Clinical questions in the Japanese Urological Association's 2024 clinical practice guidelines for urethral strictures
Abstract
Transurethral procedures such as direct vision internal urethrotomy and urethral dilation have been the traditional treatments for urethral strictures. However, transurethral procedures are associated with high recurrence rates, resulting in many uncured cases and prompting major international urological societies to recommend urethroplasty as the standard treatment owing to its high success rate. In contrast, many Japanese general urologists have little doubts about treating urethral strictures with transurethral treatment. Therefore, urethral stricture treatments in Japan are not in line with those used in other countries. To address this, the Trauma, Emergency Medicine, and Reconstruction Subcommittee of the Japanese Urological Association has developed guidelines to offer standardized treatment protocols for urethral stricture, based on international evidence and tailored to Japan's medical landscape. These guidelines target patients with a clinically suspected urethral stricture and are intended for urologists and general practitioners involved in its diagnosis and treatment. Following the Minds Clinical Practice Guideline Development Manual 2020, the committee identified eight critical clinical issues and formulated eight clinical questions using the “patient, intervention, comparison, and outcome” format. A comprehensive literature search was conducted. For six clinical questions addressed by the existing guidelines or systematic reviews, the level of evidence was determined by qualitative systematic reviews. Quantitative systematic reviews and meta-analyses were performed for the two unique clinical questions. The recommendation grades were determined using the Delphi method and consensus by the committee. These guidelines will be useful to clinicians in daily practice, especially those involved in the care of urethral strictures.
Abbreviations
-
- AUA
-
- American Urological Association
-
- CI
-
- confidence interval
-
- CUA
-
- Canadian Urological Association
-
- DVIU
-
- direct vision internal urethrotomy
-
- EAU
-
- European Association of Urology
-
- EPA
-
- excision and primary anastomosis
-
- LS
-
- lichen sclerosus
-
- ntEPA
-
- nontransecting excision and primary anastomosis
-
- PFUI
-
- pelvic fracture urethral injury
-
- PICO
-
- patient, intervention, comparison, and outcome
-
- QOL
-
- quality of life
-
- RCT
-
- randomized control trial
-
- RR
-
- risk ratio
-
- SHIM
-
- Sexual Health Inventory for Men
-
- SIU
-
- Société Internationale d'Urologie
INTRODUCTION
Traditional treatments for urethral strictures have included transurethral procedures, such as direct vision internal urethrotomy (DVIU) and urethral dilation, for many years. However, the high rate of recurrence associated with transurethral treatments has led to a significant number of uncured cases, which is problematic. Recently, major international urological societies such as the Société Internationale d'Urologie (SIU), American Urological Association (AUA), and European Association of Urology (EAU) have successively released clinical guidelines for urethral strictures that recommend urethroplasty as a standard treatment owing to its high success rate.1-11 However, many Japanese general urologists have little doubts about treating urethral strictures with transurethral treatment; as a result, urethral stricture treatments used in Japan are not in line with those used in other countries. Therefore, the guidelines delineated in this article were established to offer standardized treatment protocols for urethral strictures that are grounded in international evidence and tailored to the medical landscape of Japan. These guidelines were created by the Trauma, Emergency Medicine, and Reconstruction Subcommittee of the Japanese Urological Association with the help of appropriate urology specialists with experience in treating urethral strictures.
Target readers
The patients targeted by these guidelines are those clinically suspected of having urethral strictures based on their medical history and tests. The intended users of these guidelines are urologists and general practitioners involved in the diagnosis and treatment of urethral strictures.
Preparation methods
These guidelines were developed in accordance with the Minds Clinical Practice Guideline Development Manual 2020 version 3. The committee established domains for terminology and epidemiology, clinical symptoms and testing, diagnosis, treatment, and follow-up; responsible individuals were appointed to each domain according to the preferences of the committee members. Since urethral strictures predominantly affect male patients, the guidelines are described with respect to male urethral strictures unless otherwise stated. Eight critical clinical issues with unclear benefits and harms associated with the treatment of urethral strictures and the decision-making of both patients and healthcare providers were identified. Eight clinical questions (CQs) were formulated using the “patient, intervention, comparison, and outcome” (PICO) format. The committee members collected the outcomes for each CQ and quantified the importance of those outcomes using the Delphi method. Given the limited number of committee members and difficulty separating the systematic review team from the guideline development group, it was decided that one primary member and one secondary member in charge would undertake both the systematic review and the writing of the guideline recommendations for each CQ. With the support of the International Medical Information Center, a comprehensive literature search of articles published between January 1, 2010 and July 16, 2022 was performed using keywords related to the treatment of urethral strictures. Relevant works were extracted from PubMed, the Cochrane Library, and Igaku Chuo Zasshi by the committee members. Potential references were identified by performing an initial review of the titles, formats, and abstracts of the 2235 works that were collected. Furthermore, articles that were considered significant by the committee members were manually searched. These articles, along with those found during the initial review, underwent detailed examinations during the secondary review to determine the final selections. The levels of evidence for six CQs (CQ1, CQ2, CQ3, CQ4, CQ5, and CQ7) with existing guidelines or systematic reviews that matched the PICO format were determined through qualitative systematic reviews. However, the levels of evidence of two CQs (CQ6 and CQ8) that were unique to our guidelines and were not described by the existing guidelines were determined through quantitative systematic reviews and meta-analyses. The recommendation grades were decided based on consensus by the committee through the Delphi method. In the original Japanese version, basic, textbook-level knowledge about urethral stricture was presented as background questions, but the main purpose of this report is to present the CQs and their answers in this guideline as they appear in the original. Therefore, the inclusion of the background questions has been omitted.
CQ1. Is Urethroplasty recommended over transurethral treatments for urethral strictures?
Recommendation Statement: Transurethral treatments are preferred for strictures that meet all of the following criteria (favorable urethral stricture): strictures not associated with trauma, lichen sclerosus (LS), or previous hypospadias surgery; strictures without a treatment history; strictures with a length shorter than 2 cm and an open lumen; solitary strictures; and strictures confined to the bulbar urethra. For all other cases, urethroplasty is recommended.
Recommendation level: weak.
Evidence certainty: C (weak).
Voting results: 9 of 10 members (90%) indicated a weak recommendation.
Transurethral treatments such as DVIU and dilation for urethral strictures have been widely adopted owing to their simplicity and technical ease; however, they have low success rates.2 Urethroplasty has a higher success rate than transurethral treatments; however, urethroplasty is technically more demanding and not widely available in all regions.12-14 Whether transurethral treatments or urethroplasty should be recommended for urethral strictures without a history of prior treatment is a critical clinical issue for urologists.
Success rates and complications were considered clinically important outcomes. A systematic review was performed through a qualitative synthesis of the Cochrane review by Wong et al.,15 Canadian Urological Association (CUA) guidelines,16 and EAU guidelines8 aligned with the PICO of this CQ. According to the Cochrane review, no studies have compared urethral dilation and urethroplasty, and only one randomized controlled trial (RCT) that compared DVIU and urethroplasty was found in the conference abstracts.17 A study that compared 25 patients treated with DVIU and another 25 treated with urethroplasty for pelvic fracture urethral injury (PFUI) revealed that the success rate in the urethroplasty group (76%) was significantly higher than that in the DVIU group (19%) (risk ratio [RR], 3.39; 95% confidence interval [CI], 1.62–7.07).17 The CUA guidelines feature a meta-analysis encompassing 28 nonrandomized research studies, and five of these studies directly compared urethroplasty and transurethral treatments. For cases of untreated urethral strictures, urethroplasty has a success rate of 84.5%, surpassing that of 61.5% for transurethral treatments. However, compared to transurethral treatments, urethroplasty is associated with an approximately 4% increased risk of complications, including urinary tract infections and bleeding.16 The success rates determined by the meta-analysis of the CUA guidelines are generalized figures for urethral strictures and do not distinguish between the different stricture etiologies. Similarly, an analysis of the EAU guidelines revealed substantial variation (range, 8%–77%) in success rates for DVIU, reflecting heterogeneity in the etiology of the urethral stricture cases studied.8 Studies have found that the efficacy of transurethral treatments for urethral strictures is influenced by the characteristics of the stricture, such as its etiology, location, and length.18-20 Transurethral treatments are notably less effective for strictures that are longer than 2 cm, those located in the penile urethra, and multiple or recurrent strictures. Furthermore, strictures that do not have an open lumen, those resulting from trauma, those occurring after hypospadias surgery, and those caused by LS are less likely to benefit from transurethral treatment.2, 8, 11, 16 Therefore, leading international guidelines specify that transurethral treatments should be considered the primary option only for cases that satisfy all of the following criteria: Strictures that are not associated with trauma, LS, or previous hypospadias surgery; strictures with no prior treatment; strictures with a length less than 2 cm with an open lumen; solitary strictures; and strictures confined to the bulbar urethra. For cases that do not meet these conditions, urethroplasty is recommended as the initial treatment instead of transurethral treatments (Figure 1).2, 8, 11, 16
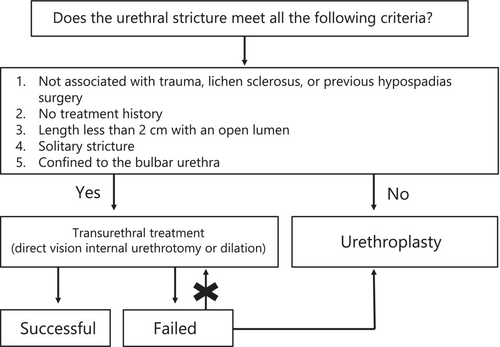
Therefore, despite the limited strength of evidence, urethroplasty offers a higher success rate than transurethral treatments for urethral strictures. Although urethroplasty may lead to more complications, the rates of severe complications associated with both approaches are comparable. Nonetheless, factors that are potentially detrimental to patients, such as extended hospitalization, extended catheterization, and treatment costs, may render urethroplasty a more challenging option. Additionally, the scarcity of reconstructive urologists who specialize in urethroplasty may limit patients' access to this treatment. Hence, variables such as age, location of residence, preferred treatment method, and the physical and financial strains of traveling for treatment can create significant differences in the preferences of patients. Japanese medical insurance covers both transurethral treatment and urethroplasty; therefore, variations in their success rates and patient preferences should be considered when selecting a treatment method.
CQ2. Is urethroplasty preferred to repeating transurethral treatment for recurrent urethral strictures after transurethral treatment?
Recommendation Statement: Urethroplasty should be considered as the first-line treatment option for recurrent urethral strictures after transurethral treatments. Repeating transurethral treatment is not recommended.
Recommendation level: strong.
Evidence certainty: B (moderate).
Voting results: 8 of 10 members (80%) indicated a strong recommendation.
Due to the need for considerable expertise to consistently achieve high success rates, the widespread availability of urethroplasty has been limited. Conversely, transurethral treatments are simpler and more accessible. Therefore, transurethral treatments have been the sole treatments of choice for the management of urethral strictures at numerous institutions. However, the relatively low success rates of transurethral treatments often lead to their repetitive use.21 The availability of experienced urologists skilled in urethroplasty is limited. Consequently, some patients must travel long distances to receive this treatment, as they seek it due to its notably high success rate.21
The essential clinical decision comprises choosing between repeated transurethral treatments and urethroplasty. The surgical success rate was established as an outcome beneficial to patients, and complications associated with treatment were established as outcomes harmful to patients. The CUA16 and EAU guidelines,8 which matched the PICO for this CQ, were qualitatively integrated into a systematic review. According to the CUA guidelines, the success rate of urethroplasty is approximately 84%, and that of transurethral treatments is 47%.16 Both treatments are associated with complications; however, transurethral treatments, which often require multiple sessions, have higher complication rates.16 Therefore, the CUA guidelines recommend urethroplasty for recurrence after the initial transurethral treatment.16
The CUA guidelines are based on the literature up to 2018 and do not include the OPEN trial.22, 23 During the OPEN trial, urethroplasty or DVIU was performed for patients with bulbar urethral strictures that were not cured with at least one transurethral treatment. No differences in the primary endpoint (patient-reported outcomes at 24 months after treatment) of these two treatments were observed. However, regarding the secondary endpoints, such as the maintenance of urinary flow and the need for additional treatment, urethroplasty resulted in significantly better outcomes than DVIU. According to the per-protocol analysis, the difference in the need for additional treatment was even more significant.22 The EAU guidelines have highlighted several issues associated with the OPEN trial.8 First, the OPEN trial included intermittent self-dilation after DVIU, which was not considered as an additional treatment, and the need for intermittent self-dilation did not indicate stricture recurrence. Importantly, the average stricture length was short (2 cm), and only cases of bulbar urethral strictures, which are relatively more responsive to transurethral treatments, were included. Regarding complications, urethroplasty was associated with postoperative pain and pain at the oral mucosal harvest site, which were less commonly observed with DVIU (urethroplasty, 70.7%; DVIU, 26.0%); however, the frequency of complications that required intervention was comparable (urethroplasty, 10.9%; DVIU, 11.3%).
The success rates of transurethral treatments decrease with each repetition.18, 24-27 A randomized controlled trial by Heyns et al. found the following success rates at 48 months: (1) Patients who did not experience a recurrence within 3 months of the first transurethral treatment had a 60% success rate. (2) Patients who experienced a recurrence at 3 months and received a second transurethral treatment had a 0% success rate. (3) Patients who experienced a second recurrence at 6 months and received a third transurethral treatment also had a 0% success rate.18 This suggests that transurethral treatments are futile for cases of early recurrence (within 3 months) after the first treatment and those involving multiple recurrences. Although no statistical significance was found during this prospective cohort study, a particularly low efficacy of transurethral treatments for penile and distal bulbar urethral strictures was observed.20
Other potential risks attributable to repeated transurethral treatments include complications associated with strictures, long treatment durations, and high medical costs. Numerous studies have indicated that transurethral treatments can complicate urethral strictures, necessitate more complex surgical techniques during subsequent urethroplasty, and reduce success rates.27-35 Repeated inappropriate transurethral treatments likely prolong the duration until the urethral strictures are cured.36 Although analyses of cost-effectiveness can be found in the international literature,37-39 no studies have considered Japanese medical insurance system. In Japan, the number of facilities capable of performing urethroplasty is limited, and the burden associated with traveling for treatment is a significant issue for patients who wish to undergo urethroplasty. Additionally, transurethral treatments are less invasive than urethroplasty. Therefore, it is likely that some patients will opt for the transurethral treatments despite the lower long-term success rates.40 Physicians and their patients must make treatment decisions together based on this evidence.
CQ3. Is cold-knife DVIU preferred to hot-knife or laser DVIU for urethral strictures?
Recommendation Statement: The success rates and complications of cold-knife DVIU are equivalent to those of hot-knife or laser DVIU. The device should be chosen based on the surgeon's experience and available equipment at the treatment facility.
Recommendation level: no recommendation.
Evidence certainty: C (weak).
Voting results: 10 of 10 members (100%) indicated no recommendation.
The devices used for DVIU include the traditional cold knife, hot knife, and various energy devices such as lasers. Whether cold-knife DVIU offers higher success rates, fewer complications, and lower costs than those associated with hot-knife or energy device-based DVIU is a significant clinical issue. As no studies have directly compared costs, the success rates and complications were considered as the outcomes. Two meta-analyses41, 42 and the EAU guidelines8 that matched the PICO for this CQ were qualitatively integrated into a systematic review.
Castellanos et al. performed a meta-analysis of four RCTs.41 The success rates 3 months after treatment were similar between both groups (RR, 0.55; 95% CI, 0.18–1.66; p = 0.29); however, at 6 months (RR, 0.39; 95% CI, 0.19–0.81; p = 0.01) and 12 months (RR, 0.44; 95% CI, 0.26–0.75; p = 0.003), the laser DVIU group had higher success rates.41 There was no difference in the complication rates between the groups, and no severe complications were reported.41 Zheng et al. added three comparative trials to the four RCTs selected by Castellanos et al. and conducted another meta-analysis; they reported no difference in success rates at 3, 6, and 12 months after treatment42 Regarding complications, there was no difference in incidence (odds ratio [OR], 0.78; 95% CI, 0.35–1.74; p = 0.54); however, the laser DVIU group had a significantly lower risk of bleeding (OR, 0.08; 95% CI, 0.01–0.43; p = 0.003). Chen et al. compared a cold-knife DVIU group (n = 22) with a laser DVIU group (n = 24) and reported that although the laser DVIU group had better success rates after 1 year, there was no difference in success rates after 2 years, and both groups had very low success rates at 5 years (cold-knife DVIU, 12%; laser DVIU, 9%).43
Comparisons between cold-knife and hot-knife DVIU (plasma kinetics) using the EAU guidelines were performed based on an RCT by Cecen et al. and a prospective cohort study by Ozcan et al.8, 44, 45 Cecen et al. reported that the success rates at 9 months after treatment were 70% for cold-knife DVIU and 86% for plasma kinetics (p = 0.025); however, there was no difference at 18 months (63% vs. 67%, respectively; p = 0.643).44 Ozcan et al. reported success rates of 63% for cold-knife treatment and 77% for plasma kinetics at 12 months after treatment (p = 0.04).45 The EAU guidelines concluded that because of these conflicting results, the lack of long-term follow-up, and series heterogeneity, there were no differences in the success rates and complication rates between the devices used for DVIU, and that it is not possible to determine the superiority of one over another.8 No studies have directly compared the medical costs of or resources necessary for these techniques. In Japan, DVIU is covered by medical insurance; however, no additional reimbursement is provided when energy devices are used. Although the differences in devices do not affect the cost to the patients, it is important to note that medical providers must bear the additional cost when energy devices are used. The SIU guidelines state that the utility of lasers is not better than that of the cold knife; therefore, they do not recommend the routine use of lasers owing to their additional costs.2 As the benefits and harms of these procedures are nearly identical, patients' preferences and choices are not applicable. Therefore, the device used for DVIU can be chosen based on the physician's preference and experience as well as the facility's available equipment.
CQ4. Among the transurethral treatment techniques for urethral stricture, which is recommended: DVIU or urethral dilation?
Recommendation Statement: The success and complication rates of DVIU and urethral dilation are almost equivalent. Either procedure can be chosen based on the facility's available equipment and the physician's experience and preference.
Recommendation level: no recommendation.
Evidence certainty: C (weak).
Voting results: 10 of 10 members (100%) indicated no recommendation.
Transurethral treatments for urethral strictures primarily include DVIU and urethral dilation; however, it is important to determine which one is more recommended. Success rates were considered outcomes beneficial to patients and complications were considered outcomes harmful to patients. A systematic review was conducted by qualitatively integrating the following three studies with the same outcomes as this CQ: an RCT by Azab et al.,46 a Cochrane review by Wong et al.,15 and the EAU guidelines8 based on the Cochrane review. All of these studies addressed the comparison between DVIU and urethral dilation. The Cochrane review by Wong et al.15 evaluated only one RCT by Heyns et al.,18 which compared DVIU and urethral dilation without conducting a meta-analysis. In the RCT by Heyns et al., 210 patients with nonobliterative urethral strictures were randomized into the DVIU (n = 104) and urethral dilation (n = 106) groups.18 The success rates at 48 months after treatment were 39% for the DVIU group and 12% for the urethral dilation group, with no statistically significant difference between the groups (p = 0.13) and no difference in the time to stricture recurrence.18 The study was criticized because of its wide CIs, uncertainty, and lack of stratification of potential confounding factors such as stricture location, cause, and length.15 Azab et al. randomized 88 cases of urethral stricture into the DVIU (n = 44) and urethral dilation (n = 44) groups and observed them prospectively for 12 months. Both groups experienced improvements in subjective symptoms, the postvoid residual volume, and the maximum flow rate.46 The study was criticized because treatment success was solely based on the Qmax and its brief observation period. The perioperative complication rate of the urethral dilation group (14%) was slightly higher than that of the DVIU group (11%); however, the difference was not statistically significant.15, 18 Urethral bleeding was more common in the DVIU group (3.8%) than in the urethral dilation group (2.8%), but false passages were equivalent (urethral dilation group, 0.94%; DVIU group, 0.96%). Extravasation and pain occurred more frequently in the DVIU group (3.8%) than in the urethral dilation group (0%).
As no high-quality studies have compared DVIU and urethral dilation, there is no evidence suggesting that one is superior to the other in terms of success and complication rates. Both treatments are covered by medical insurance in Japan. Therefore, treatment should be chosen based on the available equipment at the facility and the physician's preference.
CQ5. Is intermittent self-dilation recommended after transurethral treatments for urethral strictures?
Recommendation statement: Intermittent self-dilation can be exclusively performed for patients ineligible for urethroplasty to reduce the chance of stricture recurrence.
Recommendation level: weak.
Evidence certainty: C (weak).
Voting results: 10 of 10 members (100%) indicated a weak recommendation.
The success rates of transurethral treatments for urethral strictures are low, and stricture recurrence is common. Intermittent self-dilation has been widely adopted as an adjunct method to prevent stricture recurrence because it does not require hospitalization, and fewer physician visits are necessary.47, 48 However, the significance of intermittent self-dilation requires reevaluation.
A comprehensive literature search was conducted. Recurrent strictures were considered the outcome of this CQ. A systematic review was performed by qualitatively integrating the Cochrane review and EAU guidelines.8, 48 According to the Cochrane review, recurrent strictures were observed in 85 of 197 patients (43.1%) who underwent intermittent self-dilation during 8–24 months of follow-up after DVIU, compared to 128 of 207 patients (61.8%) in the control group who did not undergo intermittent self-dilation. Thus, it was concluded that intermittent self-dilation significantly reduces stricture recurrence (RR, 0.70; 95% CI, 0.48–1.00; p = 0.05).48 However, because all trials included in the systematic review were deemed to have a high risk of bias, the quality of the research was considered very low.48 The EAU guidelines state that intermittent self-dilation after transurethral treatment significantly reduces the rate of stricture recurrence and they weakly recommend stabilizing strictures with intermittent self-dilation after transurethral treatments only for patients who are not candidates for urethroplasty.8 No trial has rigorously studied complications associated with intermittent self-dilation. A meta-analysis of two small-scale studies found no statistically significant difference in the occurrence of adverse events between the intermittent self-dilation and control groups (RR, 0.60; 95% CI, 0.11–3.26; p = 0.56).47-50 Urinary tract infections occurred in 4.7%–18.1% of patients in the intermittent self-dilation group and in 15.3%–22.7% of those in the control group.49, 50 Urethral bleeding was observed in 7.1% of patients in the intermittent self-dilation group.51
Intermittent self-dilation can complicate strictures and delay the time to urethroplasty.8 Similar to other transurethral treatments such as DVIU and urethral dilation, intermittent self-dilation can result in more complex strictures, thus making subsequent urethroplasty more difficult.32, 33 Furthermore, the complexity of the self-dilation technique, pain during insertion, and decreased quality of life (QOL) have been pointed out, potentially harming the patient. A multicenter prospective study revealed that out of 85 patients who underwent intermittent self-dilation, 30 (35.3%) experienced moderate, and 22 (25.9%) experienced severe difficulty in catheter insertion.52 The pain during insertion and impact on QOL were moderate in 27 (31.8%) and 27 (31.8%) patients, respectively, and severe in 15 (17.6%) and 47 (55.3%) patients, respectively; additionally, a more pronounced deterioration in QOL was observed among younger patients and those with proximal urethral strictures.8, 52 Khan et al. reported that 8 of 30 (26.7%) patients discontinued intermittent self-dilation because of technical difficulties.49 Chhabra et al. reported that 71 of 144 (49.3%) patients discontinued intermittent self-dilation because of technical difficulties.53 However, some studies have reported that all included patients were able to perform intermittent self-dilation without any problems.49, 50, 54
Intermittent self-dilation after transurethral treatments, at least during the short term, can reduce the rate of stricture recurrence without a significant increase in complications, suggesting that the benefits of intermittent self-dilation for patients outweigh the harms. However, complications associated with strictures caused by intermittent self-dilation and the negative impact of intermittent self-dilation on urethroplasty can be detrimental to patients who may require urethroplasty in the future. Harms, such as pain associated with catheter insertion, difficulty mastering the technique, and decreased QOL, are also anticipated. Intermittent self-dilation is covered by Japanese medical insurance and considered medically acceptable. Therefore, similar to the AUA, EAU, and SIU guidelines, the present guidelines weakly recommend intermittent self-dilation to reduce the rate of stricture recurrence after transurethral treatments; additionally, they recommend that intermittent self-dilation should be limited to patients who cannot undergo urethroplasty because of issues such as surgical intolerance, and those who do not wish to undergo urethroplasty.2, 8, 11
Previous reports of intermittent self-dilation have not standardized the target cases, intervention methods, follow-up procedures, or definition of stricture recurrence. There have been no evaluations of long-term (more than 2 years) outcomes of intermittent self-dilation and urethroplasty. Additionally, there have been no comparisons of intermittent self-dilation and urethroplasty in terms of their value to patients and the public, impact on QOL, cost-effectiveness, and patient acceptance. Currently, urethroplasty is recognized as the standard treatment, and the limited efficacy of transurethral treatments has become clear. A discrepancy exists between cases previously subjected to intermittent self-dilation and those anticipated to be eligible for intermittent self-dilation in the future. Well-designed RCTs regarding the appropriate indications for intermittent self-dilation are necessary.
CQ 6. In what type of facility and by whom should urethroplasty be performed for urethral strictures?
Recommendation statement: Physicians with limited experience in urethroplasty should refer patients to facilities where urethroplasty is routinely performed as part of daily practice or seek support from experienced reconstructive urologists.
Recommendation level: weak.
Evidence certainty: C (weak).
Voting results: 10 of 10 members (100%) indicated a weak recommendation.
International guidelines regarding the treatment of urethral strictures recommend initially performing urethroplasty for most urethral strictures, excluding favorable urethral strictures,3, 8, 9, 11 and the present guidelines provide the same recommendation (see CQ1 and CQ2). However, many urologists do not have the skills required to perform urethroplasty, which is technically more complex and demanding than transurethral treatments.12-14, 55 Furthermore, the success rate of urethroplasty for patients who have not undergone prior treatment is higher than that for patients with stricture recurrence after previous treatment.8 Consequently, identifying the optimal facilities and surgeons to perform urethroplasty in order to maximize its therapeutic benefits is an important clinical consideration.
A comprehensive literature search of how the success rates of urethroplasty performed by single surgeons changed with accumulated case experience yielded three retrospective observational studies.56-58 These studies were subjected to a meta-analysis for this CQ. Hoare et al. reported that success rates improved over the course of 15 years (analyzed in 5-year intervals; from 86.7% to 90.0% to 93.4%), indicating a temporal improvement.57 Horiguchi et al. reported that the success rate increased from 86.1% for the first 36 cases to 97.5% for the subsequent 79 cases.58 Ekerhult et al. reported that the success rate increased from 67.3% for the first half of 109 cases to 85.2% for the second half.56 All three studies demonstrated that the success rates improved with surgical experience (Figure 2). Less adverse outcomes were associated with surgeries performed by experienced surgeons, and the complication rate tended to decrease with experience.56, 59, 60 The literature indicates that proficiency in urethroplasty, as evidenced by expert-level success rates, requires substantial experience.60, 61
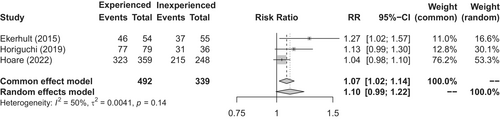
The current recommendations indicate that urethroplasty should be performed by highly experienced reconstructive urologists at facilities where the procedure is regularly performed. The AUA guidelines recommend that physicians who do not regularly perform urethroplasty should refer patients to those with experience rather than repeating transurethral treatments.11 The EAU guidelines recommend performing urethroplasty for complex cases, such as PFUIs, pan-anterior urethral strictures, and strictures related to hypospadias surgery at high-volume centers.8 Similarly, the SIU guidelines assert that urethroplasty for PFUIs should be performed by experienced reconstructive urologists who are proficient at applying ancillary techniques such as pubectomy.4
Patients who desire a cure for urethral strictures would benefit from urethroplasties performed by experienced reconstructive urologists. Given the case volume and intricacy of urethroplasty, it might not be essential for every general urologist to master this procedure. Instead, patients should be directed to specialized centers, as practiced in Western countries. However, if there are no experienced reconstructive urologists near the patient's residence, then only patients who can manage the geographical, physical, and financial challenges of traveling to receive treatment may benefit from urethroplasty. In the United States, reconstructive urologists certified by the Society of Genitourinary Reconstructive Surgeons regularly perform urethroplasties. However, these specialized physicians tend to be concentrated in metropolitan areas, underscoring the limited nationwide availability of this treatment.62 In Japan, urethroplasty is covered by medical insurance, and treatment outcomes of urethral strictures are expected to significantly improve if the medical service system is adequately equipped. However, the limited number of reconstructive urologists and lack of information regarding facilities capable of offering urethroplasty are issues that need to be addressed.
CQ7. Is nontransecting excision and primary anastomosis (ntEPA) preferable to excision and primary anastomosis (EPA) for short bulbar urethral strictures?
Recommendation Statement: Although EPA is associated with a higher incidence of sexual dysfunction, high success rates can be achieved with either procedure.
Recommendation level: no recommendation.
Evidence certainty: C (weak).
Voting results: 7 of 10 members (70%) indicated no recommendation.
EPA has been recognized as the gold standard treatment for short bulbar urethral strictures, and numerous studies have reported its high success rate. A meta-analysis of 16 studies including 1262 cases and the SIU guidelines found that the EPA success rate was 93.8%.9 However, the erectile dysfunction rate after EPA is higher than that after the onlay technique, which does not require urethral transection.63, 64 Concerns about other complications associated with sexual function after EPA have been raised as well, including temporary numbness of the glans and penile discomfort.65 A study of 308 patients treated at a single Japanese institution found that the EPA success rate was 97.1%; however, 19.1% of those patients experienced decreased erectile function (a decrease of more than 5 points in the Sexual Health Inventory for Men [SHIM score] 6 months postoperatively).66 The corpus spongiosum receives its blood supply from the antegrade flow from the bulbar artery, which is a branch of the internal pudendal artery, and the retrograde flow from the dorsal penile artery through the penile corpora and glans. Transection of the urethra during EPA, which interrupts the antegrade blood flow, is considered a cause of sexual dysfunction. Additionally, idiopathic or iatrogenic strictures, which comprise the majority of bulbar urethral strictures, do not require full circumferential excision of the corpus spongiosum owing to the smaller area of scar tissue compared to that associated with traumatic etiologies.67 Therefore, surgical techniques such as vessel-sparing EPA and nontransecting anastomotic urethroplasty, which involve excising only the stricture without transecting the urethra and maintaining the blood flow through the corpus spongiosum (collectively referred to as ntEPA in this CQ) have been developed. Virasoro reported a success rate of 95.6% with the vessel-sparing EPA technique (reported by Jordan) in 68 bulbar urethral strictures.68 Additionally, using the nontransecting anastomotic urethroplasty technique reported by Andrich, Ivaz reported a success rate of 99% in 101 patients indicating outcomes comparable to those of EPA.69 Therefore, along with EPA, ntEPA techniques are becoming the standard treatment for short bulbar urethral strictures. However, it is not yet clear which technique is preferable, thus presenting an important clinical issue. One comparison of ntEPA and EPA considered success rates, rates of complications associated with sexual function, rates of complications not associated with nonsexual function, operative duration, and blood loss as clinically important outcomes. However, most studies were single-arm case–control analyses that used only one technique, and only two retrospective observational studies have compared ntEPA and EPA for short bulbar urethral strictures.67, 70 There have been no randomized controlled trials involving these techniques. Additionally, no studies have clearly compared complications related or unrelated to sexual function. Furthermore, no studies have compared the operative duration and blood loss associated with ntEPA and EPA. Therefore, this CQ was based on the outcomes of two retrospective observational studies that compared ntEPA and EPA.
The most important benefit for patients is the success rate, and the harms are complications related to sexual function. Waterloos et al. reported no significant difference in the success rates of EPA (88.4%) and ntEPA (93.2%) (p = 0.33); however, the observation period was significantly longer for cases treated with EPA.67 Chapman et al. found no significant difference in the success rates of EPA (93.8%) and ntEPA (97.9%) (p = 0.18); however, the observation period was significantly longer for cases treated with EPA.70 These results are consistent; therefore, further studies are required to determine whether the superiority of ntEPA over EPA. As the success rates of ntEPA and EPA are considered equivalent, ntEPA is a suitable alternative to EPA. In contrast, regarding harms related to complications that are not associated with sexual function, the frequency of complications classified as Clavien–Dindo grade II or higher observed with cases treated with EPA (3.6%–8.1%) and that of those observed with cases treated with ntEPA (4.3%–6.8%) were similar.67, 70 Complications related to sexual function were only investigated by Chapman et al., who reported that EPA was associated with a higher rate (14.3%) of decreased postoperative SHIM scores than ntEPA (4.3%) (p = 0.008) for urethral transection; in other words, the use of EPA was the only factor associated with decreased SHIM scores.70
In summary, both surgical techniques offered more significant benefits than harms. In Japan, both are covered by medical insurance. Although EPA is more likely to result in complications related to sexual function, patient satisfaction remains high. However, if the blood flow of the corpus spongiosum can be preserved, then ntEPA may offer additional benefits to patients. The viability of non-transurethral EPA (ntEPA) is contingent on the degree of scarring in the corpus spongiosum. Furthermore, since ntEPA is a relatively novel technique, not all surgeons experienced in traditional EPA are necessarily capable of performing ntEPA. Consequently, when determining whether to pursue EPA or ntEPA, clinicians should carefully consider the expected success rates, potential complications, and the surgeon's level of expertise for each individual patient case.
CQ8. Is the oral mucosa preferable to the penile foreskin as a substitute tissue for urethroplasty?
Recommendation statement: The oral mucosa and penile foreskin are equally recommended as substitute tissues for urethroplasty. However, the use of the oral mucosa is recommended for cases without excess foreskin or with strictures caused by LS.
Recommendation level: strong.
Evidence certainty: C (weak).
Voting results: 9 of 10 members (90%) indicated a strong recommendation.
When anastomotic urethroplasty techniques such as EPA and ntEPA are not feasible, the reconstruction of long bulbar urethral strictures or penile urethral strictures requires the use of substitute tissue. Historically, the skin, penile foreskin, bladder mucosa, intestinal mucosa, and oral mucosa have been used as a substitute tissue for urethroplasty. However, owing to the ease of tissue harvesting, better graft take, absence of hair growth, and superior ability to prevent stone formation and infection, the use of the oral mucosa or penile foreskin has become common.8 The existing guidelines recommend the use of oral mucosal grafts for urethroplasty.3, 8, 11 However, circumcision is not a common practice in Japan, and many patients have excess penile foreskin available. If the penile foreskin can serve as an equally effective substitute tissue compared to oral mucosa, then it could be utilized to avoid the challenges and patient burden associated with harvesting the oral mucosa. This raises important questions about the relative merits of using oral mucosa versus penile foreskin as substitute tissues, as well as whether certain specific parts of the oral mucosa may be superior. Additionally, it prompts consideration of whether a penile foreskin graft or flap approach is preferable. Therefore, evaluations including the success rate of urethroplasty as a beneficial outcome and complications associated with the reconstructed urethra and donor site as harmful outcomes were performed. The evaluations were divided into the following: oral mucosal graft versus penile foreskin graft; and oral mucosal graft versus penile foreskin flap. In addition, comparisons within the oral mucosa included comparing buccal and lingual mucosal grafts. The penile foreskin graft and penile foreskin flap were also compared.
Comparing the oral mucosal and penile foreskin grafts
A comprehensive literature search identified one RCT,71 six observational studies,72-77 and one systematic review78 for the meta-analysis. An RCT by Tyagi et al. showed no significant difference in the success rate of the oral mucosal graft group (n = 48 patients) and that of the penile foreskin graft group (n = 47 patients) at 18 months postoperatively (91.7% vs. 89.4%, respectively; p = 0.74; RR, 1.03; 95% CI, 0.26–7.05).71 Additionally, there was no significant difference in the complication rates (Clavien–Dindo grade I) between the groups (16.7% vs. 12.7%, respectively; p = 0.80).71
However, a meta-analysis of observational studies showed that the oral mucosal graft group had a significantly higher success rate than the penile foreskin graft group (86.9% vs. 76.8%, respectively; p = 0.0004; RR, 1.17; 95% CI, 1.07–1.28) (Figure 3).72-77 A systematic review by Lumen et al. also showed that the oral mucosal graft group had a significantly higher success rate than the penile foreskin graft group (85.9% vs. 81.8%, respectively; p = 0.011; RR, 1.10; 95% CI, 1.02–1.19).78 However, the penile foreskin graft group included significant biases, such as having a significantly longer urethra reconstructed and longer postoperative observation periods associated with the longer existence of this technique.78 Therefore, there is no high-level evidence regarding whether the oral mucosal graft or penile foreskin graft is superior.
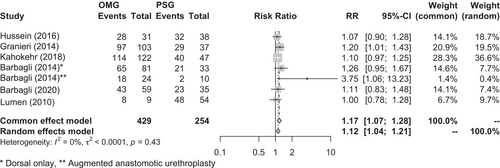
Comparing the oral mucosal graft and penile foreskin flap
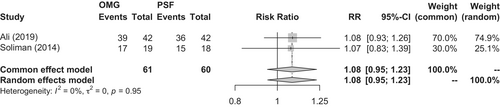
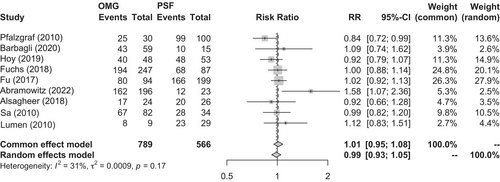
Two RCTs,79, 80 nine observational studies,72, 77, 81-87 and one systematic review88 were identified. A meta-analysis of the RCTs showed no significant difference in the success rates of the oral mucosal graft group (n = 61) and penile foreskin flap group (n = 60) (91.8% vs. 85.0%, respectively; p = 0.24; RR, 1.08; 95% CI, 0.95–1.23) (Figure 4). Nondonor site complications, such as penile curvature and rotation, were observed less frequently with oral mucosal grafts than with penile foreskin flaps (18.0% vs. 38.3%, respectively; p = 0.017; RR, 0.47; 95% CI, 0.25–0.87); however, there was no difference in the rate of donor site complications (Clavien–Dindo grade I; 15.0% vs. 10.0%, respectively; p = 0.44; RR, 1.40; 95% CI, 0.59–3.32).79, 80 A meta-analysis of observational studies showed no difference in the success rates of the oral mucosal graft group (n = 789) and penile foreskin flap group (n = 566) (80.6% vs. 83.7%, respectively; p = 0.68; RR, 1.01; 95% CI, 0.95–1.08) (Figure 5).72, 77, 81-87 Similarly, a systematic review by Ma et al. indicated no difference in the success rates of the oral mucosal graft group and penile foreskin flap group (84.9% vs. 81.9%, respectively; p = 0.39; RR, 1.04; 95% CI, 0.95–1.13).88
Comparing the buccal and lingual mucosal grafts
A systematic review based on a meta-analysis of 12 studies by Wang et al. showed no difference in the urethroplasty success rates between the buccal and lingual mucosal graft groups (80.9% vs. 84.2%, respectively; p = 0.31; RR, 1.04; 95% CI, 0.97–1.12).89 Regarding donor site complications, the lingual mucosal graft group more frequently experienced early postoperative speech impairment and difficulty in tongue protrusion, whereas the buccal mucosal graft group more frequently experienced swelling and hematoma at the donor site, early postoperative difficulty in opening the mouth, and numbness inside the mouth for approximately 6 months postoperatively.89
Comparing the penile foreskin graft and flap
An RCT involving 37 cases of anterior urethral strictures by Hussein et al. showed that although the penile foreskin flap group had a higher incidence of skin necrosis, the urethroplasty success rates of the penile foreskin flap group and penile foreskin graft group were not significantly different (78.9% vs. 72.2%, respectively; p = 0.64; RR, 1.09; 95% CI, 0.76–1.58).90
In summary, the oral mucosa and penile foreskin are considered to have equivalent benefits and risks when used as substitute tissues for urethroplasty. Additionally, the buccal and lingual mucosa, as well as penile foreskin grafts and flaps, can be considered equivalent. However, for patients with a history of hypospadias surgery or circumcision and those without excess penile foreskin, the use of the oral mucosa is recommended. Moreover, the use of the penile foreskin is contraindicated for strictures caused by LS that may extend to the foreskin; therefore, the use of the oral mucosa is recommended.8, 10, 11 No studies were found that compared cost-effectiveness of these methods. The use of oral mucosa and penile foreskin in urethroplasty is covered by insurance in Japan. Therefore, it is crucial to choose procedures and materials after thoroughly considering the patient preferences and anticipated outcomes.
AUTHOR CONTRIBUTIONS
Akio Horiguchi: Conceptualization, writing—original draft preparation. Masayuki Shinchi: Project administration. Yusuke Hirano: Project administration. Hiroshi Asanuma: Writing—original draft preparation. Yoshiyuki Ishiura: Writing—original draft preparation. Koji Inoue: Writing—original draft preparation. Akihiro Kanematsu: Writing—original draft preparation. Tadashi Tabei: Writing—original draft preparation. Yoshimi Tamura: Writing—original draft preparation. Yosuke Nakajima: Writing—original draft preparation. Kimihiko Moriya: Writing—original draft preparation. Yusuke Yagihashi: Writing—original draft preparation. Takashi Fukagai: Project administration. Yasuhisa Fujii: Project administration.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest. Takashi Fukagai and Yasuhisa Fujii are the Editorial Board member of International Journal of Urology and the co-authors of this article. To minimize bias, they were excluded from all editorial decision-making related to the acceptance of this article for publication.
APPROVAL OF THE RESEARCH PROTOCOL BY AN INSTITUTIONAL REVIEWER BOARD
Not applicable.
INFORMED CONSENT
Not applicable.
REGISTRY AND THE REGISTRATION NO. OF THE STUDY/TRIAL
Not applicable.
ANIMAL STUDIES
Not applicable.




