macrophages and actively phagocytose dying infected host cells, promoting parasite transmission between monocytes and macrophages in the lesions9-11 (Figure 1a). These primary lesions are major sites of parasite expansion or control, as well as a source of parasites that seed secondary granulomas in other dermal sites or internal organs in susceptible hosts. Significantly, parasites can persist long after natural or drug-mediated resolution of primary lesions, providing a parasite reservoir that can lead to subsequent reactivation of disease in immunocompromised individuals, many years after the initial infection.
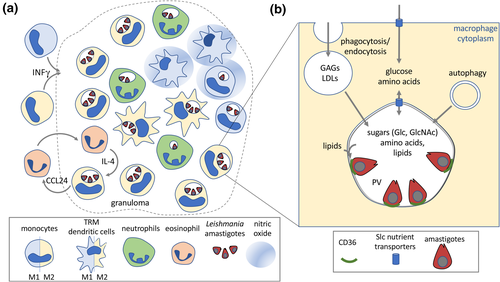
The extent to which Leishmania granulomas promote or control parasite development is dependent on several factors, including the species of Leishmania involved, host genetics as well as early innate and subsequent adaptive immune responses. In genetically resistant murine models, the development of self-resolving lesions is associated with a strong CD4+ T-helper type 1 immune response and production of proinflammatory cytokines, IFNγ and interleukin (IL)-12 that polarize infected and uninfected macrophages in lesions toward a classically activated M1 state.5 M1 macrophages secrete inflammatory mediators (e.g. IL-12, tumor necrosis factor-α) and express high levels of inducible nitric oxide synthase (iNOS) and NADPH oxidase 2, leading to production of nitrous oxide (NO) and reactive oxygen species (ROS), respectively, with concomitant containment or clearance of intracellular parasites (Figure 1a). Conversely, acute, nonresolving infections are associated with a T-helper type 2 immune response and production of anti-inflammatory cytokines (e.g. IL-4, IL-13 and/or IL-10) as well as polarization of infected macrophages toward a range of alternatively activated M2 states that promote tissue repair and constitute a permissive host cell reservoir.12 While this paradigm is supported by many studies, recent work has shown that Leishmania granulomas can contain subpopulations of macrophages in both M1- and M2-polarized states even when a strong host-protective T-helper type 1 response is induced13 (Figure 1a). M2 macrophages can be maintained under these circumstances by local feedback loops involving IL-4-producing eosinophils and/or excessive local production of NO which suppresses macrophage inflammatory responses.8, 14 These feedback loops, together with gradients in nutrients, oxygen and other factors, may lead to substantial heterogeneity in the types of host cell that are infected, as well as their replicative potential and state of polarization.
Macrophage polarization is now known to be tightly associated with changes in metabolic signaling pathways and cellular metabolism15 which underpin many key macrophage functions including motility, phagocytosis and mediator production (NO, ROS, cytokines and chemokines). Changes in intracellular metabolite levels can also directly modulate signaling transduction pathways such as hypoxia-inducible factor 1-α (HIF-1α) and induce long-term epigenetic and transcriptional programs that drive M1 and M2 polarization. Finally, changes in host cell metabolism directly impact on the availability of carbon sources and nutrients needed for growth of intracellular amastigotes. In this review, we summarize how changes in macrophage polarization and metabolism, induced by innate and adaptive immune responses as well as intracellular parasite stages, impact on the growth and survival of Leishmania. We also review the role of key macrophage metabolic signaling pathways in determining the outcome of infection.
Living in the Phagolysosome; Metabolism of Intracellular Parasite Stages
Leishmania proliferate within PVs that have the hallmark of mature acidified phagolysosomes (pH 5.5), while also containing markers for the endoplasmic reticulum/Golgi, suggesting a hybrid compartment that is continuously fusing with membrane vesicles from both the endolysosomal and secretory pathways16 (Figure 1b). Nutrient levels in the PV lumen are likely to be regulated by the rate of fusion of endocytic/phagocytic vesicles, autophagy and direct exchange of small molecules across the PV membrane (PVM; Figure 1b). Recent metabolomic analysis of macrophage lysosomes indicates that these compartments contain high levels of proteogenic amino acids, nucleotides/bases as well as sugars and lipids, consistent with their central role in degrading endogenous and exogenous macromolecules.17 These analyses also indicate that nutrient exchange across the PVM is highly dynamic and bidirectional, consistent with early studies showing that small molecules can be transported from the host cytoplasm to the lumen of the PV,18 and the finding that supplementation of the medium of Leishmania-infected macrophages with specific amino acids (arginine and ornithine) promotes intracellular amastigote growth.19 Interestingly, bidirectional transport of metabolites across the PVM may not be restricted to polar metabolites. Specifically, Leishmania amastigotes form distinct tight junctions with the PVM that may allow two-way transport of lipids between the host cell and amastigotes. These junctions have been shown to contain parasite amastin proteins, as well as the macrophage scavenger receptor, CD36, suggestive of a role in transporting fatty acids or other metabolites across the PVM.20, 21
Despite having access to a wide range of carbon sources within the macrophage lysosome, recent studies have shown that intracellular amastigotes switch to a slow growth state and activate a stringent metabolic response.22-24 The latter is associated with reduced expression of glucose and some amino acid transporters, greater dependence on glycolysis coupled with redox balancing succinate metabolism for adenosine triphosphate (ATP) synthesis and a truncated tricarboxylic acid cycle which is primarily used to synthesize amino acids, such as glutamine. This metabolic switch appears to be at least partly hardwired into amastigote differentiation, and not a direct response to nutrient limitation, as similar changes also occur in in vitro differentiated amastigotes cultivated in rich medium.22 Activation of the stringent response may confer protection against host-derived NO and ROS that target mitochondrial enzymes containing iron–sulfur clusters.25 Consistent with this hypothesis, parasite lines with reduced glucose uptake and increased dependency on amino acids are highly attenuated in vivo.24 Thus, despite having access to multiple carbon sources, amastigotes are highly dependent on sugars (glucose and glucosamine) as their major carbon source.24
Leishmania amastigotes lack a glyoxylate cycle and are unable to use fatty acids as a primary carbon source. However, fatty acid uptake and β-oxidation are also upregulated in these stages, and a recent study has shown that β-oxidation of polyunsaturated fatty acids (PUFAs) is essential for virulence.26 Fatty acid β-oxidation may provide an additional source of carbon for glutamate synthesis.22, 27 Alternatively, it may be required to prevent accumulation of toxic levels of PUFA.26 A similar function has been proposed for the Leishmania iron transporter protein, iron regulator I, which is thought to play a critical role in preventing iron toxicity within the PV.28
Impact of Macrophage Metabolism on Intracellular Leishmania Growth
While Leishmania appear to reside within a relatively nutrient-replete niche, some nutrients may become limiting and/or reach toxic levels depending on the macrophage activation state. In particular, changes in host cell amino acid and central carbon metabolism have been shown to impact significantly on amastigote growth and survival.
Host arginine metabolism
Leishmania are arginine auxotrophs and are completely dependent on host arginine for protein synthesis, as well as de novo synthesis of other essential metabolites, such as polyamines, trypanothione and the nonproteinogenic amino acid hypusine.29 Arginine levels within the PV are limiting for parasite growth as modulation of macrophage arginine levels, either by supplementation of the medium with exogenous arginine or inhibition of macrophage arginine uptake, enhances or limits amastigote growth, respectively.30 Intracellular amastigotes also upregulate the expression of their arginine transporter, AAG3, and endogenous arginase, suggesting that amastigotes need to compete against macrophage PVM arginine transporters.31 Significantly, Leishmania mutants lacking arginase are still able to cause lesions, although their growth in vivo is attenuated, suggesting that amastigotes can also scavenge essential polyamines, such as ornithine, from the PV.32-35 However, L. donovani mutants lacking enzymes downstream of ornithine (i.e. ornithine decarboxylase, spermidine synthase and S-adenosylmethione decarboxylase) are severely attenuated in virulence, suggesting that PV levels of spermidine or spermine are below those needed for parasite survival.36, 37
Changes in macrophage activation and polarization lead to significant changes in intracellular arginine pools, microbicidal NO and growth-promoting polyamines, which directly impact amastigote survival (Figure 2). IFNγ-activated M1 macrophages have elevated levels of arginine uptake, due to increased expression of the cationic amino acid transporter 2b (CAT2B) arginine transporter, as well as increased expression of iNOS38 (Figure 2). iNOS catalyzes the two step, NADPH-dependent conversion of arginine through Nω-hydroxy-l-arginine to citrulline and NO. Both Nω-hydroxy-l-arginine and NO have potent antimicrobial activities and play a key role in controlling Leishmania growth during both acute and chronic phases of murine infections.39-41 Increased expression of iNOS and NO production also strongly polarizes macrophages toward a glycolytic M1 phenotype, as NO is a potent inhibitor of mitochondrial respiration, and may prevent repolarization to an M2 phenotype.42 Interestingly, low levels of IFNγ activation can promote intracellular parasite growth by increasing arginine availability while NO levels are below a growth inhibitory threshold.38 This is particularly evident for members of the L. mexicana complex that induce large communal vacuoles and are more resistant to NO than other species of Leishmania that inhabit tight-fitting, individual vacuoles.38 Similarly, sublethal NO levels in acute skin granulomas were found to restrain but not kill intracellular amastigotes,43 and could potentially drive the parasites into a slow growth and/or metabolically quiescent state that is well adapted to survive in the granuloma tissue niche.
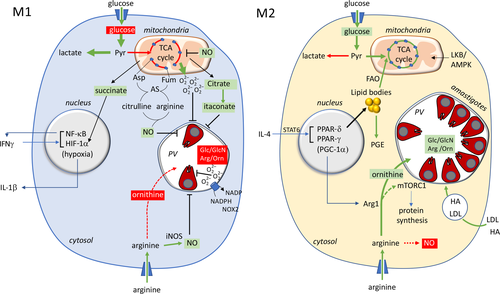
Conversely, polarization of macrophages toward an M2 phenotype by IL-4/IL-13 (via activation of STAT6), or other anti-inflammatory cytokines (e.g. IL-10/STAT3) or hypoxic tissue microenvironments, leads to increased expression of arginase-1 (Arg1), as well as the upregulation of the CAT2B transporter38 (Figure 2). Arg1 catalyzes the conversion of arginine to ornithine and urea, thereby promoting de novo glutamine, proline and polyamine synthesis which sustains collagen production and the tissue repair functions of M2 macrophages. Arg1 is reciprocally regulated with iNOS at the level of transcription (although Arg1+/iNOS+ macrophages are detected in lesions) as well as metabolically.9 Specifically, upregulation of Arg1 diverts arginine from NO production, while upregulation of iNOS results in the generation of Nω-hydroxy-l-arginine, a potent inhibitor of arginase. As a result, elevated expression of Arg1 in myeloid cells limits the antileishmanial effector functions, as well as the inflammatory effects of iNOS/NO. The upregulation of myeloid Arg1 may also deplete arginine levels in tissues and inhibit proliferation of host-protective T cells and other immune cell functions.44 Many studies have investigated whether enhanced expression of Arg1 in Leishmania-infected M2-polarized macrophages promotes parasite growth beyond antagonizing NO production. In support of such a role, parasites preferentially populate Arg1+ monocytes and macrophages in lesions, and Arg1 expression correlates with increased lesion development in susceptible BALB/c mice.41, 45 Furthermore, treatment of L. major-infected BALB/c mice with the nonselective arginase inhibitor (Nω-hydroxy-l-arginine), or conditional knockdown of Arg1 in hematopoietic and endothelial cells, showed that the hyperexpression of Arg1 may prevent uncontrolled disease progression, visceralization and lethality.19, 46 By contrast, conditional knockdown of Arg1 expression or a block in Arg1 induction in C57BL mice that form self-curing lesions had no effect on the progression of disease or parasite persistence after granuloma resolution.9, 44 Moreover, proliferating L. major parasites did not exhibit a tropism for Arg1+ macrophages in latent C57BL infections, indicating that elevated expression of host arginase is not essential for intracellular survival.47 These differences in the significance of host arginase in Leishmania infection outcomes likely reflect variability in Arg1 expression in different mouse strains (normally much higher in BALB/c mice) and in the innate microbicidal capabilities of BALB/c and C57BL mice.44 Interestingly, Arg1-dependent synthesis of putrescine has recently been shown to sustain cycles of macrophage efferocytosis and internalization of apoptotic or pyroptotic cells.48 Elevated Arg1 expression in newly recruited monocytes may therefore promote parasite transmission between granuloma phagocytes through efferocytosis.
Host tryptophan metabolism
Leishmania growth within macrophages may also be constrained by the availability of other amino acids, such as tryptophan. IFNγ-activated M1 macrophages upregulate expression of indoleamine-2,3-dioxygenase and the conversion of tryptophan to kynurenine metabolites, reducing the availability of tryptophan to amastigotes.49 Indoleamine-2,3-dioxygenase is highly expressed in cutaneous Leishmania lesions, although the impact of indoleamine-2,3-dioxygenase 1 expression on parasite burden remains poorly defined. Macrophages also utilize tryptophan for NAD+ biosynthesis, with tryptophan 2,3-dioxygenase (TDO) catalyzing the first step. Although TDO expression is not induced by IFNγ, TDO levels are negatively correlated with parasite load in L. major cutaneous lesions and TDO inhibition in ex vivo infected macrophages promotes parasite growth.49 Whether indoleamine-2,3-dioxygenase and TDO act synergistically or separately to restrict Leishmania growth needs further investigation.
Host central carbon metabolism
Infection of murine macrophages with L. donovani increased expression of glucose transporters, leading to an initial switch to enhanced aerobic glycolysis.50 However, after 24 h, infected macrophages upregulated mitochondrial respiration, consistent with a switch toward an M2-polarized phenotype. Infected macrophages in L. major lesions also exhibit elevated oxidative phosphorylation, indicative of M2 polarization in vivo.14 Although the metabolic reprogramming of Leishmania-infected macrophages has not been examined in detail, IL-4-mediated polarization of macrophages is associated with increased mitochondrial biogenesis, and the operation of a complete tricarboxylic acid cycle, which is supported by increased glutaminolysis and fatty acid β-oxidation.15, 51 M2 macrophages also express elevated levels of 3-phosphoglycerate dehydrogenase that channels glycolytic intermediates into serine, glycine and folate one-carbon metabolism.52 These metabolic shifts may promote Leishmania growth in a number of ways. First, the shift toward more efficient mitochondrial respiration may allow the accumulation of intracellular pools of glucose in the host cell which promotes amastigote growth.25, 50, 53 However, amastigotes can also utilize sugars and amino sugars generated through the breakdown of extracellular matrix hyaluronan and glycosaminoglycans in the PV, and therefore may not be dependent on the two-way exchange of sugars across the PVM.54 Indeed, uptake of extracellular matrix components is increased in M2 macrophages as part of their tissue repair function and likely contributes to enhanced amastigote growth.55 Second, Leishmania appear to be at least partly dependent on glycine and serine salvage from the PV, although they can synthesize these amino acids de novo.56 Increased levels of glycine/serine, as well as folate metabolites, in M2 macrophages may further promote parasite growth.
By contrast, IFNγ-activated M1 macrophages efficiently channel internalized glucose into aerobic (or anaerobic) glycolysis and the pentose phosphate pathway for ATP and NADPH production, which in turn is required for production of NADPH oxidase 2-driven ROS.15 Glycolytically derived pyruvate is still catabolized in the mitochondria, but in an impaired tricarboxylic acid cycle in which flux through isocitrate dehydrogenase and succinate dehydrogenase is blocked, leading to citrate/isocitrate and succinate accumulation, and increased mitochondrial ROS via reverse electron transport. Citrate accumulation drives the synthesis of fatty acids as well as the antimicrobial metabolite, itaconate, while increased succinate levels stabilize HIF-1α, promoting the transcription of glycolytic enzymes and inflammatory cytokines (i.e. IL-1β) that are collectively detrimental to Leishmania proliferation.
Lipid body accumulation
Infection of murine and human macrophages with different Leishmania species leads to the accumulation of lipid bodies in the host cytoplasm57, 58 as well as transcriptional changes in many lipid biosynthetic genes.59 Lipid body accumulation is most pronounced in macrophages from susceptible BALB/c background, and the hyperaccumulation of lipid bodies in HIF-1α-negative macrophages strongly promotes intracellular growth of L. donovani amastigotes.60 Macrophage lipid bodies often accumulate around Leishmania PVs and, in some cases, are even present in the PV lumen. These lipid bodies may provide amastigotes with a source of PUFAs that can be used as a carbon source26 and/or may protect parasites from oxidative stress.
Lipid bodies are also sites for synthesis of omega-3 and omega-6 PUFA-derived lipid mediators that have profound anti-inflammatory and inflammatory activities. A number of studies have shown that enzymes involved in the synthesis of anti-inflammatory prostaglandin E2 synthesis, such as cyclooxygenase 2 and phospholipase A2, are upregulated in L. donovani-infected macrophages,61 while levels of both omega-6-derived prostaglandins and leukotriene (prostaglandin F2 and leukotriene B4) and omega-3-derived resolving D1 (RvD1) are increased in the serum of human patients with visceral or diffuse cutaneous leishmaniasis.62 Both prostaglandin E2 and the resolvins are potent immune modulators that inhibit T-helper type 1 immune response and polarize macrophage toward an M2 phenotype through activation of a peroxisome proliferator activated receptor (PPAR) signaling (see below). Addition of RvD1 to L. amazonensis-infected macrophages was also associated with increased expression of heme oxidase-1 which promotes parasite growth by reducing ROS production.63
Role of Macrophage Metabolic Signaling Pathways in Leishmania Infection
Macrophage polarization within different tissue niches is regulated by local levels of cytokines, growth factors/chemotactic proteins, TLR ligands and nutrient levels that, in turn, lead to activation of metabolic signaling pathways and the reprogramming of metabolism geared to different effector cell functions. Key metabolic signaling pathways in macrophage activation include the mammalian target of rapamycin (mTOR), adenosine monophosphate (AMP) kinase (AMPK) and HIF-1α pathways.64 There is increasing evidence that these pathways play important roles in regulating Leishmania granuloma formation as well as the polarization of macrophages within these tissues to form both permissive and nonpermissive niches within these tissues as outlined in the following sections.
mTOR signaling and regulation of Leishmania growth
Mechanistic target of rapamycin (mTOR) is a key regulator of central carbon metabolism in most eukaryotes. Macrophages express two mTOR multiprotein complexes, mTORC1 and mTORC2, which have nonredundant signaling functions and differ in regulating adaptor proteins and in their sensitivity to rapamycin. The mTORC1 complex is recruited to the lysosome (and other organellar) membranes together with the central adaptor protein, Raptor, through interactions with the Rag GTPase, which in turn is activated by the availability of amino acids in the cytoplasm and the lysosome lumen.65 mTORC1 activation occurs via GTP-bound RHAB which is, in turn, inhibited by tuberous sclerosis complex (TSC1/2). Genetic loss of the TSC1/2 proteins therefore leads to constitutive activation of mTORC1 in the presence of adequate nutrient levels.66 Additional regulation is provided by cross signaling from other metabolic sensors, such as AMP kinase (AMPK), that negatively regulate mTORC1 by phosphorylation of the TSC2 protein.
Activation of murine macrophages with anti-inflammatory cytokines, such as IL-4 or IL-13, promotes mTORC1 activation through the PI3K/protein kinase B (Akt) pathway, leading to increased protein synthesis and a switch to anabolic metabolism that is needed to sustain the energy-demanding functions of cell migration, cytokine production and tissue repair.51, 64 Infection of murine macrophages with L. donovani also leads to the activation of mTOR and polarization toward a permissive M2 phenotype, while inhibition of mTORC1 (via rapamycin treatment) limits parasite growth in ex vivo infected macrophages and granuloma development in mice.67-69 Other studies have shown that mTORC1 activates a number of key downstream targets and transcription factors, including HIF-1α, peroxisome proliferator-activated receptor gamma coactivator 1-α, sterol regulatory element-binding protein-1 and PPAR-γ, that lead to transcriptional upregulation of proteins involved in glucose uptake, glycolysis, mitochondrial biogenesis, oxidative phosphorylation, and fatty acid and cholesterol biosynthesis that individually or collectively may promote intracellular Leishmania growth.51, 64 Remarkably, constitutive mTORC1 activation in murine macrophages (via TSC2 knockdown) resulted in spontaneous formation of macrophage-rich granulomas in a murine model for sarcoidosis,66 suggesting that activation of mTORC1 by L. donovani may stimulate this innate host immune response.
Activation of mTORC1 by Leishmania is likely mediated by PI3K–Akt signaling which is strongly linked to intracellular growth of Leishmania amastigotes.65, 70, 71 Akt phosphorylates (i.e. inactivates) TSC proteins that negatively regulate mTORC1,65 as well as activating enzymes such as ATP citrate lyase. Increased acetyl-coenzyme A biosynthesis is required for histone acetylation and the transcription of a subset of M2 genes (e.g. ARG1), while suppressing nuclear factor-κB signaling, the production of proinflammatory cytokines (e.g. IL-12), iNOS and apoptosis.72 Pharmacological inhibition or genetic knockdown of PI3K/Akt in human macrophages inhibits L. amazonensis growth.70 While activation of PI3K by phagocytosis of microbial pathogens is often transient, Leishmania appear to induce sustained activation of this pathway by recruiting the inositol lipids, phosphatidylinositol 3,5-bisphosphate and phosphatidylinositol (3,4,5)-trisphosphate, to the PVM leading to aberrant recruitment of Akt to the PV.71 The accumulation of phosphatidylinositol phosphates on the PVM may reflect increased fusion of phosphatidylinositol-4-phosphate-containing endoplasmic reticulum/Golgi-derived vesicles with the PVM, although this remains to be confirmed.16
Activation of mTOR also leads to the nuclear translocation of sterol regulatory element-binding protein-1c, and the transcriptional upregulation of fatty acid synthase and acetyl-coenzyme A carboxylase, which are both involved in de novo fatty acid synthesis and the accumulation of lipid droplets in splenic and liver macrophages.69 Interestingly, the mTORC1-induced increase in fatty acid synthesis in L. donovani-infected murine macrophage was exacerbated following genetic loss of HIF-1α.69 HIF-1α is activated by mTORC1 and may redirect the metabolic switch of infected macrophages toward a more glycolytic/M1-like phenotype. Consistent with this possibility, the permissiveness of macrophages isolated from different mouse strains to L. donovani infection was inversely related to HIF-1α expression.69 Furthermore, macrophages isolated from humans carrying mutations in HIF-1α and consequently expressing lower HIF-1α levels have increased lipid production and are more permissive to L. donovani infection, highlighting a novel genetic susceptibility to leishmaniasis.
Paradoxically, infection of murine BALB/c macrophages with L. major was associated with the proteolytic cleavage of the mTOR kinase by the parasite surface protease gp63.73 Inhibition of mTORC signaling in L. major-infected macrophages led to activation of the translational repressor 4E-binding protein-1 and a global reduction in host protein synthesis. These were in turn associated with a reduced secretion of type-1 IFNs, IFNα/IFNβ,73 which can polarize macrophages toward an M1 phenotype with an impaired tricarboxylic acid cycle that generates succinate, citrate and itaconate. These metabolites activate HIF-1α and/or deleterious for Leishmania growth.42, 64, 74 In contrast to the situation in L. donovani-infected macrophages and animal models, mTORC1 activation in the L. major-BALB/c model appears to be host protective, as rapamycin treatment resulted in enhanced parasite growth in vivo, while mice lacking 4E-binding protein-1 had lower parasite burdens and pathology than wild-type mice.73 How gp63 accesses the host mTOR complex and the universality of this mechanism in other Leishmania–macrophage interactions remain to be defined.
AMPK signaling also modulates Leishmania growth
Macrophage polarization is also regulated by AMP kinase (AMPK), a key component in a second metabolic signaling complex that is often reciprocally regulated with mTOR.64 AMPK is normally activated under nutrient limiting conditions and is involved in sustaining homeostasis and catabolic metabolism in nongrowing cells.64 In mammalian cells, AMPK is activated by changes in the bioenergetic state, particularly the ratio of AMP to adenosine diphosphate relative to ATP and glucose deprivation,64 as well as by upstream regulators, such as sirtuin 1 (SIRT1) and liver kinase B (LKB).64 Infection of murine macrophages with L. infantum was associated with AMPK activation and a switch to increased mitochondrial oxidative phosphorylation.50 Activation of AMPK was dependent on LKB/SIRT1, with SIRT1 being upstream of LKB.50 AMPK activation led to increased expression of the glucose transporter Slc2a4 as well as the co-regulator of PPAR-γ (PGC-1α),50 which stimulates mitochondrial biogenesis in both ex vivo cultivated and granuloma macrophages. The AMPK-dependent metabolic reprogramming of L. infantum-infected macrophages may promote intracellular parasite growth by increasing the availability of glucose through more efficient mitochondrial metabolism.50, 53 L. infantum lesion development in AMPK-, SIRT1- or LKB1-knockout mice was attenuated, suggesting that the AMPK–LKB1–SIRT1 axis is important for intracellular parasite growth in vivo.73 Activation of AMPK could also account for an observed increase in autophagy in L. donovani-infected macrophages.68 Increased autophagy promotes intracellular Leishmania growth, presumably by increasing nutrient levels in the PV compartment.75 This is in contrast to the situation for many other intracellular bacterial pathogens where increased autophagy results in their more efficient delivery to and degradation within the lysosome.15, 76
How AMPK is activated in Leishmania-infected macrophages remains unclear. Leishmania infection can lead to the efflux of ATP,77 which would alter intracellular pools of AMP/adenosine diphosphate/ATP and potentially activate AMPK in the host cell. The released ATP is converted to adenosine by the surface-expressed ectonucleotidases, CD39/CD73 and adenosine deaminase (ADA2), leading to the activation of G-protein receptors, A2AR and A2BR, which have also been shown to promote M2 polarization.77 AMPK can also be activated by cytoplasmic galectins that detect lysosomal damage,78 although whether Leishmania amastigotes activate this pathway is unknown. More broadly, it remains unclear whether visceralizing Leishmania species simultaneously activate both AMPK and mTORC1 signaling pathways to their advantage. AMPK and mTOR negatively regulate each other by multiple mechanisms, and also drive opposing catabolic and anabolic pathways. However, both signaling networks have been shown to support M2 macrophage polarization and Leishmania may have evolved mechanisms for exploiting aspects of both pathways that benefit amastigote survival.
Downstream pathways: PPAR signaling and lipid metabolism
The PPARs (PPAR-α, -γ, -δ) are nuclear receptors and master regulators of lipid metabolism in macrophages. Activation of PPAR-γ results in increased expression of genes involved in macrophage fatty acid uptake (via CD36), lipid storage, lipolysis and β-oxidation. Activation of PPAR-γ downstream of mTORC1 signaling also leads to increased mitochondrial biogenesis, oxidative phosphorylation and Arg1 expression characteristic of M2 polarization.53, 79, 80 A number of studies have shown that activation of macrophage PPAR-γ in both cutaneous and visceral infections promotes disease progression, while pharmacological inhibition or genetic silencing of PPAR-γ delays lesion development and reduces the size of cutaneous and visceral granulomas.79-81 In an important study, Beattie and colleagues showed that a transcriptional network centered around retinoid X receptor-α (RXR-α) is aberrantly sustained in infected macrophages in L. donovani-induced liver granulomas, but downregulated in uninfected macrophages from the same tissue.82 RXR-α forms functional heterodimers with PPAR proteins, suggesting that sustained RXR-α/PPAR signaling is important for parasite growth and survival in liver granulomas, and that infected macrophages are less susceptible to being polarized toward an M1 phenotype in this inflammatory tissue environment. Consistent with RXR-α/PPAR signaling being parasite supportive, pharmacological inhibition of RXR-α results in reduced parasite load in the spleen and liver.82 The role of other PPAR isoforms in Leishmania granulomas has not been extensively studied.
PPAR-γ–δ are activated by anti-inflammatory cytokines (IL-4, IL-13, IL-10), TLR receptors and a range of lipids and other stimuli, including oxidized low-density lipoprotein, PUFA (arachidonic acid, 5-hydroxyeicosatetraenoic acid and 5-oxo-eicosatetraenoic acid), oxidized linoleic acid (13-HODE) and prostaglandins (D2, prostaglandin E2, prostaglandin F2, thromboxane B2), that are variously present in Leishmania granulomatous tissues.83 Interestingly, Leishmania-derived PUFAs polarize murine macrophages toward an M2 phenotype when added exogenously.84 As noted earlier, Leishmania amastigotes form a tight junction with the PVM, raising the possibility that parasite lipids may be directly transported to the host cell and modulate host cell signaling. Moreover, Leishmania have recently been shown to express an aldo–keto reductase that functions as a prostaglandin F2α synthase. This enzyme is exported to the host cell cytoplasm, possibly via released exosomes and may hijack host responses by inducing the synthesis of anti-inflammatory prostaglandins.85, 86
Future Perspectives
Considerable progress has been made in understanding how changes in host cell metabolism impact on the outcome of granulomatous diseases caused by pathogens such as Mycobacterium tuberculosis, Salmonella typhimurium and Schistosoma spp.15 While fewer studies have been undertaken on the immunometabolic aspects of Leishmania granulomas, these protozoal diseases represent an excellent model system for studying granuloma dynamics given the extensive literature on host immunity to Leishmania (including the first description of T-helper type 1/T-helper type 2 immune response), the availability of robust animal models for both dermal and liver granuloma induction and the relative simplicity of Leishmania granuloma histology compared with other infectious granulomas. As in other granulomatous diseases, the extent to which Leishmania granulomas function to restrain or allow parasite expansion is dependent in large part on the extent to which macrophage/monocytes are polarized toward an M1 or M2 phenotype. Importantly, macrophages can exist in different polarization states within the same granuloma, reflecting spatial and temporal heterogeneity in the ontology of different macrophage/monocyte populations (i.e. tissue-resident macrophages versus recruited monocytes), and the levels of cytokines, chemokines and host immune factors (e.g. NO), as well as gradients in extracellular nutrients and oxygen levels. Future studies on the metabolic reprograming of host cells in Leishmania granulomas therefore need to take into account this phenotypic diversity of host cells in the granuloma microenvironment and the orthogonal metabolic signals that contribute to polarization.
Macrophage polarization in Leishmania granulomas has also been shown to be dependent on key metabolic signaling hubs, such as Akt/mTOR, AMPK, PPAR and HIF-1α. While activation of some of these pathways is commonly associated with macrophage polarization toward M1 or M2-like phenotypes, there is increasing evidence that crosstalk between these pathways can lead to a spectrum of different metabolic responses in vivo. In particular, the observation that activation of both mTOR and AMPK promotes liver granuloma formation induced by visceralizing species of Leishmania is of interest as these pathways are normally reciprocally regulated and antagonize each other. Similarly, while activation of mTOR signaling in macrophages can lead to an M2 phenotype that is permissive for Leishmania growth, it can also lead to M1 polarization under conditions that also activate HIF-1α signaling and/or increased secretion of inflammatory cytokines that constrain Leishmania growth. Therefore, dissection of the role of host cell metabolic signaling in Leishmania granuloma development will require a holistic examination of antagonistic/synergistic interactions between different pathways.
Finally, consideration of the impact of macrophage metabolic reprograming on Leishmania pathogenesis will require a detailed understanding of the metabolic requirements of intracellular amastigote stages, as well as the nutritional environment in the PV. In particular, while amastigotes can clearly access a variety of metabolites from the macrophage cytoplasm, it remains unclear whether this occurs through host PVM transporters and/or through PVM-amastigote tight junctions, and the extent to which amastigotes are directly responsive to changes in macrophage metabolism. Conversely, there is increasing evidence that transport of parasite proteins and metabolites across the PVM modulates host signaling pathways. While it has been speculated that parasite proteins may be transported into the host cytoplasm in membrane-bound exosomes, how these proteins are released into the cytoplasm is unknown. Finally, it is worth noting that Leishmania amastigotes, unlike nearly all other bacterial, fungal and protist pathogens, survive within a phagolysosome-like compartment of macrophages. As such, it is likely that Leishmania will be dependent on different macrophage signaling and metabolic pathways for survival compared with other intracellular microbes. For example, while activation of autophagy restricts intracellular growth of M. tuberculosis, it is beneficial for Leishmania. Overall, these studies highlight the intimate interconnection between host and parasite metabolism which ultimately determines the outcome of infection. Further progress in understanding immunometabolic signaling and programming in Leishmania granulomas is likely to open new opportunities for developing adjunct therapies that target host processes.
Acknowledgments
We thank Dr Katrina Binger and Ms Erin McGowen for discussions. This work is supported by an NHMRC Principal Research Fellowship (#1154540) and NHMRC Project Grant (#1183085) to MJM.
Author Contribution
Eleanor Claire Saunders: Writing-original draft; Writing-review and editing. Malcolm McConville: Funding acquisition; Writing-original draft; Writing-review and editing.
Conflict of Interest
The authors have no conflict of interest to declare.





