Summary of the Clinical Practice Guidelines for Upper Tract Urothelial Carcinoma 2023 by the Japanese Urological Association
Abstract
This article is an English translation of the Clinical Practice Guidelines for Upper Tract Urothelial Carcinoma (2nd edition) published in June 2023. The Japanese Urological Association's (JUA) Guidelines Committee on Upper Tract Urothelial Carcinoma (UTUC) created a 2023 update guideline to support clinicians' current evidence-based management of UTUC and to incorporate its recommendations into clinical practice. The new guideline adhered as closely as possible to the Minds Manual for Guideline Development 2020 ver. 3.0. Findings related to epidemiological, pathological, diagnosis, treatment, and follow-up were reviewed. In addition, seven clinical questions (CQs) were set to determine the grade of recommendation and level of evidence. Preconceptions and biases were removed from the preparation process, the overall evidence was evaluated appropriately, and recommendations were made after fully considering the balance between benefits and harms. Although the evidence is still insufficient to be taken up as a CQ, the latest important information is described in seven columns, and clinical issues that should be resolved in the future related to the CQ are described as recommendations for tomorrow. We hope that these guidelines will help medical professionals, patients, and their families involved in the treatment of UTUC in their decision-making, and hope that a critical review of these guidelines will lead to further refinements in the next edition.
Abbreviations & Acronyms
-
- ADC
-
- antibody-drug conjugate
-
- AGREE
-
- appraisal of guidelines research & evaluation
-
- BCG
-
- Bacillus Calmette-Guérin
-
- BSC
-
- best supportive care
-
- CI
-
- confidence interval
-
- CIS
-
- carcinoma in situ
-
- COI
-
- conflict of interest
-
- CQ
-
- clinical question
-
- CT
-
- computed tomography
-
- CTU
-
- CT urography
-
- EAU
-
- European Association of Urology
-
- GC
-
- gemcitabine+cisplatin
-
- GCarbo
-
- gemcitabine+carboplatin
-
- GFR
-
- glomerular filtration rate
-
- HNPCC
-
- hereditary non-polyposis colon cancer
-
- HR
-
- hazard ratio
-
- JMLA
-
- Japan Medical Library Association
-
- JSCO
-
- Japan Society of Clinical Oncology
-
- JUA
-
- Japanese Urological Association
-
- Minds
-
- Medical Information Network Distribution Service
-
- MMC
-
- mitomycin C
-
- M-VAC
-
- methotrexate, vinblastine, doxorubicin, and cisplatin
-
- NCCN
-
- National Comprehensive Cancer Network
-
- ORR
-
- objective response rate
-
- OS
-
- overall survival
-
- POUT
-
- Peri-Operative chemotherapy versus sUrveillance in upper Tract urothelial cancer
-
- PS
-
- performance status
-
- RCT
-
- randomized controlled trial
-
- RNU
-
- radical nephroureterectomy
-
- THP
-
- pirarubicin
-
- TNM
-
- tumor-node-metastasis
-
- UC
-
- urothelial carcinoma
-
- UTUC
-
- upper tract urothelial carcinoma
-
- WHO
-
- World Health Organization
INTRODUCTION
This article is an English translation of the Clinical Practice Guidelines for Upper Tract Urothelial Carcinoma (2nd edition) published in June 2023. The first edition was published in April 2014, so, it will be the first revised edition in 9 years. During this period, new therapeutic agents for urothelial carcinoma were covered by insurance one after another in Japan, and nephroureterectomy was covered by the insurance as a robot-assisted surgery. In addition, the 2nd edition of the Rules for Treatment of Renal Pelvis, Ureter and Bladder Cancer1 was published in August 2021. Considering this situation, we started the revision work of these guidelines in August 2021. Until now, the treatment of advanced urothelial carcinoma has relied on platinum agents, and the development of new drugs has been delayed for a long time. In the last 5 years, new drug therapies have been covered by the insurance in Japan. Thus, although it has been revised 9 years after the first edition, this revision is timely.
Although UTUC and bladder cancer are histopathologically the same urothelial cancer, their differences pose a problem. Since there are various differences between UTUC and bladder cancer, such as differences in oncological outcomes and gene profiling, we believe that examining the differences between them is an important issue for future research. Since most randomized controlled trials have included both bladder cancer and UTUC as urothelial cancers, the extent to which the results of subgroup analysis should be reflected in recommendations was addressed by the revision committee. The frequency of UTUC is about 1/10th of that of bladder cancer, and belongs to the category of rare cancers. In the future, it is unlikely that a randomized controlled trial limited to UTUC will be planned, so it goes without saying that verification using real-world data is essential.
We hope that this guideline will help medical professionals, patients, and their families involved in the treatment of UTUC in their decision-making, and hope that a critical review of the guidelines will lead to further refinements in the next edition.
TARGET AND OBJECTIVES
The main purpose of this guideline is to clarify the recommendations for clinical questions related to the treatment of UTUC, with the aim of improving treatment outcomes, safety, and quality of life for patients with UTUC in Japan. The goal is to disseminate it widely in daily clinical practice as a standard clinical practice guideline. This guideline was edited for patients suspected of having UTUC and patients diagnosed with UTUC. We hope that these guidelines will help them understand UTUC, and select and execute to provide better medical care based on mutual understanding between medical professionals and those in a position to receive medical care.
METHODS
Basic concept of revision
This revision complies with the Minds Manual for Guideline Development 2020 ver. 3.0. published in March 2021.2 Preconceptions and biases were removed from the preparation process, the overall evidence was evaluated appropriately, and recommendations were made after fully considering the balance between benefits and harms.
Findings related to epidemiological, pathological, diagnosis, treatment, and follow-up were reviewed. In addition, seven clinical questions (CQs) were set to determine the grade of recommendation and level of evidence. When setting CQs, if clear guidelines are given in the 1st edition of the Clinical Practice Guidelines for UTUC, or if evidence has been accumulated since then, items for which sufficient consensus has been established described in the general statement as Background Questions. Prior to making Background Questions, we carefully selected items that were still controversial (Foreground Questions) and took them up as clinical questions (CQ). Although the evidence is still insufficient to be taken up as a CQ, the latest important information is described in seven columns, and clinical issues that should be resolved in the future related to the CQ are described as recommendations for tomorrow.
Process for preparation
- The JUA Guidelines Committee elected the chairman of the revision committee.
- Members of the revision committee (in charge of revision and cooperation) were elected, and an evaluation committee was established.
- The revision committee decided on the overall chapter structure, CQ, and keywords.
- An exhaustive search of important papers by the Japan Medical Library Association was conducted. Based on the determined keywords, papers published from January 2006 to February 2022 were extracted from the search data. Targets are PubMed, The Cochrane Library, and Japan Medical Central Journal.
- Revision committee members and cooperation committee members conducted primary screening based on the abstracts and secondary screening based on the whole paper, and made final decisions.
- Revision committee members evaluated the evidence for each of the accepted papers, summarized the individual evaluations for each outcome, and evaluated the overall evidence.
- Revision committee members created the evidence evaluation sheet, overall evidence evaluation sheet, and a summary document for making recommendations.
- The revision committee held discussions at the recommendation decision meeting and decided on recommendations by voting.
- The revision committee finalized the recommendations and completed the draft of the guideline.
- The evaluation committee of the Japanese Urological Association and Minds evaluated the draft and responded.
- The revision committee made revisions based on the responses, and made further revisions based on public comments.
- The revision committee finalized the guidelines.
- JUA gave final approval.
All these meetings were conducted via e-mail or online due to the COVID-19 pandemic. Since online meetings limit the number of people who can speak, we made it possible to watch recordings and provided opportunities for question-and-answer sessions through the mailing list. The Guideline Office also responded to individual questions.
Evidence certainty of the overall evidence and recommended decision voting choices
-
Evidence certainty of the overall evidence were below.
- A (strong): Strong confidence in the adequacy of the effect estimate to support the recommendation.
- B (medium): Moderate confidence in the adequacy of the effect estimates to support the recommendation.
- C (weak): limited confidence in the adequacy of the effect estimate to support the recommendation.
- D (very weak): little confidence in the adequacy of the effect estimate to support the recommendation.
-
Recommended decision voting choices (orientation and strength) were below.
- Recommended (strongly recommended)
- Propose to do (weak recommendation)
- Propose not to do it (weak recommendation)
- Not recommended (strongly recommended)
- The strength of the recommendation cannot be determined (not graded)
- Abstain from voting due to COI
Patient engagement
Since UTUC has a variety of pathologies and a wide range of treatment methods, it was difficult to reflect the opinions of individual patients and their families in the development of clinical practice guidelines as the views of the patient as a whole. As a result, two patients with UTUC who underwent diagnosis, perioperative adjuvant drug therapy, radical surgery, and postoperative follow-up, participated in the revision committee.
Although the important clinical issues from the patient's side were proposed, the relevant literature was limited, and it was determined that it would be difficult to make recommendations through a systematic review. Therefore, we decided to create a column related to this, “Optimal follow-up after nephroureterectomy: minimal frequency of cystoscopy.”
Insurance approval
This guideline was started for treatments that were initially covered by insurance in Japan. However, although CQ4 is actually implemented in comprehensive medical care, it is not covered by health insurance for UTUC. After confirming the descriptions of the clinical practice guidelines for pancreatic cancer and the views of the insurance committee of the Japanese Urological Association, it was decided that “Even if there is no insurance approval in Japan, it should be stated that the evidence level is high, and in that case, it should also be stated that the insurance has not been approved.”
Subgroup analysis
Clinical trials for UTUC are almost always included in urothelial carcinoma. Therefore, there was an opinion that the clinical practice guideline for UTUC should interpret the results of UTUC subgroup analysis among urothelial carcinomas to make recommendations. There was also an opinion that the overall results of urothelial carcinoma should be directly reflected in the recommendations. In addition, various opinions were raised as to what to do when UTUC subgroup analysis is not performed, and what to do when the Hazard Ratio is more than 1 as a result of UTUC subgroup analysis. Ultimately, the Minds Manual2 states, “A strong recommendation is that most patients will want this recommended course of action. Only a minority of patients will not. Clinicians should, in principle, accept this recommended course of action”, and Minds' view is, “When in doubt about a decision, make a decision in a weak and reserved direction,” in addition, after actually inquiring Minds, they answered that interpretation is fine, “If it is effective for all subjects, we will recommend it first. If we are not confident about the results of the subgroup analysis, we decide whether the recommendation is strong or weak, which is often a weak recommendation.” Thus, before the voting, we communicated this point to the revision committee members and started the voting process. We also asked the revision committee members to accurately describe the results of the subgroup analysis.
Overall structure and CQs
Chapter 1. Epidemiology and pathology
- Lists only the general statement.
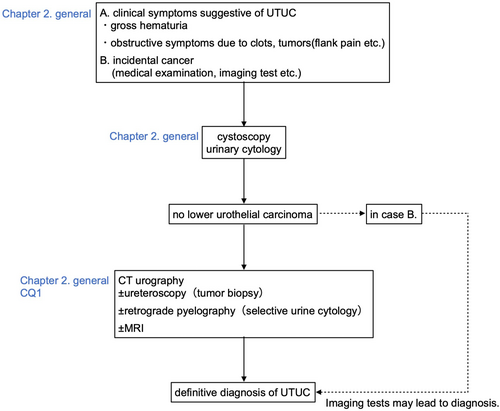
Chapter 2. Diagnosis
- General statement.
- Column1: Bladder cancer and UTUC. Same? or Something similar?
- Column2: Significance of urinary tumor markers and DNA FISH tests used in the diagnosis of bladder cancer in UTUC.
- CQ1: Is ureteroscopic tumor biopsy recommended for the diagnosis of UTUC?
Chapter 3. Surgical treatment
- General statement.
- Column3: Optimal follow-up after nephroureterectomy: minimal frequency of cystoscopy.
- CQ2: Are laparoscopic or robot-assisted surgeries recommended for total nephroureterectomy?
Chapter 4. Drug therapy
- General statement.
- Column4: Current status and issues of upper tract drug infusion therapy.
- CQ3a: Is perioperative systemic drug therapy recommended for nephroureterectomy? Is postoperative adjuvant chemotherapy recommended in total nephroureterectomy?
- CQ3b: Is perioperative systemic drug therapy recommended for nephroureterectomy? Is nivolumab recommended as adjuvant drug therapy in total nephroureterectomy?
- CQ4: Is intravesical therapy recommended for prevention of intravesical recurrence after nephroureterectomy?
- CQ5: Is pembrolizumab recommended for metastatic or unresectable UTUC that has relapsed or progressed after first-line anticancer chemotherapy?
- CQ6: Is maintenance avelumab recommended for metastatic or unresectable UTUC treated with first-line anticancer chemotherapy?
- CQ7: Is enfortumab vedotin recommended for unresectable or metastatic UTUC previously treated with platinum-containing anticancer chemotherapy and immune checkpoint inhibitors?
- Column5: Radiation therapy for UTUC.
- Column6: Interpretation of subgroup analysis in RCTs, especially when the results of the subgroup analysis differ from those of the overall population.
- Column7: Identity of UTUC.
Results of External Evaluation of the 2023 Clinical Practice Guideline for UTUC
- Evaluation results by JUA evaluation committee and reply by the revision committee.
- Evaluation results by Minds according to AGREE II Instrument and reply by the revision committee.
- Public comments by the Japanese Urological Association, the Japanese Society of Oncology and Minds web sites, and reply by the revision committee.
RESULTS AND DISCUSSION
Chapter 1. Epidemiology and pathology
Epidemiology
UTUC is a malignant tumor that arises from the urothelium of the renal pelvis and ureter, but it is rarer than bladder cancer, which arises from the same urothelium, accounting for 5%–10% of all urothelial tumors.3-5 According to the national cancer registration incidence and rate report in Japan, the age-adjusted incidence rate (per 100 000 population) in 2019 was 1.4 for renal pelvic cancer and 1.2 for ureteral cancer.6 By gender, renal pelvic cancer is 2.2 in men and 0.7 in women, and ureter cancer is 1.8 in men and 0.7 in women, about three times higher in men.6
Urothelial carcinoma is clinically characterized by spatial and temporal multiplication throughout the urinary tract. Patients with UTUC often have antecedent or synchronous bladder cancer, and the incidence of bladder cancer (intravesical recurrence) after surgery for upper urothelial carcinoma is high.7 The entire urinary tract should be screened in the presence of UTUC or bladder cancer.
Etiology
Many environmental factors, such as smoking, exposure to chemical carcinogens, drugs, and chronic inflammation, have been reported as risk factors for the development of UTUC.8 Cigarette smoking is the most important risk factor for UTUC, with smokers having a three-fold higher risk than non-smokers, those with a history of smoking in the past having a two-fold increase in risk, and those with a history of smoking for more than 45 years having a 7.2-fold increase in risk.9
Upper urothelial carcinoma is an autosomal dominant disorder associated with Lynch syndrome.10 Lynch syndrome is also called hereditary non-polyposis colon cancer (HNPCC), the expression of mismatch repair proteins is lost due to germline mutations in MLH1, MSH2, MSH6, and PSM2, which are genes involved in mismatch repair during DNA replication (mismatch repair genes).11 Lynch syndrome is known to be a risk factor for both upper and lower urothelial carcinomas, and about 9% of upper and lower urothelial carcinomas and about 1% of bladder cancers have germline mutations in mismatch repair genes.12 It is desirable to conduct screening based on the age of onset, medical history, family history, and consider genetic testing and genetic counseling.13, 14
Histology
Most of the histologic types of UTUC develop urothelial carcinoma, but non-urothelial cancers include squamous cell tumors, glandular tumors, and neuroendocrine tumors.1 Squamous cell carcinoma and adenocarcinoma are diagnosed only when there is no urothelial carcinoma component.1 Although the relationship between subtypes and prognosis is controversial, it has been pointed out that some subtypes may be factors that affect the recurrence rate and disease-specific survival rate,15 and it is desirable to specify its existence in the pathological diagnosis.1 An analysis using cancer registries in Japan revealed that urothelial carcinoma accounted for 92.9%, squamous cell carcinoma 3.4%, adenocarcinoma 1.3%, neuroendocrine carcinoma 0.4%, and others 2.0%.16
UTUC is classified into non-invasive papillary urothelial carcinoma, carcinoma in situ, and invasive urothelial carcinoma, like bladder cancer.1 The grade of urothelial carcinoma is classified into two grades, low grade and high grade, depending on the tumor structure and degree of cellular atypism. Carcinoma in situ of the urothelium is excluded from the classification of dysplasia in the WHO classification. Cellular atypism in most cases of invasive urothelial carcinoma is high grade, but there are rare cases of invasive urothelial carcinoma with low grade. The grade of invasive urothelial carcinoma is evaluated at the invasive site. In Europe, the 3-grade grade assessment method based on the 1973 edition of the WHO classification is still mainly used as a method for assessing the grade of urothelial carcinoma, and it is supposed to be written together in the general rule.1
TNM classification1, 17
T classification of UTUC
pTx primary tumor cannot be evaluated.
pT0 no primary tumor.
pTa papillary non-invasive carcinoma.
pTis carcinoma in situ.
pT1 Tumor invading mucosal epithelial connective tissue.
pT2 Tumor invading muscle layer.
pT3 Renal pelvis: Tumor invades peripelvic adipose tissue or renal parenchyma beyond muscular layer*.
Ureter: Tumors that invade the periureteral fatty tissue beyond the muscular layer.
pT4 Tumor invading perirenal adipose tissue beyond adjacent organs or kidney.
*There are cases of renal pelvic carcinoma in which tumor cells progress into collecting ducts or renal tubules without interstitial invasion, in such cases, collecting ducts or intratubular lesions should be determined as carcinoma in situ components, diagnose pT3 only when renal parenchymal infiltration is observed.
N classification of UTUC
NX Inability to assess regional lymph node metastasis.
N0 No regional lymph node metastasis.
N1 Solitary lymph node metastasis ≤2 cm in greatest dimension.
N2 Single lymph node metastasis >2 cm in greatest dimension or multiple lymph node metastases.
M classification of UTUC
M0 No distant metastases.
M1 Distant metastasis.
Molecular classification of UTUC
Molecular biological classification is also performed for UTUC,18, 19 but it has been reported that the frequency of luminal type is higher and the frequency of basal type is lower in high-grade UTUC compared with bladder cancer.18 It has also been reported that it can be classified into five types based on gene expression profile and gene mutation profile.20
Chapter 2. Diagnosis
Selective urine cytology test, urine marker test
Selective urine cytology using renal pelvis and ureter urine collected by ureteral catheterization has been shown to have a higher cancer detection rate than spontaneous urine cytology.21 In addition, in patients diagnosed with high-grade UTUC by biopsy, urinary cytology showed high grade in 50%, whereas selective urinary cytology has reportedly revealed high-grade findings in 90% of cases.22 It has been reported that the brushing method is the best method for collecting renal pelvis and ureter urine, and that it detects 91% of cancers.23 In addition, when collecting renal pelvis and ureter urine, it is recommended that it can be performed before injecting a contrast medium because the effect on the sample reduces diagnostic accuracy.24 As of 2022, among the urine marker tests covered by insurance for bladder cancer, NMP-22 (nuclear matrix protein-22) can also be administered to UTUC.25
Flexible ureteroscopy
Flexible ureteroscopy not only evaluates the morphology and size of UTUC, but also enables the definitive diagnosis of cancer by ureteroscopic biopsy. Ureteroscopic biopsy can be used to evaluate the degree of malignancy and depth of tumor invasion, but the size of the sample that can be collected is limited, and sampling of the submucosal layer and muscular layer is difficult. Thus, accurate diagnosis of malignancy and depth of invasion is difficult.26 According to a meta-analysis, it has been reported that when ureteroscopic biopsy revealed submucosal tissue invasion (cT1 or higher), positive predictive value for muscle invasion (≥pT2) in total resection specimens was 94%, and when diagnosed as high-grade by ureteroscopic biopsy, the positive predictive value for muscle invasion (pT2 or higher) in total resection specimen was 60%.27 The cTa findings and low grade on ureteroscopic biopsy may be useful in selecting eligible patients for kidney-sparing surgery.
Imaging test
Recently, CT urography (CTU) is recommended when UTUC is strongly suspected.3 CTU is a CT examination that evaluates the urinary tract with thin slices before and after contrast, including images in the excretory phase when the renal pelvis and ureter are filled with contrast medium. CTU makes it easier to understand the relationship between lesions and the urinary tract by performing 3D image processing on cross-sectional image data.
Concerning microscopic hematuria, the detection rate of UTUC by CTU is as low as 0.02% in a meta-analysis, and there is an opinion that CTU should be used only in patients aged 50 years or older with risk factors.28
Staging is based on the General Rule for Clinical and Pathological Studies on Renal Pelvic, Ureteral and Bladder Cancer (2nd edition) published in 2021 by the Japanese Urological Association, the Japanese Society of Pathology, the Japan Radiological Society, and the Japanese Society of Clinical Oncology.1 The basic diagnostic policy for the T classification is as below.
T2 or lower: Wall thickening of the renal pelvis/ureter, or cancer is observed as an enhancement defect image inside. The lateral surfaces of the renal pelvis and ureteral walls are smooth.
T3: Irregularity of the renal pelvis and ureteral walls in the cancerous area, fluffing of the surrounding adipose tissue, and increased absorption values are observed. In renal pelvic carcinoma, infiltration into the renal parenchyma is observed.
T4: Continuity between cancer and adjacent organs is observed. In renal pelvic carcinoma, infiltration into the perinephric adipose tissue is observed.
The staging of UTUC is also based on evaluation by CT, but there are few reports on its diagnostic ability. Multi-slice detector CT has an accuracy of 96.6% in evaluating muscle-invasive cancer (pT2 ≤), 66.6% in diagnosing invasion to surrounding organs and lymph node metastasis (pT4 or N+), and overall staging accuracy rate is reported to be 87.8%.29
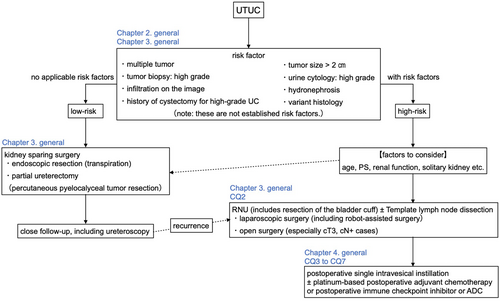
Risk classification
UTUC is difficult to clinically evaluate for tumor stage. It is considered useful to stratify and evaluate the risk of progression to determine the indications for kidney-sparing surgery, preoperative chemotherapy, and lymphadenectomy. There is currently no established risk classification model for the diagnosis of UTUC.
Molecular diagnosis
Bladder cancer is often heterozygous with various gene mutations, whereas UTUC is classified into five subtypes according to the mutation status of TP53, MDM2, RAS, and FGFR3, and found to be largely recapitulated in each independent cohort.20 It has been demonstrated that these genetic abnormalities can be detected with high sensitivity and specificity not only from tumor specimens but also from urinary DNA.20, 30 However, it has been found that the sensitivity drops to about 50% in cases with severe hydronephrosis, these cases refer to the need to collect upper tract urine, such as by ureteral catheterization.20
Table 1 shows CQ1 and its answer.31-34
| CQ1 | Is ureteroscopic tumor biopsy recommended for the diagnosis of UTUC? |
|---|---|
| Statement | Propose to perform ureteroscopic tumor biopsy only when imaging and urine cytology are inadequate for diagnosis. |
| Recommendation grade | Propose to do (weak recommendation) |
| Evidence certainty | C(weak) |
| Explanation | Ureteroscopic tumor biopsy is useful for tumor diagnosis, however, it is necessary to recognize the possibility of increased intravesical recurrence and carefully consider indications. Therefore, ureteroscopic tumor biopsy should be considered only in cases in which imaging tests and urine cytology are insufficient for diagnosis. Considering the limited indications for ureteroscopic tumor biopsy and the increased incidence of intravesical recurrence, the strength of the recommendation was weak. All papers covered by this CQ were retrospective observational studies, therefore the certainty of the evidence was C(weak). |
Chapter 3. Surgical treatment
Indications and approaches for total nephroureterectomy and perioperative pharmacotherapy
Radical nephroureterectomy (RNU) is the standard surgical treatment for UTUC. This includes resection of the bladder cuff. However, due to the progression of chronic kidney disease and deterioration of renal function after RNU, it has been pointed out that postoperative adjuvant chemotherapy may not be suitable for cisplatin,35, 36 therefore consideration of kidney-sparing surgery is recommended in possible cases.
The current standard surgical procedure for RNU for high-risk cases is open surgery.3, 37 Laparoscopic surgery is also widely performed as minimally invasive surgery,38 and there are reports that cancer control similar to that of open surgery can be obtained.39, 40 However, the only randomized prospective study showed that the survival rate of laparoscopic surgery was inferior to that of open surgery in patients with locally advanced pT3 cancer.41 For this reason, open surgery is currently recommended for locally advanced cancer.3
In recent years, robot-assisted surgery has become popular in Europe and the United States,38, 42 and it is covered by insurance in Japan from 2022 and will become more popular in the future. One meta-analysis showed that cancer control, including recurrence-free survival, overall survival, intravesical recurrence-free survival, positive margins, and number of lymph nodes removed, was almost equivalent to that of open surgery.43 Robot-assisted surgery is superior in complication rate, blood transfusion rate, and has been shown to be less invasive.43 However, it remains to be verified whether long-term cancer control equivalent to open surgery can be achieved even in locally advanced cancers of pT3 or higher.
In patients with UTUC, RNU may reduce renal function and render cisplatin unsuitable,35 as it is reasonable to perform neoadjuvant chemotherapy. In fact, it has been reported that there are cases in which preoperative chemotherapy leads to pathological down stage and complete disappearance.44, 45 However, as there are still no results from randomized trials, at present, no recommendation has been obtained in EAU guidelines 2020 update.3
Indications and outcome of lymphadenectomy in total nephroureterectomy
It appears that the presence or absence of lymph node metastasis can be truly determined by performing template dissection, which is based on dissecting lymph nodes corresponding to the site of occurrence.46-48 There is consensus that lymphadenectomy at the time of RNU for UTUC is meaningful for staging purposes.3, 49 However, at present, it is difficult to say that its therapeutic significance has been established. Currently, a phase II clinical trial in which high-risk patients are assigned to a template dissection group and a group to remove only enlarged lymph nodes diagnosed by preoperative imaging (planned enrollment: 504 patients, primary endpoint: 3-year non-recurrence rate) is ongoing (NCT03474926) and is due to end in 2023.
Since the number of lymph nodes varies greatly from patient to patient, the absolute number does not guarantee the completeness of the dissection. When evaluating extended dissection with an increased number of lymph nodes compared with standard dissection, the Will Rogers phenomenon (stage migration and apparent improvement in prognosis due to the introduction of new treatments and surgery) must be considered.48
Indications and outcome of kidney-sparing surgery
According to the NCCN49 and EAU guidelines3 classification, kidney-sparing surgery is an option even for patients with bilateral kidneys in low-risk cases, and should be considered in patients with chronic renal failure and patients with a single kidney even in high-risk cases.3, 49 Ureteroscopic biopsy is the main pathologic diagnosis for this standard, but in patients who underwent RNU after biopsy, it should be noted that there are 33%–37% cases of upgrading.50, 51
Endoscopic resection (transpiration) treated tumor by performing laser ablation using holmium yttrium-aluminum-garnet (Ho-YAG), neodymium YAG (Nd-YAG), thulium YAG (Thu-YAG), etc. through a transurethral approach using an ureteroscope. According to a long-term follow-up study, the non-recurrence rate after endoscopic resection (transpiration) was 53.4% at 5 years and 20.5% at 10 years, indicating that recurrence may occur even after 5 years. Long-term follow-up is considered necessary.52 Although there is no unified protocol for follow-up after endoscopic resection (transpiration), considering the high recurrence rate, cystoscopy and CT urography should be performed in low-risk cases. It is recommended that ureteroscopy should be performed after 1 month, 6 months, and then annually for 5 years, and ureteroscopy should be performed once after 3 months. In high-risk cases, it is recommended that cystoscopy, urine cytology, CT urography, and chest CT should be performed at 3 months, 6 months, and annually thereafter, and ureteroscopy and renal urine cytology should be performed 3 and 6 months later.3
Table 2 shows CQ2 and its answer.3, 41, 53, 54
| CQ2 | Are laparoscopic or robot-assisted surgeries recommended for total nephroureterectomy? |
|---|---|
| Statement | Propose to perform laparoscopic or robot-assisted surgery in total nephroureterectomy. However, locally advanced cases should be considered on a case-by-case basis. |
| Recommendation grade | Propose to do (weak recommendation) |
| Evidence certainty | C(weak) |
| Explanation | There is only one randomized controlled trial comparing open surgery with robot-assisted or laparoscopic surgery in RNU, and the other reports are either retrospective studies or systematic reviews. At this time, the certainty of the evidence regarding the efficacy of robot-assisted surgery and laparoscopic surgery was judged to be C (weak). Therefore, we propose to perform laparoscopic or robot-assisted surgery in total nephroureterectomy. However, locally advanced cases should be considered on a case-by-case basis. Based on a comprehensive judgment, the certainty of the evidence was C(weak), and the recommendation was weak. |
Chapter 4. Drug therapy
Intravesical therapy to prevent intravesical recurrence after nephroureterectomy
Intravesical recurrence after radical surgery for UTUC has a high frequency of 20%–50%, and is often observed within 2 years after surgery.3, 55 It is caused by intraluminal dissemination of cancer cells from UTUC or pan-urothelial field defect.56 Intravesical instillation of anticancer drugs is a method for suppressing intravesical recurrence after RNU. A meta-analysis investigating the effect of postoperative single intravesical instillation of mitomycin C (MMC) or pirarubicin (THP) showed that postoperative intravesical recurrence was significantly suppressed, although the certainty of the evidence was weak.57 It should be noted that this is not covered by insurance in Japan.
Upper tract infusion therapy for renal preservation
Total nephroureterectomy is the standard treatment for UTUC, but nephrectomy inevitably leads to deterioration of renal function. For this reason, papillary Ta/T1 tumors of the upper tract such as single kidney, renal hypofunction, and bilateral papillary Ta/T1 tumors of the upper tract and CIS cases are considered to be treated with BCG or MMC upper tract infusion therapy with the aim of preserving renal function. Recently, a prospective study demonstrated complete tumor disappearance in 59% of patients with low-grade upper urothelial carcinoma after a total of six retrograde injections of MMC-containing reverse thermal gel once weekly.58 It should be noted that these are not covered by insurance in Japan.
Perioperative adjuvant drug therapy before and after nephroureterectomy
Neoadjuvant chemotherapy
For cT3-4 and cN+ UTUC, which are considered to be at high risk of recurrence, there is a history that neoadjuvant chemotherapy has been performed with a regimen similar to that for muscle-invasive bladder cancer, because both are urothelial cancers. However, there are no randomized controlled trials investigating the efficacy of neoadjuvant chemotherapy for UTUC, and prospective trials are extremely limited. Therefore, The EAU guideline3 does not recommend the administration of neoadjuvant chemotherapy.
After total nephroureterectomy, renal function declines, making platinum ineligible, and postoperative adjuvant chemotherapy may be difficult to apply. Furthermore, the effect of postoperative adjuvant therapy with nivolumab has been observed only in patients who underwent neoadjuvant chemotherapy for UTUC.59 Therefore, the use of neoadjuvant chemotherapy for high-risk UTUC is expected to increase in the future, but it is essential to accumulate knowledge with a high level of evidence regarding the efficacy of neoadjuvant chemotherapy for UTUC.
Adjuvant therapy
Adjuvant chemotherapy has been considered for pathologically unfavorable pathological findings such as pT3 or higher and pN+ in RNU specimens. It has the disadvantage that its application is limited in some cases due to the deterioration of renal function due to nephrectomy, but it has the advantage of being able to select patients with a high risk of recurrence based on pathological examination and avoid overtreatment in patients pT1pN0 or lower.
The POUT trial is a phase III randomized controlled trial that investigated disease-free survival as the primary endpoint.60 Adjuvant chemotherapy significantly improved disease-free survival compared with surveillance.60 In addition, in a meta-analysis including retrospective studies of real-world data, overall survival, cancer-specific survival, and disease-free survival were significantly better in the adjuvant chemotherapy group.61 However, based on the results of subgroup analysis of POUT study, the efficacy of GCarbo therapy in patients with a GFR of 30 to 49 mL/min may not be sufficient.60
The CheckMate 274 trial is a phase III randomized controlled trial comparing the effects of PD-1 immune checkpoint inhibitor nivolumab with placebo as adjuvant drug treatment after radical surgery for muscle-invasive urothelial carcinoma.62 Disease-free survival was significantly better in the nivolumab group.62 In Japan, postoperative adjuvant drug therapy with nivolumab were covered by health insurance in March 2022. It is noted that the HR (95% CI) for disease-free survival by primary tumor site suggested that the recurrence prevention effect of adjuvant nivolumab therapy for urothelial carcinoma may differ depending on the primary site.62 And in UTUC, adjuvant therapy with nivolumab may be effective in preventing recurrence in patients who have undergone neoadjuvant chemotherapy.60 However, as this was a subgroup analysis of an extremely small number of cases, the interpretation of the results should be carefully considered. On the other hand, for patients who have not undergone neoadjuvant chemotherapy, the package insert of nivolumab states that treatment with postoperative platinum agents should be given priority in patients who can be treated with postoperative platinum agents.63
Systemic drug therapy for advanced/metastatic UTUC
First-line setting
Cisplatin-containing chemotherapy is the standard of care for first-line treatment of unresectable metastatic UTUC. However, there are no reports with strong scientific evidence demonstrating the efficacy of chemotherapy only for advanced/metastatic UTUC. In a phase III study of dose-dense M-VAC therapy, bladder cancer and a small number of UTUC were analyzed together, and the difference in results between them was not clarified.64 Several clinical trials are underway. However, in the KEYNOTE-361 trial in patients with unresectable, locally advanced, or metastatic urothelial carcinoma, including untreated UTUC, pembrolizumab alone or pembrolizumab plus standard chemotherapy was not statistically superior to standard chemotherapy.65 The results of future clinical trials are awaited.
Maintenance therapy after chemotherapy
The JAVELIN Bladder 100 trial is a phase III randomized controlled trial comparing the anti-PD-L1 antibody avelumab maintenance therapy + BSC group and BSC alone group.66 The subjects were unresectable/metastatic urothelial carcinoma including UTUC who had undergone four to six cycles of first-line chemotherapy with GC therapy or GCarbo therapy without disease progression.66 The avelumab maintenance therapy group showed a significantly longer overall survival compared to BSC alone group.66 In Japan, insurance coverage for avelumab maintenance therapy was approved in February 2021.
Second-line treatment after first-line chemotherapy
KEYNOTE-045 is a phase III randomized controlled trial investigating overall survival and progression-free survival as primary endpoints between pembrolizumab (up to 2 years) and chemotherapy (either paclitaxel, docetaxel, or vinflunine) in patients with recurrent or advanced urothelial carcinoma including UTUC after first-line platinum-based chemotherapy.67 An analysis of all patients showed no statistically significant difference in progression-free survival between the two groups, but overall survival was significantly better in the pembrolizumab group.67 In Japan, insurance coverage for this second-line pembrolizumab therapy was approved in December 2017. In a subgroup analysis of the KEYNOTE-045 study, the HR for OS of pembrolizumab for UTUC was 0.53 (95% CI: 0.28 to 1.01).67
Third-line treatment
In the EV-301 study, enfortumab vedotin, antibody-drug conjugate (ADC), or chemotherapy (paclitaxel, docetaxel, or vinflunine) was administered to patients with urothelial carcinoma, including UTUC, who had failed both platinum-containing chemotherapy and immune checkpoint inhibitors, and overall survival was investigated as the primary endpoint.68 Since overall survival was significantly improved in the enfortumab vedotin group, this drug was covered by health insurance in Japan in December 2021. A subgroup analysis of overall survival showed a HR of 0.85 (95% CI: 0.57 to 1.27) for UTUC compared with 0.67 (95% CI: 0.51 to 0.88) for bladder cancer.68
Table 3 shows CQ3 and its answer.60-62 Table 4 shows CQ4 and its answer.57, 69-72 Table 5 shows CQ5 and its answer.67, 73, 74 Table 6 shows CQ6 and its answer.66, 75, 76 Table 7 shows CQ7 and its answer.68, 77, 78
| CQ3a | Is perioperative systemic drug therapy recommended for nephroureterectomy? Is postoperative adjuvant chemotherapy recommended in total nephroureterectomy? |
|---|---|
| Statement | Recommend to perform postoperative systemic platinum-based chemotherapy after total nephroureterectomy for patients with high-risk nonmetastatic UTUC. |
| Recommendation grade | Recommended (strongly recommended) |
| Evidence certainty | B (medium) |
| Explanation | The POUT trial, a phase III randomized controlled trial, showed adjuvant chemotherapy significantly improved disease-free survival compared with surveillance. In addition, in a meta-analysis including retrospective studies of real-world data, overall survival, cancer-specific survival, and disease-free survival were significantly better in the adjuvant chemotherapy group. Based on the above, postoperative systemic platinum-based chemotherapy is effective for patients with high-risk non-metastatic UTUC, the certainty of the evidence is B(medium), and the recommendation was strong. |
| CQ3b | Is perioperative systemic drug therapy recommended for nephroureterectomy? Is nivolumab recommended as adjuvant drug therapy in total nephroureterectomy? |
|---|---|
| Statement | Propose the use of nivolumab as postoperative adjuvant drug therapy in total nephroureterectomy. |
| Recommendation grade | Propose to do (weak recommendation) |
| Evidence certainty | C(weak) |
| Explanation | The CheckMate 274, a phase III randomized controlled trial, showed disease-free survival was significantly better in the nivolumab group compared with placebo group. Nivolumab is proposed as postoperative adjuvant drug therapy in total nephroureterectomy. However, it has also been suggested that the efficacy of nivolumab may differ depending on the primary site. In addition, in patients with UTUC (particularly in patients without a history of neoadjuvant systemic platinum-based chemotherapy), the efficacy of nivolumab compared with existing treatments is unclear. And prospective randomized trial is CheckMate 274 only, therefore the certainty of the evidence is C(weak), and the recommendation was weak. |
| CQ4 | Is intravesical therapy recommended for prevention of intravesical recurrence after nephroureterectomy? |
|---|---|
| Statement | Propose a single intravesical instillation of anticancer drugs (not covered by health insurance in Japan) to prevent intravesical recurrence after nephroureterectomy. |
| Recommendation grade | Propose to do (weak recommendation) |
| Evidence certainty | C(weak) |
| Explanation | To date, the results of three randomized controlled trials (RCTs) have been reported, and multiple meta-analyses including case series studies have been reported. These reports indicate that postoperative single intravesical instillation of anticancer drugs is effective in suppressing intravesical recurrence, although the certainty is weak. However, it should be noted that this method of use is not covered by health insurance in Japan. Based on the above, single-dose intravesical instillation of anticancer drugs was effective in preventing intravesical recurrence after nephroureterectomy, and the certainty of the evidence was C(weak), and the recommendation was weak. |
| CQ5 | Is pembrolizumab recommended for metastatic or unresectable UTUC that has relapsed or progressed after first-line anticancer chemotherapy? |
|---|---|
| Statement | Propose the use of pembrolizumab for metastatic or unresectable UTUC that has relapsed or progressed after first-line anticancer chemotherapy. |
| Recommendation grade | Propose to do (weak recommendation) |
| Evidence certainty | C(weak) |
| Explanation | The KEYNOTE-045 trial showed that overall survival was significantly better in the pembrolizumab group compared with chemotherapy group. In a subgroup analysis of the KEYNOTE-045 study, the HR for OS of pembrolizumab for UTUC was 0.53 (95% CI: 0.28 to 1.01). However, results for progression-free survival, ORR and serious adverse events are uncertain in UTUC subgroup. Based on the above, the use of pembrolizumab is effective for recurrent or advanced UTUC after first-line anticancer chemotherapy, and the certainty of the evidence was C (weak), and the recommendation was weak. |
| CQ6 | Is maintenance avelumab recommended for metastatic or unresectable UTUC treated with first-line anticancer chemotherapy? |
|---|---|
| Statement | Propose maintenance therapy with avelumab for patients with unresectable UTUC who have undergone first-line anticancer chemotherapy and have not seen disease progression. |
| Recommendation grade | Propose to do (weak recommendation) |
| Evidence certainty | C(weak) |
| Explanation | The JAVELIN Bladder 100 trial showed overall survival was significantly longer in the avelumab maintenance therapy group compared with BSC alone group. However, the study did not initially use anatomic tumor location as a stratification factor. In a subgroup analysis of this study, the avelumab maintenance therapy group in patients with UTUC had a significantly longer prognosis (HR 0.89, 95% CI: 0.578–1.373). Based on the above, the avelumab maintenance therapy is effective for patients with unresectable UTUC who have received first-line anticancer chemotherapy and have not seen disease progression, and the certainty of the evidence was C(weak), and the recommendation was weak. |
| CQ7 | Is enfortumab vedotin recommended for unresectable or metastatic UTUC previously treated with platinum-containing anticancer chemotherapy and immune checkpoint inhibitors? |
|---|---|
| Statement | Propose enfortumab vedotin treatment for unresectable or metastatic UTUC who have been treated with platinum-based anticancer chemotherapy and immune checkpoint inhibitors. |
| Recommendation grade | Propose to do (weak recommendation) |
| Evidence certainty | C(weak) |
| Explanation | EV-301 study showed overall survival was significantly improved in the enfortumab vedotin group. However, in a subgroup analysis of this study, overall survival showed a HR of 0.85 (95% CI: 0.57 to 1.27) for UTUC. And there are no prospective studies or high-quality retrospective clinical studies limited to UTUC. Based on the above, although the usefulness of enfortumab vedotin for urothelial cancer as a whole has been demonstrated, the certainty of the evidence was C(weak), and the recommendation was weak. |
CONCLUSIONS
This article is an English translation of the Clinical Practice Guidelines for Upper Tract Urothelial Carcinoma (2nd edition) published in June 2023. The contents of this guideline may be revised according to clinical research results to be published in the future and developments and changes in future clinical practice. We hope that this guideline will be useful for healthcare professionals involved in the clinical practice of UTUC in Japan, and we hope that a critical review of the guideline will lead to further improvements in the next edition.
SPECIAL CONSIDERATIONS
The guidelines are the most standard guidelines at the time of creation, and do not force actual medical practice. Ultimately, taking into account the situation of the facility (personnel, experience, equipment, etc.) and the individuality of each patient, the treatment should be decided through discussions with the patient, family, doctor, and other medical personnel. The Japanese Urological Association is responsible for the content of the guidelines, but the responsibility for the results of medical care should belong to the person in charge of medical care. The Japanese Urological Association and the Guidelines Revision Committee are not responsible. The doses of drugs used in the text are for adults, and include some drugs that are not approved in Japan and doses in overseas clinical trials.
-
Public item on the website of the Japanese Urological Association (in Japanese).
- Scope for UTUC Clinical Practice Guideline
- Outcome Importance List
- Summary of evidence evaluation results
- Evidence evaluation criteria
- Summary of overall evidence evaluation
- Draft recommendations for each CQ (recommendation decision meeting explanatory materials)
- Outline of each CQ (recommendation decision meeting explanatory material)
ACKNOWLEDGMENTS
The Japanese Urological Association covered all funding for the development of this guideline. In accordance with the standards set by the Japanese Urological Association, we received these financial contributions for online meetings and the acquisition, preparation, and preservation of materials, but these support did not affect the development of guidelines.
We would like to thank JSCO and Minds for their cooperation in preparing these guidelines. We would also like to thank JMLA for providing literature search services and Igaku Tosho Shuppan Co., Ltd. for publishing support. We would also like to express our heartfelt gratitude to everyone who cooperated in the creation of these guidelines, especially, two patients A.M. and S.Y., and Ms. Minako Oikawa, JUA Guidelines Committee Office, and to cooperation members who participated in the drafting and the external evaluation committee who evaluated the content.
Cooperation members—Takashi Kawahara: Department of Urology, Faculty of Medicine, University of Tsukuba; Hiroaki Sato: Department of Urology, Chiba University Graduate School of Medicine; Toshiki Kijima: Department of Urology, Dokkyo Medical University; Taketo Kawai: Department of Urology, Graduate School of Medicine, The University of Tokyo; Shuichi Tatarano: Department of Urology, Graduate School of Medical and Dental Sciences, Kagoshima University; Susumu Kageyama: Department of Urology, Shiga University of Medical Science; Masayuki Nagasawa: Department of Urology, Otsu City Hospital; Tetsuya Yumioka and Masashi Honda: Division of Urology, Department of Surgery, Faculty of Medicine, Tottori University; Kazutoshi Fujita and Takafumi Minami: Department of Urology, Kindai University Faculty of Medicine; Junichi Inokuchi and Fumio Kinoshita: Department of Urology, Graduate School of Medical Sciences, Kyushu University; Takuto Hara and Yuto Matsushita: Department of Urology, Hamamatsu University School of Medicine; Atsunari Kawashima and Akinaru Yamamoto: Department of Urology, Osaka University Graduate School of Medicine; Nozomi Hayakawa and Koichiro Aida: Department of Urology, St. Marianna University School of Medicine.
Members of the Evaluation Committee of JUA: Chair: Nobuo Shinohara, Department of Urology, Graduate School of Medicine, Hokkaido University. Members: Akihiro Ito, Department of Urology, Tohoku University School of Medicine. Hiroshi Kitamura, Department of Urology, Faculty of Medicine, University of Toyama, Toyama, Toyama. Keiji Inoue, Department of Urology, Kochi Medical School, Kochi University, Nankoku, Kochi.
Members of the Evaluation Committee of Minds: Eiji Ishikawa, Internal Medicine and Nephrology Center, Saiseikai Matsusaka General Hospital. Atsuko Kitano, Department of Oncology, St. Luke's International Hospital. Hiroshi Koga, Department of Dermatology, Shinshu University School of Medicine. Nobumasa Takagaki, Nobumasa Clinic. Hiroshi Noto, Department of Endocrinology and Metabolism, St. Luke's International Hospital.
AUTHOR CONTRIBUTIONS
Kazuyuki Mori: Writing—original draft; Writing—review and editing; Project administration. Shingo Hatakeyama: Writing—original draft; Writing—review and editing; Project administration. Hideki Enokida: Writing—original draft; Project administration. Hideaki Miyake: Writing—original draft; Project administration. Eiji Kikuchi: Writing—original draft; Project administration. Hiroyuki Nishiyama: Writing—original draft. Tomohiko Ichikawa: Writing—original draft. Takao Kamai: Writing—original draft. Yasushi Kaji: Writing—original draft. Haruki Kume: Writing—original draft. Tsunenori Kondo: Writing—original draft. Hideyasu Matsuyama: Writing—original draft. Naoya Masumori: Writing—original draft. Akihiro Kawauchi: Writing—original draft. Atsushi Takenaka: Writing—original draft. Hirotsugu Uemura: Writing—original draft. Masatoshi Eto: Writing—original draft. Norio Nonomura: Writing—original draft. Yasuhisa Fujii: Investigation; Project administration. Shiro Hinotsu: Investigation; Project administration. Chikara Ohyama: Writing—original draft; Writing—review and editing; Project administration; Supervision.
CONFLICT OF INTEREST STATEMENT
This guideline was formulated for the purpose of contributing to society, and its content is strictly based on scientific grounds. COI declarations by all members involved in the development of these guidelines have been carefully scrutinized by COI Committee of JUA and have concluded that there is no material COI-related interference. COI for each member was managed by JUA and published on the JUA website. In order to eliminate competing interests with specific organizations, products, and technologies, we have taken steps such as banning certain potential COI members from voting on CQ.




