Revisiting the secretion mechanism(s) of macrophage migration inhibitory factor—welcome to the “UPS club”
Graphical Abstract
See also: Original Article by Dankers et al.
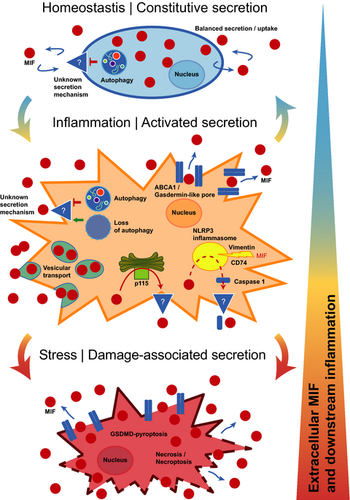
Cytokines are protein mediators regulating intercellular communication. They orchestrate the immune response and play pivotal roles in numerous diseases such as inflammatory and autoimmune diseases, cardiovascular conditions and cancer. Understanding their expression and secretion mechanisms therefore is important and conditional to the identification of therapeutic strategies blocking the release of pathogenic cytokines. Most cytokines carry an N-terminal signal peptide and are secreted via the conventional endoplasmic reticulum (ER)-Golgi route. However, a number of important inflammatory mediators including the interleukin (IL)-1 family of cytokines do not express a signal peptide and are released by alternative pathways, collectively termed unconventional protein secretion (UPS). Macrophage migration-inhibitory factor (MIF) is one of the oldest cytokines discovered. It is a key upstream regulator of innate immunity and an important host defense protein, whereas dysregulated MIF is a pivotal mediator of acute and chronic inflammatory conditions, atherosclerosis, rheumatoid arthritis and systemic lupus erythematosus (SLE).1-3 MIF is an “atypical” cytokine; this notion refers not only to its high evolutionary conservation across kingdoms, but also to molecular characteristics such as an N-terminal tautomerase pocket, its chemokine-like behavior and engagement of CXC chemokine receptors, lack of a signal peptide and abundant cytosolic expression as well as presumed additional intracellular functions.4 In fact, MIF was suggested to be released by unconventional protein secretion routes previously,5 but ultimate evidence has been missing and the precise mechanisms have remained incompletely understood. This study by Dankers et al. adds an important new facet to its secretion mechanism, linking MIF release to the processes of necroptosis and nucleotide-binding domain, leucine-rich-repeat-containing (NLR) family pyrin domain containing 3 (NLRP3) inflammasome-dependent pyroptosis.6 The data contribute to establish MIF as a member of the “unconventional protein secretion UPS club of proteins.”
MIF was discovered as a T-cell and macrophage cytokine, but it is now clear that it is broadly expressed in most cell types. Abundant cytosolic storage pools of MIF and baseline (nonpathologic) circulating MIF levels in the 1–10 ng mL−1 range are considered to be part of its atypical cytokine profile. Under inflammatory conditions, circulating MIF levels are rapidly and drastically increased, reaching levels up to 500–1500 ng mL−1. The actual local tissue levels of released MIF protein under disease conditions are poorly defined, but in vitro cell culture-based studies suggest a similar rate of elevation by up to several 10- to 100-fold. While MIF secretion studies have mainly been performed in immune cells, it has become clear that secretion can also occur in response to a variety of stimuli from endothelial cells and platelets, parenchymal cells such as cardiomyocytes, vascular smooth muscle cells and fibroblasts as well as tumor cells. That the mechanism(s) underlying the upregulation of MIF release is unconventional and endoplasmic reticulum/Golgi independent was initially insinuated by the lack of a signal peptide in the MIF sequence and was experimentally demonstrated by studies using inhibitors of the classical ER/Golgi pathway.7, 8 Protein interaction studies and the application of pharmacological inhibitors implicated the ABC transporter ABCA1, the signalosome subunit JAB1/CSN5 and the cytosolic, Golgi-associated protein p115 in the unconventional protein secretion route of MIF.9 JAB1/CSN5 has been suggested to have a sequestration function and p115 facilitates MIF secretion, but it has remained unclear whether these proteins are directly or indirectly involved, and the actual mechanism mediating the release or transport of MIF from the intracellular compartment into the extracellular space has remained undefined.4 Studying immortalized mouse bone marrow-derived macrophages (BMDM) and human THP-1 monocyte-like cells, Dankers et al. now offer evidence that MIF release from monocytes/macrophages may be mediated by necrosis, necroptosis and pyroptosis.6
Necrosis was induced by hydrogen peroxide, ethanol or ultraviolet light, and led to a 3- to 100-fold increase in extracellular MIF over a treatment time of 6 h. Necrosis-induced release of MIF was shown before for cardiomyocytes and neutrophils10, 11 and actually is expected for an atypical cytokine like MIF that is semiconstitutively expressed and stored intracellularly at relatively high concentrations. In fact, the release pattern following necrotic stimulation of macrophages mirrored that of lactate dehydrogenase (LDH) as a representative of an intracellular house-keeping protein.6
Necroptosis is a programmed form of necrosis, also considered as regulated inflammatory cell death. Unlike in necrosis, permeabilization of the cell membrane during necroptosis is tightly regulated and is mediated by caspase-independent receptor-interacting kinase-1(RIPK1)/RIPK3/ripoptosome/mixed lineage kinase domain-like pseudokinase (MLKL)-mediated pore formation. Dankers et al. treated their bone marrow-derived macrophages and THP-1 monocytes with lipopolysaccharide (LPS)/pan-caspase inhibitor Z-VAD and TNF inhibitor/inhibitor of apoptosis (IAP) family of proteins BV-6/Z-VAD, respectively, to induce necroptosis. They observed a rapid massive release of MIF, which was specifically dependent on the necroptotic signaling machinery as verified by experiments using the RIPK1 inhibitor necrostatin-1. This is the first report causally linking MIF release from immune cells to necroptosis (Figure 1). Interestingly, a connection between MIF and necroptosis has been suggested before. We previously showed that MIF signaling increases necroptosis in cardiac fibroblasts but protects from necroptosis in tubular epithelial cells in the setting of acute kidney injury.12, 13 Barnes et al. implicated necroptosis in ethanol-induced receptor-interacting kinase-3 RIPK3-dependent MIF release from liver cells.14 In conjunction with these studies, the current work by Dankers et al. may be suggestive of an intriguing broader role of MIF in necroptotic cell death processes. MIF release is rapidly facilitated by necroptosis, while extracellular MIF in turn controls this programmed inflammatory cell death process.

Pyroptosis is another form of programmed necrotic cell death, shares similarities with necroptosis, but is restricted to immune cells and has a much higher inflammatory potential. Key mechanistic differences are (i) the initiation by the NLRP3 inflammasome (the “pyroptosome”) by intracellular danger signals instead of an extracellular trigger; (ii) the involvement of caspases (caspase-1/4/5 in humans and caspase-11 in mice), which lead to the processing and activation of IL-1 family cytokines and (iii) proteolytic generation of gasdermin D (GSDMD), which is the pore-forming protein in pyroptotic processes in contrast to MLKLs mixed lineage kinase domain-like pseudokinases in necroptosis.15 Dankers et al. triggered NLRP3-mediated pyroptosis in their macrophage cell models by a combination of LPS priming and the potassium ionophore and NLRP3 activator nigericin. They observed marked MIF release under these conditions and verified pyroptosis specificity by the NLRP3-specific inhibitor MCC950, which abrogated MIF release. While MIF has been previously linked to NLRP3-associated inflammation (Figure 1), the current paper by Dankers et al. in Immunology & Cell Biology is the first report establishing mechanistic causality between NLRP3 inflammasome activity and MIF secretion. The same authors previously found that MIF functions as an upstream activator of NLRP3, enhancing IL-1β secretion.16 Surprisingly, this was directly mediated by intracellular MIF, which is required for the interaction between NLRP3 and the intermediate filament protein vimentin. Kang et al. uncovered an alternative mechanism of upstream control of NLRP3 by MIF in human monocytes in the context of lupus autoimmunity. They showed that U1 small-nucleotide ribonucleoparticle immune complex is a specific trigger of MIF secretion, which subsequently activates NLRP3, caspase-1 and downstream IL-1β in a CD74-dependent manner.17 This upstream control mechanism of NLRP3 thus is a function of autocrine, extracellular MIF (Figure 1). In addition, a potential connection between MIF and the inflammasome had become apparent in a previous iTRAQ mass spectrometry secretome screen, in which MIF was one of the top-ranked proteins cosecreted with caspase-1. Although no follow-up biochemistry was undertaken to confirm that MIF interacts with caspase-1 and/or that MIF secretion was causally caspase-1 dependent, the study implicated the NLRP3 inflammasome in MIF secretion18 (Figure 1). Together with the current work by Dankers et al., these studies establish MIF as both an upstream regulator of NLRP3 activity and a novel target of the NLRP3-dependent pyroptotic cytokine secretion machinery.
This leads over to an intriguing question that neither Dankers et al. nor any of the other studies have yet addressed: how, mechanistically, does intracellular MIF protein that is released during necroptosis or pyroptosis (or through the other suggested pathways; Figure 1) cross the plasma membrane barrier to reach the extracellular compartment? The membrane-permeabilizing pore that enables for the release of intracellular expressed danger-associated molecular patterns (DAMPs, also termed alarmis) during necroptosis is MLKL. The release of IL-1β, other IL-1 cytokines and DAMPs from inflamed macrophages in pyroptotic cell activation is mediated via membrane pores formed by GSDMD. According to a recent concept, “small” GSDMD pores can act as channels that facilitate the release of IL-1β and other small leaderless cytokines from viable cells with otherwise intact plasma membranes, whereas “larger” GSDMD pores would be associated with lytic forms of cell content release in more advanced stages of cell hyperactivation.19, 20 The pore molecule executing the release of MIF in activated monocyte/macrophages has yet to be discovered. Figure 1 (middle panel) summarizes the mechanistic options. As Dankers et al. find MIF release to be NLRP3 dependent, GSDMD pores are likely to form concurrent with MIF release, and future studies will need to determine whether GSDMD can function as the MIF-transporting pore. Alternatively, the previously implicated ABCA1 transporter or an unknown channel/pore may serve as a MIF-releasing pore. Previous studies with pharmacological inhibitors also have implicated a vesicular export mechanism, the details of which have remained unclear.5 Moreover, some of the experiments performed by Dankers et al. suggest that MIF release may significantly increase in more the advanced, “lytic,” stages of pyroptosis and/or necroptosis. At this stage, membrane translocation of MIF may be executed by larger GSDMD pores or eventually exacerbated membrane integrity and cell death (Figure 1, bottom panel).
The study by Dankers et al. touches upon additional facets of MIF secretion biology. Following up on their previous work showing that loss of autophagy further enhances LPS-stimulated MIF secretion,21 they here confirm in their BMDM model that autophagy inhibition promotes MIF release similar to that of IL-1β. Thus, autophagy inhibition may contribute to MIF release during pyroptosis, but it might also be one of the mechanisms relevant in constitutive MIF secretion under homeostatic conditions. However, the actual pore or channel that enables MIF secretion at baseline remains unknown (Figure 1, upper panel). Alternatively, a vesicular mechanism or exosome-mediated export of MIF as hypothesized previously may be responsible for MIF release under such conditions, which would be in line with coordinated secretion/reuptake processes ensuring a homeostatic extracellular MIF pool, and the growth factor-like characteristics of MIF that Dankers et al. also speculate about.
In summary, the current study fills a gap in our understanding about the release mechanisms of MIF proteins during various stages of inflammation. Most importantly, it adds necroptosis and pyroptosis to the list of relevant pathways mediating MIF release from macrophages, offers initial mechanistic explanations and suggests a staggered sequence of cell activation from homeostasis to marked inflammation and damage-associated cell stress (Figure 1). Some interesting mechanistic questions remain to be clarified in the future. For example: (i) what is the role of p1159 in these release processes? (ii) what is the molecular identity of the pore? and (iii) what are the relative contributions of the different mechanisms in early stage, low-grade inflammatory stimulation versus elevated inflammation versus exuberant hyperstimulation associated with cell damage? The answers will also help to address open questions regarding LPS-triggered MIF secretion from fully viable macrophages such as RAW264.7 or peritoneal exudate macrophages versus BMDMs or human monocytes,6, 22 MIF release mechanisms in other nonimmune cell types or the intriguing observation from single-cell secretion profiles, suggesting the existence of stimulus-dependent MIF secretion-competent cells.23 Regardless of these open mechanistic aspects, our gained knowledge of the MIF secretion mechanism furthers the concept that MIF is a key upstream mediator of monocyte/macrophage inflammation.
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft (DFG) grant SFB1123-A3 and by DFG under Germany’s Excellence Strategy within the framework of the Munich Cluster for Systems Neurology (EXC 2145 SyNergy – ID 390857198) to JB. AH is supported by a Metiphys scholarship of LMU Munich. Open access funding enabled and organized by Projekt DEAL.
Author Contribution
Jürgen Bernhagen: Conceptualization; Writing—original draft; Writing—review and editing. Adrian Hoffmann: Conceptualization; Writing—original draft; Writing—review and editing.