Surveying purine biosynthesis across the domains of life unveils promising drug targets in pathogens
Abstract
Purines play an integral role in cellular processes such as energy metabolism, cell signaling and encoding the genetic makeup of all living organisms—ensuring that the purine metabolic pathway is maintained across all domains of life. To gain a deeper understanding of purine biosynthesis via the de novo biosynthetic pathway, the genes encoding purine metabolic enzymes from 35 archaean, 69 bacterial and 99 eukaryotic species were investigated. While the classic elements of the canonical purine metabolic pathway were utilized in all domains, a subset of familiar biochemical roles was found to be performed by unrelated proteins in some members of the Archaea and Bacteria. In the Bacteria, a major differentiating feature of de novo purine biosynthesis is the increasing prevalence of gene fusions, where two or more purine biosynthesis enzymes that perform consecutive biochemical functions in the pathway are encoded by a single gene. All species in the Eukaryota exhibited the most common fusions seen in the Bacteria, in addition to new gene fusions to potentially increase metabolic flux. This complexity is taken further in humans, where a reversible biomolecular assembly of enzymes known as the purinosome has been identified, allowing short-term regulation in response to metabolic cues while expanding on the benefits that can come from gene fusion. By surveying purine metabolism across all domains of life, we have identified important features of the purine biosynthetic pathway that can potentially be exploited as prospective drug targets.
PURINES AND THE ORIGIN OF LIFE
The most widely accepted model for the origin of life is that it arose via the production of organic molecules by simple prebiotic chemical reactions. The Miller–Urey experiment showed that amino acids essential for creating proteins could be formed from simple molecules such as water, methane, ammonia and hydrogen.1 Further investigations established that the purine nucleotides adenine and guanine, key components of both RNA and DNA, could also form under conditions likely present on primitive Earth.2 Over 3.5 billion years later, all living things from the simplest unicellular life to more complex multicellular organisms require purines for survival. Purines play an integral role in diverse processes including energy metabolism, cell signaling and encoding the genetic makeup of all organisms—providing strong selective pressure that ensures the purine de novo biosynthetic pathway is maintained across all domains of life.
Purines contain a six-membered pyrimidine ring fused to a five-membered imidazole ring. The first of these heterocyclic compounds was discovered in urinary calculi; uric acid (from the French urique for “urine”) in 1776 and xanthine (from the Greek xanthos for “yellow”) in 1838.3, 4 Guanine was identified in 1846 and named for the bird guano in which it was found, and 4 years later hypoxanthine (xanthine with less oxygen) was isolated from cow spleen.5, 6 It was not until 1884 that the term “purine” was coined from the Latin purum (pure) and urinae (urine) by Fischer, who was awarded a Nobel Prize for his achievements in the field of purine synthesis.7 Experiments on ox pancreas in 1886 led to the identification of adenine (from the Greek aden for “gland”) that in turn led to Kossel being awarded a Nobel Prize for the discovery that nucleic acids were composed of the purines adenine and guanine alongside the pyrimidines thymine, cytosine and uracil.8
The biochemical processes underpinning the synthesis of purines would not be identified until over half a century later. The determination of the intermediates involved in de novo purine biosynthesis began in the 1930s with the discovery that hypoxanthine could be detected in the livers of pigeons but not chickens, ducks, rats or guinea pigs.9 Key intermediates in the pathway were subsequently elucidated by feeding isotopically labeled substrates to pigeons and analyzing uric acid crystallized from their droppings.10 These in vivo experiments were followed by in vitro studies purifying and assaying individual enzymes from pigeon liver, cow liver and Saccharomyces cerevisiae, together laying the foundation for our current understanding of the biochemical processes required for the de novo biosynthesis of purines in the Eukaryota.11-13
THE BIOCHEMISTRY OF PURINE BIOSYNTHESIS IN THE EUKARYOTA
De novo purine and pyrimidine biosynthesis both require the adenosine triphosphate (ATP)-dependent phosphorylation of ribose-5-phosphate by ribose-phosphate diphosphokinase (EC 2.7.6.1) to produce phosphoribosylpyrophosphate (PRPP).14
The first dedicated step of de novo purine biosynthesis is the hydrolysis of l-glutamine and transfer of the liberated amine group to PRPP by PRPP amidotransferase (EC 2.4.2.14), creating phosphoribosylamine (PRA) (Figure 1).15 Next, phosphoribosylglycinamide (GAR) synthetase (EC 6.3.4.13) catalyzes the ATP-dependent ligation of l-glycine to phosphoribosylamine (via a phosphorylated intermediate) to yield GAR.16 N10-fTHF-GAR transformylase (EC 2.1.2.2) then ligates the formyl group from 10-formyltetrahydrofolate (N10-fTHF) to GAR, producing phosphoribosylformylglycinamide (FGAR).17 The subsequent activation of the FGAR amide oxygen by the ATPase domain of phosphoribosylformylglycinamidine (FGAM) synthetase (EC 6.3.5.3) produces an iminophosphate intermediate. From the glutaminase domain, ammonia is then channeled via a structural domain, that amidates the intermediate to create FGAM.18 Finally, phosphoribosylaminoimidazole (AIR) synthetase (EC 6.3.3.1) catalyzes the ATP-dependent activation of the FGAM formyl oxygen that reacts with a nearby nitrogen to close the five-membered imidazole ring of the purine base and form AIR.19
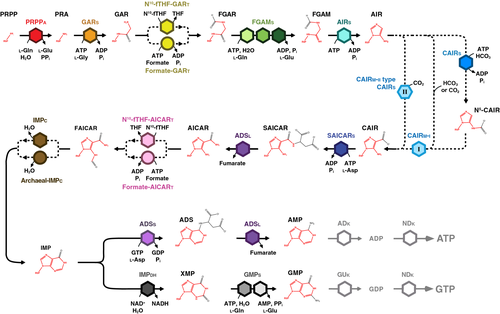
Formation of the pyrimidine ring then begins with phosphoribosylcarboxyaminoimidazole (CAIR) synthetase (EC 4.1.1.21) carboxylating AIR using CO2 to form CAIR.20 Phosphoribosylaminoimidazolesuccinocarboxamide (SAICAR) synthetase (EC 6.3.2.6) then mediates the ATP-dependent ligation of l-aspartate to CAIR forming SAICAR; the following β-elimination of fumarate from SAICAR by adenylosuccinate (ADS) lyase (EC 4.3.2.2) produces phosphoribosylaminoimidazolecarboxamide (AICAR).21 N10-fTHF-AICAR transformylase (EC 2.1.2.3) then ligates the formyl group from N10-fTHF to AICAR to form phosphoribosylformamidocarboxamide (FAICAR).22 Closure of the pyrimidine ring (completing formation of the purine base) occurs via elimination of water from FAICAR by inosine monophosphate (IMP) cyclohydrolase (EC 3.5.4.10) and cyclization to produce IMP.23
Purine de novo biosynthesis bifurcates after IMP. In the adenosine monophosphate (AMP) biosynthesis branch, ADS synthetase (EC 6.3.4.4) transfers the γ-phosphate from guanosine triphosphate (GTP) to IMP, forming the intermediate 6-phosphoryl IMP; the 6-phosphoryl group is then displaced by the α-amino group of l-aspartate to form ADS.24, 25 The conversion of ADS to AMP follows the same β-elimination mechanism by ADS lyase that it performs earlier in the pathway.26, 27 AMP can be converted to adenosine diphosphateby adenylate kinase, then to ATP by nucleoside diphosphate kinase.28, 29 In the guanosine monophosphate (GMP) biosynthesis branch, IMP dehydrogenase (EC 1.1.1.205) catalyzes the NAD+-dependent hydrolysis of IMP to form xanthosine monophosphate (XMP).30, 31 Next, the ATP pyrophosphatase domain of GMP synthetase (EC 6.3.5.2) adenylates XMP to form an XMP intermediate, while the glutamine amidotransferase domain produces ammonia to add an amine group to the intermediate and yield GMP.27 GMP can be converted to guanosine diphosphate by guanylate kinase, then to GTP by nucleoside diphosphate kinase.29, 32
In addition to the de novo pathway, exogenous purines can also be scavenged from the environment via a salvage pathway through the action of phosphoribosyltransferases that convert adenine, hypoxanthine, xanthine and guanine to AMP, IMP, XMP and GMP, respectively.31, 33
THE HISTORY OF PURINE METABOLISM AS A DRUG TARGET
Given the requirement for significant quantities of nucleic acids in highly proliferative cells such as cancers, immune cells and infecting pathogens, it was proposed that targeting of specific biological processes in the purine metabolism pathway may lead to effective therapeutic treatments.34, 35 This premise led to the conception of rational drug design, earning Hitchings and Elion a Nobel Prize.36
Pharmaceuticals that act through the purine biosynthetic pathway were subsequently developed. Allopurinol, an inhibitor of the purine degradation enzyme xanthine oxidase, blocks the accumulation of uric acid and, by extension, the occurrence of gout.37 Azathioprine is converted into the purine analog 6-mercaptopurine before it is salvaged by hypoxanthine–guanine phosphoribosyltransferase to disrupt purine metabolism; originally designed as an anticancer drug, this compound was found to be more effective as an immunosuppressant in organ transplant recipients.38 Acyclovir is sequentially metabolized by viral thymidine kinase, host guanylate kinase and host nucleoside diphosphate kinase to create acyclovir triphosphate, a purine analog that selectively incorporates into, and terminates synthesis of, viral DNA in the treatment of viral infections such as herpes.39 Beyond these synthesized compounds, natural products have also been found that interfere with purine metabolism. Mycophenolic acid from Penicillium brevicompactum inhibits IMP dehydrogenase in a wide range of species, including in humans where it is used as an immunosuppressant.33, 40
Despite the medical advances that have been made by targeting purine metabolism, many of the enzymes in the pathway remain unexplored, particularly in the field of antibiotic development. To better understand why some biochemical reactions may serve as superior targets, it is worthwhile to look beyond the Eukaryota and consider purine metabolism across all domains of life.
ARCHAEA AND AN ALTERNATIVE PURINE LIFESTYLE
The original characterization of purine biosynthesis in the 1950s did not include studies of the Archaea, unsurprising given that this domain (that contains no known pathogens) was not recognized until two decades later. However, with the exception of proposed symbionts that are unable to create purines de novo, studies of these autochthons of diverse habitats reveal that while all of the classic elements of the canonical eukaryotic purine metabolic pathway are present in the domain, a subset of the familiar biochemical roles is performed by unrelated proteins in many species (Figure 2).
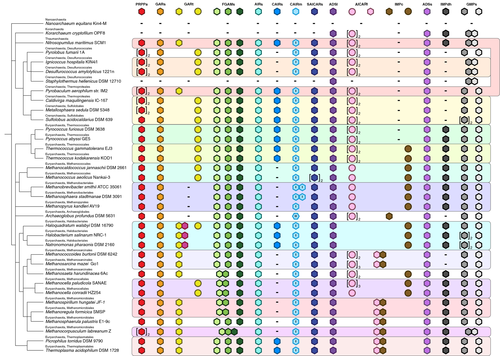
The most widespread examples of alternative purine metabolism enzymes in the Archaea are AICAR transformylase and GAR transformylase. In the Eukaryota, these enzymes use N10-fTHF as a formyl donor, but in the Archaea the ability to synthesize this cofactor is not universal. Consistent with this, the eukaryotic forms of these enzymes are only found in certain species capable of synthesizing N10-fTHF.41, 42 Many Archaea instead employ formate-dependent variants of AICAR transformylase (formate-AICARt, EC 6.3.4.23) and GAR transformylase (formate-GARt, EC 2.1.2.-) to generate formyl phosphate that supplies the formyl group for ligation to AICAR and GAR, respectively (Figure 1).43, 44 Formate is abundant in hydrothermal vents where extreme thermophilic Archaea thrive, and it has been proposed that the formate-dependent enzymes are beneficial in this type of niche.45, 46
In contrast to the biochemically distinct formate- and N10-fTHF-dependent mechanisms of the transformylases, there are two types of IMP cyclohydrolases in the Archaea that appear to possess the same enzymatic activity. One of these IMP cyclohydrolases is of the type identified in early eukaryotic studies, and the other is an unrelated protein with the same function (archaeal-IMPc, EC 3.5.4.10) (Figure 1).47, 48 Each are equally common in the species we have investigated (Figure 2).
Members of the Archaea carboxylate AIR to CAIR using one of three distinct paths (Figure 1). The first path is a two-step process that requires a CAIR synthetase (EC 6.3.4.18) that catalyzes ATP-dependent phosphorylation of bicarbonate to create a carboxyphosphate intermediate that is then ligated to AIR, yielding N(5)-phosphoribosylcarboxyaminoimidazole. A class I CAIR mutase (CAIRm-i, EC 5.4.99.18), the dominant class of the enzyme in the Archaea, rearranges the position of this carboxylate group to form CAIR.49, 50 The second path occurs in species that have class I CAIR mutase but not CAIR synthetase. It has been proposed that in these species, during growth in CO2- or HCO3-- replete niches the Class I enzyme catalyzes the direct formation of CAIR on its own, analogous to the suggested environmental link associated with the formate-requiring transformylases.51, 52 The third path is a single-step CO2-dependent carboxylation reaction initially identified in the Eukaryota, that in the Archaea has only been reported in members of the genus Archaeoglobus.53 Performed by an enzyme appropriately deemed a CAIR synthetase based on its biochemical function, at the sequence level this protein more closely resembles the class I PurE CAIR mutase of Escherichia coli. Referred to in the literature as a “class II PurE,” this enzyme could therefore also be described as a “class II CAIR mutase-type CAIR synthetase” (CAIRm-ii type CAIRs, EC 4.1.1.21).
Overall, alternative purine metabolism enzymes are a major theme in the Archaea, and intriguingly, this is a story that appears to be incomplete. Multiple species lack open reading frames likely to encode homologues of GAR transformylase, IMP cyclohydrolase, IMP dehydrogenase, or a combination of these. Whether novel, undiscovered alternative proteins exist that perform these essential biochemical reactions, or whether the biochemical step is bypassed (as with CAIR synthetase) remains unknown.53
FUSION-POWERED PURINE METABOLISM IN THE BACTERIA
As with the Archaea, some species of the Bacteria lack the de novo pathway altogether; however, in this case they are normally obligate pathogens that rely entirely on scavenging purines from the host.54, 55 Alternative purine biosynthetic enzymes also occur in the Bacteria, only to a lesser extent than the Archaea (Figure 3). Formate-dependent AICARt appears to be absent and archaeal IMPc is rare. By contrast, formate-dependent GARt is present in one-third of the species investigated which also include the ubiquitous N10-fTHF-dependent GAR transformylase. The metabolic redundancy of retaining two different forms of GAR transformylases has been demonstrated by deletion studies in E. coli where either GAR transformylase alone is sufficient for FGAR biosynthesis and survival in purine-free media.56
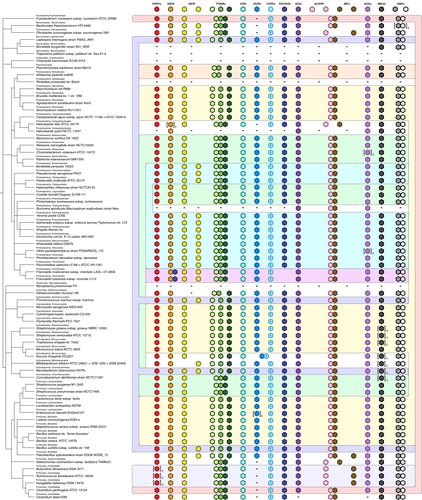
The major differentiating feature of the de novo purine biosynthetic pathway in the Bacteria is an increasing prevalence of gene fusions where two or more purine biosynthesis enzymes are encoded by a single gene. These are almost exclusively fusions of genes that encode enzymes performing consecutive biochemical functions in the pathway.
The effect of selective pressure on the fusion of genes in the purine biosynthesis pathway is highlighted by analysis of steps that require multiple distinct biochemical activities. The conversion of FGAR to FGAM requires three proteins: an ATPase protein that activates the FGAR amide oxygen, a glutaminase that produces ammonia to amidate the substrate and produce FGAM and a structural protein that channels the ammonia from the glutaminase to the ATPase protein. In some bacterial species, these are encoded by separate genes, in others the ATPase and structural genes are fused to create a single larger protein and in most the glutaminase joins this fusion to create a triple domain protein (Figure 3). Each of these arrangements can also be found in the Archaea, albeit with the fused forms least common (Figure 2). Likewise, the conversion of XMP to GMP requires two separate enzymatic activities—ATP pyrophosphatase for XMP adenylation, and glutamine amidotransferase to add an amine group to create GMP.27, 57 In the Bacteria these are fused into a single bifunctional GMP synthetase protein (Figure 3), whereas in the majority of the Archaea they exist as separate genes (Figure 2).
In a similar fashion, genes that encode enzymes performing discrete but consecutive steps in the pathway can also be fused. The genes encoding N10-fTHF-dependent AICAR transformylase (which ligates a formyl group to AICAR formingphosphoribosylformamidocarboxamide) and IMP cyclohydrolase (which cyclizes phosphoribosylformamidocarboxamide to form IMP) sometimes exist as separate genes in the Bacteria, but far more commonly occur as a single-gene fusion (Figure 3). The same fusion occurs in the Archaea, but is much rarer.
Every example of fusion involving a purine biosynthesis gene that we have observed in the Bacteria is always to another purine biosynthesis gene, and this is also true in the Archaea, indicating that these fusions are not arbitrary. Furthermore, the three most predominant purine biosynthesis gene fusions in the Bacteria entail the joining of genes that encode proteins performing consecutive biochemical steps in the pathway. In keeping with applications in synthetic biology where sequentially scaffolding enzymes can facilitate substrate channeling and significantly increase flux through a pathway, the most parsimonious explanation of these data is that there is a strong preference toward gene fusion for more efficient purine metabolism.58
PROFUSION OF PURINE METABOLIC GENE FUSION IN THE EUKARYOTA
As with the Archaea and Bacteria, some members of the Eukaryota lack the de novo purine biosynthesis pathway, usually obligate parasites or pathogens that are not free-living.59, 60 However, the majority of the Eukaryota are capable of de novo purine biosynthesis, and these contain the most common fusions evident in the Bacteria: the triple-domain FGAM synthetase, the bifunctional GMP synthetase fusion and the bifunctional N10-fTHF-dependent AICAR transformylase/IMP cyclohydrolase (referred to as “ATIC” in the Metazoa; Figure 4).
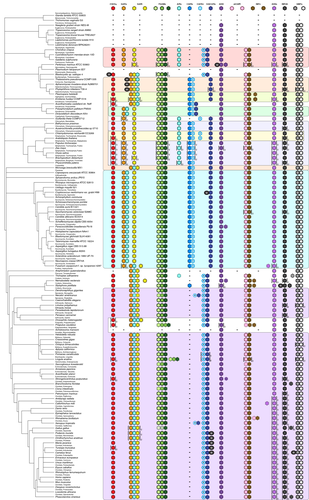
The theme of gene fusions encoding multidomain enzymes that perform sequential pathway steps goes even further in the Eukaryota. In the Fungi, the Viridiplantae and many of the protists, the carboxylation of AIR to CAIR occurs via the sequential action of CAIR synthetase and class I CAIR mutase which are now fused into a single protein; this arrangement also occurs in the bacterium Kocuria rhizophila. In the Amoebozoa, this fusion is further expanded to include the enzyme that catalyzes the next step in the pathway, SAICAR synthetase, which ligates l-aspartate to CAIR to create AICAR. The Metazoa have a simpler form of this fusion that only includes the metazoan class II CAIR mutase-type CAIR synthetase and SAICAR synthetase (dubbed “PAICS”).
One enzyme fusion present in all Fungi and a subset of Amoebozoa occurs between two enzymes responsible for nonconsecutive steps of the pathway: GAR synthetase (ligation of l-glycine to PRA creating GAR) and AIR synthetase (cyclization of FGAM to form AIR). However, this gene fusion expands in the Metazoa with the addition of N10-fTHF-dependent GAR transformylase that catalyzes the consecutive step after GAR synthetase (ligation of a formyl group to GAR to produce FGAR), forming the trifunctional protein “TrifGART.” While this example initially deviates from the trend of fusion of consecutive steps, the third addition follows the theme, bringing together the enzymes for conversion of PRA to GAR and GAR to FGAR into a single protein.
Unlike the Archaea and the Bacteria, there are multiple examples in the Eukaryota where purine biosynthesis genes have fused with genes whose function is not associated with this primary metabolic process (Figure 4; Table 1). For example, a range of animals possess a fusion of class II CAIR mutase-type CAIR synthetase/SAICAR synthetase with a predicted Myb-like DNA-binding domain, and several protists have the trifunctional GAR synthetase/GAR transformylase/AIR synthetase fused to an MGS-like domain protein. Interestingly, the coral Stylophora pistillata contains a fusion of IMP dehydrogenase to aspartyl-transfer RNA synthetase, and the fungi of the order Tremellales, which includes the pathogenic Cryptococcus species complex, exhibits a similar fusion, this time between SAICAR synthetase and tyrosyl-transfer RNA synthetase.
| Species | Purine biosynthesis enzyme | Fused protein |
|---|---|---|
| Perkinsus marinus | ADS lyase | Conserved protein domain DUF2009 |
| Blastocystis spp. | PRPP amidotransferase | Cwf15/Cwc15 cell cycle control protein |
|
Thalassiosira pseudonana Aphanomyces invadans Phytophthora infestans |
Trifunctional GAR synthetase, GAR transformylase and AIR synthetase | MGS-like domain protein |
| Cryptococcus neoformans | SAICAR synthetase | Tyrosyl-tRNA synthetase |
| Stylophora pistillata | IMP dehydrogenase | Aspartyl-tRNA synthetase |
|
Podarcis muralis Ornithorhynchus anatinus Manis javanica Tursiops truncatus Camelus ferus |
Bifunctional class II CAIR mutase-type CAIR synthetase and SAICAR synthetase | Myb-like DNA-binding domain |
| Camelus ferus | GMP synthase | Uncharacterized protein (150 amino acids) |
- ADS, adenylosuccinate; AIR, phosphoribosylaminoimidazole; CAIR, phosphoribosylcarboxyaminoimidazole; GAR, phosphoribosylglycinamide; GMP, guanosine monophosphate; IMP, inosine monophosphate; PRPP, phosphoribosylpyrophosphate; SAICAR, phosphoribosylaminoimidazolesuccinocarboxamide; tRNA, transfer RNA.
Finally, across the Eukaryota domain there are multiple examples of purine biosynthesis genes being duplicated, most often the Viridiplantae (Figure 4). While there are a limited number of changes in purine biosynthesis gene copy number in the Archaea (Figure 2) and the Bacteria (Figure 3), in the Eukaryota the frequency of gene duplications drastically increases. However, there have been no reports of neofunctionalization to date.
The prevalence and size of purine biosynthesis gene fusions in the Eukaryota support the hypothesis that close proximity of these enzymes enhances efficiency, raising the question of whether this process will continue toward all enzymatic activities of the pathway existing within a single protein. Such a protein could conceivably evolve; the combined lengths of the purine biosynthetic enzymes in humans is approximately 6000 amino acid residues, whereas the largest known protein (Titin) can reach almost 36 000 residues in length.61 While theoretically possible, a different mechanism for temporally and spatially bringing the components of the pathway together appears to already exist.
THE HIGHER-ORDER STRUCTURE OF PURINE BIOSYNTHESIS IN HUMANS
Just as the fusion of enzymes that function in sequential steps of a pathway can enable substrate channeling, facilitate regulation of metabolic flux, raise catalytic efficiency and prevent substrate degradation and diffusion, so can the formation of multiprotein complexes.62, 63 Over the last decade, studies in human cell lines have enabled the detection of a dynamic mesoscale protein assembly responsible for the production of ATP and GTP.
Studies of fluorescently tagged de novo purine biosynthesis enzymes in the human HeLa cell line revealed subcellular colocalization in response to purine availability; the enzymes clustered together into a higher-order structure dubbed the “purinosome,” a metabolon that formed in the absence of exogenous purines.64 Furthermore, cells derived from patients suffering from either ADS lyase deficiency or AICA-ribosiduria (ATIC deficiency) were unable to form these multienzyme assemblies under purine-depleted conditions, nor could HeLa cells in which individual de novo purine biosynthesis genes had been disrupted. Together, these data suggest that all components of the pathway are required for purinosome formation.65-67
Fluorescence recovery after photobleaching and cytoplasmic protein–protein interaction studies revealed the existence of a stable core complex consisting of the first half of the de novo biosynthesis pathway: PRPP amidotransferase, GAR synthetase/AIR synthetase/GAR transformylase (TrifGART) and FGAM synthetase.68 The enzymes from the second half of the pathway appear to transiently interact with this core to form the purinosome.69 Found in the cytoplasm in association with mitochondria and microtubules, formation is regulated by G-protein coupled receptor signaling, phosphorylation and interaction with molecular chaperones such as HSP90.70-73 Purinosomes form primarily, but not exclusively, during the G1 phase of the cell cycle, preceding S phase when purine demand is high because of DNA replication.74
Unlike gene fusion, compartmentalization of purine biosynthesis is a reversible biomolecular assembly of enzymes that allows short-term regulation in response to metabolic cues. As such, the existence of the purinosome provides an additional level of control over a process that is critical to numerous functions in the cell. However, all studies so far have taken place in vitro, and exclusively in human cells. The prevalence of this metabolon in vivo, particularly with regard to tissue specificity, remains to be shown. Perhaps more importantly, the question whether the purinosome exists beyond humans, beyond the Metazoa or even beyond the Eukaryota remains to be answered.
THE FUTURE OF PURINE METABOLISM AS A DRUG TARGET
The most well-known therapeutic agents that function through purine metabolism were developed during the golden age of drug discovery between the 1950s and 1980s.36 These studies were performed from a reductionist standpoint: focusing on a specific target in a specific organism was the cornerstone of the rational drug design strategy. In the postgenomic world where this important primary metabolic process can be considered across all of the domains of life, rational drug design can now exploit similarities or contrasts between species, genera, orders, phyla or even domains. From this viewpoint, the pathway can be targeted to inhibit purine biosynthesis in few species, in many or potentially in all depending on the desired outcome.
If the goal is to target just a few species, characteristics unique to the purine de novo biosynthesis pathway in one organism or a small group of closely related species could be exploited. An excellent example is the fungal pathogen Cryptococcus neoformans of the Tremellales. The fungi of this order possess an unusual gene fusion that joins SAICAR synthetase to the cytoplasmic tyrosyl-transfer RNA synthetase, providing two levels of vulnerability: the conditional lethality associated with loss of purine biosynthesis, and the essential ability to synthesize proteins (Figure 5). The fusion has created an irreversible link between these otherwise unrelated processes, providing the opportunity for the development of therapeutic agents that are organism specific while interfering with two critical cellular processes simultaneously. To our knowledge, the concurrent inhibition of two pathways by targeting a fusion protein has never been demonstrated.
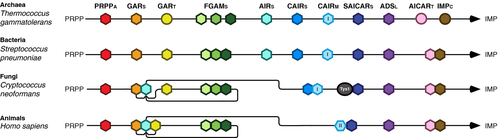
The development of a drug that targets a single pathogen is less useful than the development of a drug that can target many. Analysis of purine metabolism across all domains reveals prospective targets shared by many species. The alternative enzymes that are prevalent in the Archaea do not make good targets; this domain contains no known pathogens, and the Bacteria that have the most common of these (the formate-dependent GARt) appear to have metabolic redundancy that makes the protein nonessential. By contrast, the CAIR synthetase in most organisms is a much better target because it is unrelated to its counterpart in the Metazoa (Figure 5). Furthermore, the metazoan enzyme exists as a fusion with SAICAR synthetase, providing an additional level of differentiation.
Targeting all free-living organisms could be most easily achieved through the inhibition of those enzymes that are universally shared but do not have the potentially confounding influence of gene fusions. PRPP amidotransferase and ADS lyase are present in all free-living species and are not part of a common fusion. Inhibition of either could yield a new series of antibacterials, herbicides, antifungals, anticancer agents or immunosuppressants.
By surveying purine metabolism across all domains of life, we have identified important features which could inform each of these goals, and the strategies that could be employed to address them. An antimicrobial that also targets a human enzyme is not necessarily undesirable. As typified by mycophenolic acid, a natural product used by P. brevicompactum to combat other microbes, an antimicrobial drug that also inhibits a human purine biosynthesis enzyme, can still be valuable in the clinic as an immunosuppressant or anticancer drug. Furthermore, beyond the insights that can be gained from genomic analyses, the purinosome represents another avenue of investigation. Which of these strategies provide the most effective approach is dependent on developing a deeper understanding of this evolutionarily conserved pathway.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTION
Sheena MH Chua: Conceptualization; Data curation; Formal analysis; Investigation; Writing-original draft; Writing-review & editing. James A Fraser: Conceptualization; Funding acquisition; Investigation; Supervision; Writing-original draft; Writing-review & editing.





