Dendritic cells in Th2 immune responses and allergic sensitization
Abstract
Allergic responses are characterized by the activation of a specific subset of effector CD4+ T cells, the T-helper type 2 (Th2) cells, that respond to harmless environmental antigens causing inflammation and pathology. Th2 cells are also found in the context of parasite infections, where they can mediate parasite clearance and expulsion, and support tissue repair. The process that leads to the activation of Th2 cells in vivo is incompletely understood: while it has become clear that “conventional” dendritic cells are essential antigen-presenting cells for the initiation of Th2 immune responses, the molecules that are expressed by dendritic cells exposed to allergens, and the mediators that are produced as a consequence and signal to naïve CD4+ T cells to promote their development into effector Th2, remain to be defined. Here we summarize recent developments in the identification of the dendritic cell subsets involved in Th2 responses, review potential mechanisms proposed to explain the generation of these immune responses, and discuss the direct and indirect signals that condition dendritic cells to drive the development of Th2 responses during allergen or parasite exposure.
Introduction
Allergic disease is now the most common chronic inflammatory disease in Western countries, affecting up to 8% of the population often seriously and with a severe impact on quality of life.1 Allergic disease is defined by the inappropriate activation of immune responses to otherwise harmless stimuli such as pollens, house dust mites (HDMs), chemicals or foods, such that the disease is a result of the damage caused by the immune response itself rather than the triggering stimulus.
The inappropriate immune responses that underlie allergic disease are characterized by the presence of a CD4+ T-cell population defined as “T-helper type 2” or “Th2” which produces a characteristic set of cytokines especially interleukin (IL)-4, IL-5, IL-9, IL-13 and IL-31. These cytokines initiate antibody switching to immunoglobulin E, accumulation of mast cells and eosinophils, mucus production at mucosal surfaces or itchiness and loss of barrier function in skin. Th2 cells, their cytokines and patterns of inflammation similar to those found in allergy are also found in association with infections with multicellular parasites including intestinal helminths, in which case the resulting inflammation is thought to favor parasite expulsion and subsequent tissue repair.2
While allergic responses are well recognized and characterized, the process that leads to the differentiation of allergy-mediating Th2 cells is not fully defined. IL-4 was originally described as the essential cytokine that drives Th2 differentiation in vitro;3 however, IL-4 is not always necessary in vivo and its requirement appears to vary across different Th2 subpopulations.4 Other cytokines have also been reported to promote the development of Th2 cells but their contribution is often additive and may not extend across several different models of allergic response. In this review, we discuss the role of a specific population of antigen-presenting cells (APCs), the dendritic cells (DCs), the signals that condition DCs to prime Th2 immune responses and the signals they transmit to CD4+ T cells to initiate their differentiation into Th2.
Conventional DCs: Heterogeneity, Properties and Function
Conventional DCs (cDCs) are specialized APCs that perform a crucial role as tissue sentinels to alert the immune system to foreign antigen including pathogens and noninfectious agents such as allergens. cDCs are constantly replenished from the bone marrow through committed precursors,5 which reach secondary lymphoid organs either directly via blood or indirectly via blood and peripheral tissues. These two subsets of cDCs are usually referred to as “(lymphoid) resident” and “migratory” (migDCs) DCs, respectively, to reflect their different developmental stages. During their residence in peripheral tissues, migDCs actively sample their environment by taking up proteins, dying cells as well as foreign antigens, to finally migrate through the lymphatics to the draining lymph node (dLN) in a CCR7-dependent manner.6 DC migration from peripheral tissue to dLN can be spontaneous as in steady-state conditions, or stimulated by inflammatory signals or infection.
Two major lineages of cDCs have been identified that differ in their developmental requirements7 and function,8, 9 and share phenotypic similarities across different tissues in mouse and human.10 cDC1 development requires expression of the transcription factors interferon regulatory factor (IRF) 8, ID2 and BATF3.11 cDC1s can be identified by their selective expression of XCR1, and can cross-present exogenous antigen to CD8+ T cells via major histocompatibility complex class I. Because of their expression of high levels of Il12b transcripts, these DCs have also been implicated in the induction of Th1 responses.
cDC2s, by contrast, require expression of the transcription factors ZEB2 and IRF4,11 are highly endocytic and their predominant role is to present antigen to naïve CD4+ T cells and support their differentiation into different functional phenotypes.12 cDC2s are often more abundant than cDC1s, especially in tissues such as skin and intestinal tract. Unlike cDC1s which form a homogeneous and well-defined population expressing similar markers regardless of location, cDC2s are heterogeneous and vary in phenotype and marker expression depending on the tissue of origin13, 14 such that their unequivocal identification can be difficult because of many shared surface markers with inflammatory monocytes and macrophages. This is the case in the steady state and especially during inflammation.15 It is not clear whether the cDC2 phenotypic heterogeneity across tissues also reflects differences in function, as the functional characterization of these cDC2 subsets has been limited by the availability of suitable subset- or tissue-specific depletion models. By using single-cell RNA-sequencing, further transcriptional heterogeneity has been revealed within cDC2s in murine spleen and human blood, which correlates with a varying propensity to produce inflammatory cytokines upon Toll-like receptors (TLRs) stimulation.16
In addition to cDCs, other DC and APC populations exist in both humans and mice that differ from cDCs on the basis of dependence on growth factors or specific transcription factors. Plasmacytoid DCs are specialized for type I interferon (IFN-I) production and play an important role in antiviral immunity.17 Plasmacytoid DCs have been reported to suppress allergic sensitization and/or airway allergic immune response, a function they may carry out indirectly by promoting the induction of regulatory T cells in mice18, 19 or, in human patients, by protecting the lung environment during viral infections in early life.20 Epidermal Langerhans cells are DC-like cells that originate from a skin-resident precursor in the steady state, or from monocytes during inflammation, and share properties of both DCs and macrophages.21, 22 As the evidence linking these cell types to allergy development is relatively limited, they will not be discussed in further detail in this review.
cDCs are essential APCs in Th2 and allergic immune responses
The first formal demonstration that DCs are essential for the priming of Th2 immune responses was in 2010, when Phythian-Adams and colleagues showed that inducible depletion of CD11c+ cells, including all cDCs as well as plasmacytoid DCs and some monocytes, during infection with the parasite Schistosoma mansoni or immunization with schistosome egg antigen resulted in decreased production of Th2 cytokines including IL-4, IL-5 and IL-13.23 This study resolved much existing confusion on the identity of the initiating APCs in Th2 immune responses, which had been proposed to be a basophil one year earlier.24-27 The notion of DCs as an essential APC in allergic responses is also supported by studies where the transfer of purified migDCs from allergen-primed mice was sufficient to prime IL-4 responses in naïve recipients.28 A single-cell RNA-sequencing study also highlighted that, compared with bacterial and fungal pathogens, the uptake of helminth allergens was mostly restricted to migDC2s rather than other immune cell populations.29
Additional studies in 2013 provided further information on the phenotypic identity of Th2-inducing DCs in the context of allergic inflammation and parasite infection (Table 1). Inducible deletion of cells expressing CD301b (macrophage galactose N-acetyl-galactosamine-specific lectin 2, MGL2), a lectin receptor on some cDC2s and macrophages, did not affect Th1 responses but caused the loss of response to various Th2 stimuli.30 In addition, expression of IRF4 and PDL2 was necessary for Th2 responses31 and for allergic airway inflammation after intranasal or epicutaneous sensitization.32, 33 This requirement was because of the incomplete development of migDC2s, which are unable to reach the dLN when Irf4 is conditionally deleted in CD11c+ cells.34-36 Consistent with these observations, migDC2s acquire extensive transcriptional changes after allergen immunization,37 whereas migDC1s show few transcriptional changes and do not impact on the magnitude of the IL-4 response if deleted. Further studies identified a subset within the cDC2 population which requires the transcription factor KLF4 for its development, and is characterized by expression of low CD11b in skin and CD301b in lung. This KLF4-dependent cDC2 population was also necessary for the generation of Th2 responses to HDM allergen and schistosome egg antigen, but not for responses to Th1 or Th17 stimuli,38 suggesting that the functions previously ascribed to cDC2s may be dependent on this KLF4-dependent cDC2 subset.
| Mouse strains used | DC subset/s targeted by mutation | References |
|---|---|---|
| CD11c-DTR/OVA/EGFP | All CD11c+ cells: DC subsets, LCs, monocytes, some macrophages | 23 |
| CD11c-Cre, Irf4-flox | IRF4+ migratory cDC2s in lymph nodes, lung cDC2s, spleen cDC2s | 31-33 |
| CD301b-DTR | CD301b+ subsets of cDC2s and macrophages | 30 |
| CD11c-Cre, Klf4-flox | CD11b-low cDC2s in skin-dLN and CD301b+ cDC2s in lung and lung dLN | 38 |
| FLT3-Ligand-KO | cDC subsets, pDCs | 42 |
| CCR2-KO | Monocytes, moDCs, monocyte-derived macrophages, skin migDC1s | 42 |
- Mouse models used to demonstrate a contribution of DC and APC populations to Th2 immune responses in vivo, and the DC subset/s that are targeted in each model are presented. Only experiments reporting a positive contribution to the Th2 response are included. Please refer to the text for more detail.
- cDC, conventional dendritic cell; DC, dendritic cell; dLN, draining lymph node; KO, knockout; LC, Langerhans cell; migDC, migratory dendritic cell; moDC, monocyte-derived dendritic cell; OVA, ovalbumin.
In addition to their role in the priming of Th2 responses, cDCs are essential at the “effector” phase of the response when secondary exposure to allergen triggers local secretion of cytokines by memory Th2 cells, and allergic inflammation. In airway allergy models, depletion of CD11c+ cells at the time of intranasal challenge prevented the accumulation of eosinophils in the airway, and the development of mucus production and airway hyper-responsiveness.39, 40 It is presently unknown whether other types of allergic disease, such as those involving the skin or intestinal tract, also display a similar cDC requirement at the effector stage.
Finally, in addition to cDCs, monocyte-derived DCs41 and their monocyte precursors are a clear feature of some allergic responses and are necessary for the full expression of airway inflammation.42 It is presently unclear whether the role of these cells is to support the priming of Th2 cells at the time of initial allergen exposure, or to promote production of inflammatory cytokines upon secondary challenge, or both.
How Do Conventional DCs Drive the Development of Th2 Cells?
Several models have been proposed over the years to explain how cDCs might drive the development of Th2 cells, but as of today none of these models has gained the consensus of the immunology community or been able to encompass all available data. One early model proposes that Th2 cell differentiation occurs by default, because of the failure of cDCs to activate other cell differentiation programs such as Th1 or Th17. Indeed, so far there are no identified pathogen-associated molecular patterns or TLRs known to activate a Th2-inducing programme in cDCs, and no cDC-derived cytokines known to trigger the development of Th2 cells. In addition, this model is supported by the observation that some expression of GATA3, the transcription factor defining the Th2 lineage, and signal transducer and activator of transcription (STAT)5 activation by IL-2 or other cytokines, which is also essential for Th2 differentiation, are already found in naïve CD4+ T cells.43 Initial studies in mice have supported some aspects of this model and shown that Myd88-KO (knockout) mice immunized with ovalbumin in complete Freund’s adjuvant generated immunoglobulin E responses, and T cells that produce IL-13 instead of the IFNγ produced in wild-type mice. This finding was explained by the observation that, unlike wild-type bone marrow-derived DCs (BMDCs), Myd88-KO BMDCs exposed to mycobacteria failed to upregulate costimulatory molecules or produce IL-12.44 However, later experiments in IL-12p40-KO45 thus questioning one essential prediction of the default model.
A more recent model proposes that Th2 development requires a specialized cDC subset which is distinct from the Th1- or Th17-inducing cDC subsets. As mentioned previously, cDCs comprise two main populations, namely, IRF8+ cDC1 and IRF4+ cDC2. The IRF4-dependent, CD301b+ cDC2s have been reported as necessary for Th2 differentiation but not for Th1 responses.30, 31 Within the cDC2 subset, Th2-inducing cDC2s that are dependent on KLF4 for their development have been reported in several tissues including skin, lung and liver,38 whereas a second subset, which requires Notch2 for its development, is reportedly necessary for Th17 differentiation through its ability to produce IL-23.46, 47 By contrast, cDC1 subsets would promote Th1 and limit Th2 responses by producing IL-12.48 It is still unknown which specific molecules enable the IRF4-dependent, KLF4-dependent cDC2 subset to induce Th2 responses. IRF4 was initially suggested to be this essential molecule.49 However, later studies suggested this may not be the case, as cDC2s in which Irf4 had been conditionally deleted were able to prime normal Th2 responses when microinjected directly into the LN.50 Additional studies comparing Th responses in different tissues will be required to resolve this question.
A third model, which is not necessarily exclusive of previous ones, is that Th2 responses could be driven by an instructive “signal 3” expressed by cDC2s upon exposure to allergen. This process would correspond, for example, to the TLR-dependent expression of IL-12 by cDCs exposed to Th1 stimuli. Allergens could signal either directly, by engaging specific receptors expressed on cDCs, or in a bystander fashion by inducing cytokine production by surrounding tissue stromal or inflammatory cells, with these cytokines then conditioning cDC2s to induce Th2 responses. Several lines of evidence support this possibility, some of which will be discussed in more detail in later sections. For example, the epithelial cytokine thymic stromal lymphopoietin (TSLP) is released during allergen exposure and is known to activate cDCs to upregulate major histocompatibility complex class II and costimulatory molecules, migrate to dLN and produce increased amounts of T-cell-attracting chemokines.51 Interestingly, it was reported that TSLP does not affect all DC populations equally, but preferentially activates CD11blow cDC2s in skin-dLN compared with CD11bhigh cDC2s, or cDC1s,52 suggesting some degree of DC specialization. While an instructive signal for Th2 immune responses may exist, it is not clear whether any of the signals identified so far can fill this role. Depending on the model and type of allergen used, KO mice in which these putative Th2-instructive signals are deleted can still be able to generate Th2 responses, thereby suggesting that other, still unidentified signals remain functional and able to drive Th2 responses in these mice.
Endocytic and signaling receptors involved in allergen uptake
Although allergens are not recognized as classical or powerful TLR ligands, there is evidence that they can engage these receptors through mimicry, and that the consequent signaling can contribute to the resulting Th2 response. Similarly, C-type lectin receptors have been implicated in the induction of Th2 responses by cDCs. These receptors are discussed in the following and summarized in Figure 1.
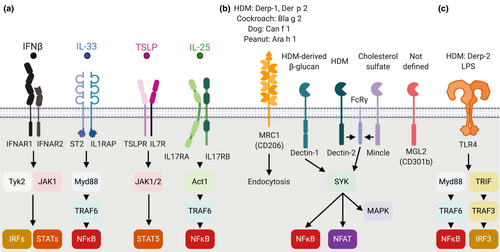
TLR4 is expressed at low levels on cDCs in vivo.29, 53 In addition, these receptors are highly expressed on macrophages, monocytes and monocyte-derived DCs, and can be activated by several allergens. The major HDM allergen Der p 2 is a functional mimic of the TLR4 cofactor MD2 that drives lung cDC activation and airway inflammation.54 TLR4 can also recognize helminth products such as glycans, glycolipids or proteinases.55 At low doses, TLR4 ligands can amplify Th2 responses: coadministration of a low dose of lipopolysaccharides, a ligand for TLR4, was necessary for the induction of Th2 responses and allergic airway inflammation after intranasal ovalbumin administration, whereas high lipopolysaccharide doses induced Th1 response.56 Low doses of intranasal lipopolysaccharides were recently shown to promote neutrophil extracellular trap formation, which favored antigen uptake by monocyte-derived DCs and allergic airway inflammation.57 Similarly, intradermal co-injection of the helminth parasite Nippostrongylus brasiliensis nonviable larvae together with low numbers of Gram-negative bacteria increased the number and proportion of IL-4+ T cells in dLN by an IFN-I dependent mechanism.58
CD301b is expressed by a subset of cDC2s that promotes Th2 responses against different allergens, suggesting a role of this molecule in the detection of glycosylated allergens.30, 59 In a single-cell RNA-sequencing study, expression of this receptor has been associated with a noninflammatory cDC2 phenotype in mice and humans.16 The same cDC2 subset coexpresses the endocytic receptor CD206 (or mannose receptor, MRC1),60 also expressed by macrophages,61 which mediates the uptake of HDM, cockroach or peanut allergens by IL-4/granulocyte-macrophage colony-stimulating factor (GM-CSF) BMDCs in vitro. In coculture experiments, the silencing of the CD206-encoding MRC1 gene on human BMDCs loaded with Der p 1 switches CD4+ T-cell polarization from IL-4+ to IFNγ+,62 suggesting a key role of this receptor in allergen uptake and Th differentiation.
cDC2s also express Dectin-1 (Clec7a), Dectin-2 (Clec4n in mouse and CLEC6A in human) and Mincle (Clec4e) which sense allergens as well as fungal and bacterial components. These molecules are also expressed on several other myeloid populations and their characteristics and signaling pathways have been recently reviewed.63 Dectin-1 is involved in the recognition of HDM-derived β-glucans. Experiments in KO mice suggest that Dectin-1 engagement promotes the migration of lung CD11b+ cDCs to the dLN and the development of Th2 cells.64 In a second study of airway responses to HDMs, signaling through Dectin-1 expressed on lung epithelial cells was found to inhibit the release of IL-33 and the consequent cDC activation.65 Further experiments using conditional inactivation of Dectin-1 in selected cell populations are needed to reconcile these findings. HDM extracts can also activate BMDCs via Dectin-2 recognition of glycan mannose,66 which is necessary for BMDCs to induce airway allergic inflammation at both the priming or effector stage.67, 68 Interestingly, TSLP can induce BMDC maturation while preserving antigen uptake ability via the upregulation of FcRγ (Fcer1g), the signaling component of Dectin-2.69 In vivo, Dectin-2 or FcRγ total KO mice showed a marked decrease in Th2 cytokines and HDM-induced allergic airway inflammation.70, 71 Mincle was originally associated with recognition of mycobacterial glycolipids, but a recent paper also reports its role in inflammatory responses in skin. Mincle is upregulated after cutaneous wounding and senses cholesterol sulfate, a molecule produced at epithelial barrier sites, resulting in further release of inflammatory mediators. Accordingly, Mincle-KO mice generate reduced inflammatory responses to skin sensitizers.72 It will be interesting to establish whether this finding also extends to models of allergic inflammation.
The need for epithelial and stromal factors: TSLP, IL-33, IL-25 and IFN-I
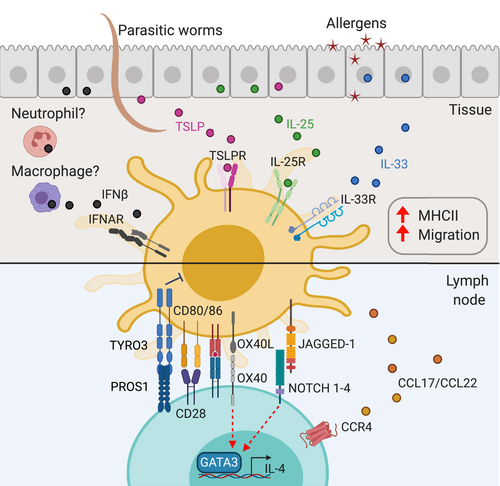
Many allergens as well as parasitic worms possess proteolytic activity that can damage epithelia, eliciting the secretion of cytokines and alarmins to orchestrate the innate response at barrier sites. Epithelial factors can activate cDCs as well as several other innate and adaptive immune populations. The production and functions of these epithelial factors have been extensively reviewed.51 Here we will focus on their impact on cDCs and their ability to initiate Th2 responses (Figure 2).
TSLP, a member of the IL-2 cytokine family, binds a heterodimeric receptor consisting of cytokine receptor-like factor 2 (Crlf2, TSLPR) and IL7R that activates mainly STAT5 but also other STAT transcription factors (Figure 1). Both receptor chains are widely expressed at steady state on migDCs and especially cDC2s, whereas splenic cDC2s express low levels of IL7R and are therefore unresponsive to TSLP unless exposed to IL-4.73 In coculture experiments, TSLP-conditioned human blood cDCs are able to induce the production of IL-4, IL-5, IL-13 and IL-21 by naïve CD4+ T cells.74, 75 In vivo, TSLP activates skin migDCs to upregulate expression of costimulatory molecules, migrate to dLN and promote Th2 immune responses in a STAT5-dependent manner.52, 76-78 In skin, the dermal CD11blow cDC2 subset appears especially responsive to TSLP treatment,52 which is consistent with the essential role of CD11blow cDC2s in Th2 induction.38
IL-25, also known as IL-17E, belongs to the IL-17 cytokine family. Its heterodimeric receptor is composed of IL17RA and IL17RB (Figure 1). The cellular effects of IL-25 are mediated via a broad range of transcription factors including NF-κB, STAT6, NFATc1, GATA3 and AP-1. In addition to epithelial cells, IL-25 is constitutively produced by “Tuft” cells, a rare cell population in thymus and intestinal tract.51 The role of IL-25 in Th2 responses has been difficult to identify79 however, Claudio et al. recently showed that the conditional deletion of Il17rb on CD11c+ cells or ZBTB46+ cDCs decreased production of Th2 cytokines, accumulation of mast cell and lung inflammation in an HDM-induced airway inflammation model,80 suggesting a direct requirement for IL-25 signaling in cDCs during Th2 immunity.
In addition to epithelial-derived cytokines, IFN-I can also contribute to the generation of type II immunity. The IFN-I family comprises 13 IFNα and one IFNβ which are produced by most cell types after stimulation through intracellular nucleic acid sensors including STING, TLR3 and 9, RIG-I and MDA-5, or endotoxin recognition via surface TLR4.84, 85 All IFN-I molecules bind to the same heterodimeric receptor composed of IFN alpha and beta receptor subunit IFNAR1 and IFNAR2 which signal through the JAK–STAT pathway and transcription factors of the IRF family (Figure 1). Intradermal injection of Nippostrongylus brasiliensis triggers a robust IFN-I signature in cDC2s, and injection of IFNAR1-blocking antibodies reduces this signature and the induced IL-4 response in dLN.37 A similar IFNAR-dependent increase in the Th2 response was also observed after immunization with BMDC cocultured with HDM or Schistosoma mansoni eggs before injection.86 Recently, whole lung transcriptomic analyses have confirmed that the induction of IFN-I response genes occurs in a broad range of lung diseases including viral infection as well as in HDM-dependent allergic airway inflammation.87 Reports that IFN-I can suppress IFNγ responses88 and regulate the balance of T-follicular versus T-effector CD4+ T-cell differentiation89 may offer potential mechanisms for the enhancement of Th2 responses by IFN-I.
IL-33 is an alarmin belonging to the IL-1 superfamily that binds a heterodimeric receptor comprising ST2 (IL1RL1) and IL-1 receptor accessory protein (IL1RAP) and activates NF-κB and MAP kinase pathways (Figure 1). IL-33 protein is constitutively stored as a precursor in the nucleus of epithelial cells. Upon damage or stress, IL-33 is released in the extracellular milieu and is activated by proteases to act as a cytokine.81 In vitro, IL-33 acts directly on BMDCs via ST2 to promote BMDC activation and Th2 differentiation in culture.82 In vivo, IL-33 conditions cDCs to support Th2 cytokine production and effector function in mouse models of allergic airway inflammation.82 In an MC903-induced model of skin atopic dermatitis, ST2 and expression of the adaptor protein MYD88 in CD11c+ DCs were necessary for epidermal thickening and skin inflammation.83
GM-CSF, or CSF2, has also been implicated in cDC activation during allergic lung inflammation. The binding subunit GM-CSF receptor alpha chain (CSF2RA) forms a heterodimer with the signaling subunit beta chain (CSF2RB) that activates the STAT5 and ERK pathways. Studies using allergens from HDM or cockroach found that GM-CSF was released by airway epithelia in an IL-1α-dependent fashion, and was critical for lung allergic responses.33, 90, 91 GM-CSF signaling is important for cDC homeostasis and survival in tissues including the lung,92, 93 and GM-CSF neutralizing antibodies impair cDC antigen uptake, migration and activation after intranasal sensitization with cockroach or HDM allergens.90, 91 Eosinophil accumulation in airway also requires GM-CSF, suggesting that this cytokine also plays an important function at the effector stage of airway inflammation, independently of DCs.93 Thus, the precise role of GM-CSF in allergic sensitization remains unclear, as also suggested by reports that GM-CSF-KO mice generate normal protective Th2 responses to the helminth parasite Nippostrongylus brasiliensis.94
cDC-expressed molecules that are associated with the priming of Th2 responses
Several molecules have been shown to be expressed in cDCs during allergic sensitization and are associated with an improved ability to prime allergic responses (Figure 2).
The chemokines CCL17 and CCL22 bind to the receptor CCR4 that is expressed on regulatory T cells and on effector Th2 cells. In the steady state, these chemokines are expressed mostly by migDCs, with CCL22 expressed by all subsets and CCL17 preferentially expressed by CD11b+ cDC2s.53 Upon treatment with TSLP74, 95 or IL-4,96 these chemokines can also be expressed by other populations of cDCs as well as myeloid cells and, at least in vitro, on CD4+ T cells activated in the presence of IL-4.97, 98 Both Ccl17 and Ccl22 are upregulated in migDC2s during Th2 responses,37 but their effect during the priming of Th2 responses in vivo remains to be determined.
cDC2s activated by epithelial Th2 cytokines also express a range of membrane-anchored ligands that are required for Th2 responses and could modulate DC–T-cell interaction. TLSP induces the expression of OX40L in cDC2s, which is thought to promote Th2 and T follicular type II responses.75, 99 However, it is still unclear whether the function of OX40L in Th2 responses is as a polarization signal or simply as a costimulatory molecule.100
NOTCH ligands have also been implicated in Th2 polarization. Four NOTCH receptors have been identified, NOTCH1–4, that bind ligands from two families, the Delta-like and Jagged families (DLL1–4 and Jagged1–2). DLL and Jagged are expressed by cDCs under various conditions including TLR signaling or exposure to cytokines. It was originally proposed that Jagged1 instructs Th2 cell development, whereas DLL1–4 drive Th1 while limiting Th2 responses (reviewed by Tindemans et al.101). However, much of this work was carried out using cultured BMDC or whole KO mouse models where functional effects cannot be clearly ascribed to expression on a certain cell type. Careful studies using mice, in which NOTCH was inactivated only in T cells, suggest that this pathway also regulates retention in LN, preventing T-cell migration to the periphery. Therefore, the Notch pathway regulates multiple aspects of the T-cell response rather than mediating a specific instructive signal for Th cell differentiation.102
In addition to positive regulators of Th2 responses, negative regulators have been described. TYRO3 is a DC-expressed molecule that interacts with the Th2-expressed ligand PROS1. Deletion of either TYRO3 on ovalbumin-loaded BMDCs or PROS1 in T cells increases Th2 cytokine production and the consequent inflammation.103 The IL-4-dependent expression of PROS1 in differentiated Th2 cells suggests that this mechanism is likely relevant to the effector phase of the response rather than during priming.
Bulk transcriptomic analysis of ex vivo migDC2s after two different Th2 stimuli, dibutyl phthalate–fluorescein isothiocyanate application or injection of Nippostrongylus brasiliensis nonviable larvae, both of which require cDC2s to trigger robust Th2 responses but are differentially dependent on TSLP, showed that in each case cDCs differentially expressed several hundred genes compared with mock-treated controls, but very few of these genes were expressed in both models. One of these shared genes was Ccl17.37 This observation raises the question of how cDC2s integrate different antigen and environmental signals to translate into a common instructive signal that drives Th2 differentiation.
Metabolic Pathways Involved in Th2 Differentiation
Metabolic pathways have an important role in determining DC responses. The majority of investigations have been performed using GM-CSF or FLT3L BMDC cultures which contain some DCs together with significant proportions of other myeloid cell populations.104 Despite this, several interesting potential insights have been derived.
At steady state, DCs in general are in catabolic state, including processes such as active oxidative phosphorylation, the tricarboxylic acid cycle, fatty acid oxidation, glutaminolysis and use of intracellular glycogen.105, 106 With immune activation, the metabolic output of DC switches to glycolysis and lactic acid fermentation, as well as anabolic metabolism for production of substrates.104 For example, human blood-derived cDC2s stimulated by TLR7/8 agonists require glycolysis for activation.107 In mouse splenic cDCs, TLR3-dependent production of IFN-I signals via IFNAR to induce hypoxia-inducible factor 1A, a known oxidative phosphorylation modulator, resulting in metabolic conversion to glycolysis.108
The phosphatidylinositol-3-kinase/mammalian target of rapamycin/5′ AMP-activated protein kinase signaling pathway has been shown to regulate DC activation and proliferation via metabolic pathway modulation109 and can affect the proportions of different subsets. For example, inhibiting fatty acid oxidation and 5′ AMP-activated protein kinase signaling results in increased cDC2s, whereas inhibiting reactive oxygen species production increases cDC1s.110 As such, the metabolic pathway may predispose the type of immune response to an antigen; if there are more cDC2s present than normal, a propensity toward the Th2 response may be present. In addition, treatment with rapamycin, an inhibitor of the mammalian target of rapamycin pathway, results in attenuation of several hallmarks of allergic airway inflammation including immunoglobulin E production and airway hyperreactivity in a mouse model.111 In partial agreement, chimeric mice in which the mammalian target of rapamycin pathway was conditionally deleted in CD11c+ cells showed decreased IL-4 production in lung, although immunoglobulin E and eosinophilia were not decreased. These mice also displayed a switch from IL-4 to IL-17 and airway neutrophilia specifically attributed to CD11b+ DCs.112 Therefore, metabolic function affects the ability of cDC2s and other cell populations to sustain allergic inflammation in lung.
Conclusions
Many years after Th2 cells were first described, the APC-derived signals that drive their differentiation remain incompletely defined. Several factors that condition cDCs to promote Th2 responses have been identified. These include epithelial-derived cytokines and alarmins that are produced after allergen exposure and of which TSLP is an especially important example. Given the many cellular targets of epithelial-derived cytokines, it will be absolutely essential to use models where the effects of these factors can be examined in selected cell populations. In addition, a better understanding is needed of whether these epithelial factors are equally important across different tissues and which specific functions they might fulfill in each. Regardless, none of these epithelial factors appears to be essential across different models of Th2 immune responses, perhaps suggesting that other, yet to be identified mediators also exist.
cDCs and in particular cDC2s are now firmly identified as the APC population driving Th2 immune responses in vivo, thus defining the target population of interest. Better markers and lineage tracing models are being used to define cDCs in comparison to other phenotypically similar populations such as macrophages and other myeloid cell populations, and ongoing progress is being made in generating mouse models to specifically delete specific cDC2 populations so that their function can be precisely defined. Understanding cDC2’s diversity across different tissues and how their function is affected by the local environment may provide new information on the propensity of different allergen exposure routes to result in sensitization. The ability to study these cells in detail by deep sequencing and single-cell transcriptomics will certainly provide new essential information on the mechanisms of Th2 cell differentiation in vivo.
Acknowledgments
This work was funded by grants from the Health Research Council of New Zealand and the Royal Society of New Zealand Marsden Fund. The authors thank Ms Greta Webb for reading the manuscript and for her useful suggestions.
Author Contribution
Olivier Lamiable: Visualization; Writing-original draft; Writing-review & editing. Johannes U Mayer: Writing-original draft; Writing-review & editing. Luis Munoz-Erazo: Writing-original draft; Writing-review & editing. Franca Ronchese: Writing-review & editing.
Conflict of Interest
The authors declare no conflicts of interest.





