Mediterranean diet and obesity polygenic risk interaction on adiposity in European children: The IDEFICS/I.Family Study
Summary
Background and Objectives
To examine whether changes in the Mediterranean Diet (MD) or any of its MD food groups modulate the genetic susceptibility to obesity in European youth, both in cross-sectional and longitudinal analyses.
Methods
For cross-sectional analysis, 1982 participants at baseline, 1649 in follow-up 1 (FU1) and 1907 in follow-up 2 (FU2), aged 2–16 years of the IDEFICS/I.Family studies were considered. For the longitudinal design, 1254 participants were included. Adherence to MD was assessed using the Mediterranean Diet Score (MDS), and genetic susceptibility to high BMI was assessed with a polygenic risk score (BMI-PRS). Multiple linear regression models were fitted to estimate gene × MD effects on markers of obesity.
Results
In cross-sectional analyses, at baseline, higher MDS was associated with higher BMI in children with high genetic susceptibility (β = 0.12; 95% CI = [0.01, 0.24]). However, 6 years later, at FU2, higher MDS was associated with lower BMI (β = −0.19; 95% CI = [−0.38, −0.01]) in children with high genetic susceptibility, showing an attenuating MDS effect. Also in FU2, vegetables and legumes (V&L) showed inverse associations with BMI (β = −0.01; CI = [−0.02, −0.00]) and WC (β = −0.02; CI = [−0.03, −0.00]) regardless of the obesity genetic risk, although the effect sizes were small. In the longitudinal analyses, no MDS-obesity associations or gene × diet interaction effects were observed.
Conclusions
In cross-sectional analysis (baseline and FU2), the MD modulated the association between obesity susceptibility and adiposity indicators in European youth, having an exacerbating effect in children measured during infancy years and an attenuating effect in early adolescent years.
1 INTRODUCTION
The prevalence of childhood obesity has dramatically increased in recent years, affecting males and females equally.1 Over the years, higher Mediterranean diet (MD) adherence has consistently shown multiple health benefits across all age groups.2, 3 In European children, MD adherence has been associated with a healthier body composition and cardiometabolic profile.4
However, these beneficial effects might be partly influenced by some biological determinants, such as genetic variation, which could potentially modulate the predisposition to obesity.5 The combination of obesity-related single-nucleotide polymorphisms (SNPs) forming polygenic risk scores (PRS) could contribute to a higher ability to predict obesity susceptibility through their polygenic architecture.6 Different obesity-specific genetic scores have been previously proposed to quantify the risk of obesity through a weighted or non-weighted sum of body mass index (BMI)-related risk alleles, showing good predictive ability as early obesity risk indicators in children and adolescents.7 Therefore, the use of PRSs might help to identify children who are more likely to be genetically susceptible to developing early obesity. A recent study conducted on European adolescents assessed gene × MD effects on adiposity indicators, showing that higher MD attenuates the genetic susceptibility to obesity.8 Similarly, a gene × diet study in European children9 showed that the exposure to a healthy environment in early life partially attenuates the genetic risk to obesity later in life.10 However, studies assessing the effect of changes in diet and the inherited susceptibility to obesity on obesity in youth remain scarce.
The prevention of chronic diseases, in the transition from childhood into adulthood, is of increasing relevance to public health policymakers. The interplay of both modifiable environmental factors, such as diet, and non-modifiable genetic traits in the development of adiposity gains enough relevance to assess in the present study whether changes in MD adherence, or to any of the MD food groups, modulated the genetic susceptibility to obesity in terms of adiposity in European children and adolescents. We hypothesized that the effect of higher MD adherence, or higher consumption of any of the MD food groups, could attenuate the effects of genetic susceptibility to obesity on obesity markers cross-sectionally. Furthermore, we hypothesize that associations between changes in the MD over time and obesity markers could be modulated by genetic susceptibility to obesity.
2 MATERIALS AND METHODS
2.1 Study population
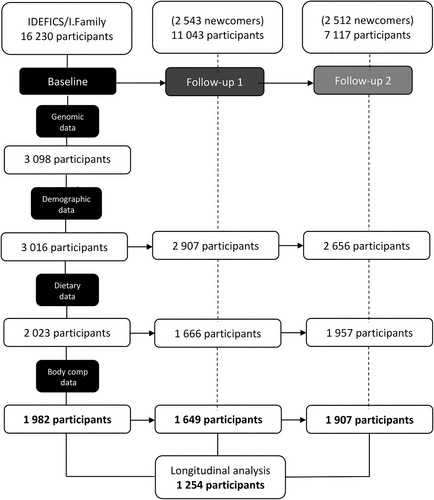
The IDEFICS/I. Family cohort is a multicentre, prospective study designed to identify anthropometric, biochemical, social and behavioural factors related to European children's health status.11 Participants were recruited through kindergartens or school settings in eight countries from Southern (Spain, Italy and Cyprus), Centre (Germany, Belgium and Hungary) and Northern (Estonia and Sweden) Europe. A total of 16 230 children aged between 2 and 9.9 years old participated in the baseline survey (2007/2008). Follow-up surveys were filled by parents and carried out at two time points: 2 years after baseline at follow-up 1 (FU1, N = 11 043, incorporating 2543 newcomers from the same participant centers) and 6 years later at follow-up 2 (FU2, N = 7117, incorporating 2512 newcomers) after baseline.12 In FU2, participants aged 12 years old or over completed the required information independently/themselves. The study was approved by all local committees and followed the ethical guidelines of the Declaration of Helsinki. Oral and written consent was obtained by the parents or legal guardians of all participants. In the present analysis, a total of 1982 participants at baseline, 1649 in FU1, 1907 in FU2, and 1254 with complete information at all time points were included, with complete genomic, demographic, dietary (quantitative) and body composition information (Figure 1).
2.2 Physical examination and adiposity measurements
All anthropometric measurements were performed by trained personnel. Height was measured barefoot, aligning the Frankfort plane horizontally, with a telescopic instrument (SECA 225 Stadiometer) to the nearest 0.1 cm. Body weight, measured in a fasting state, was taken in underwear and barefoot on a calibrated scale accurate to 0.1 kg (Tanita BC 420 SMA scale). BMI, as a measure of obesity, was calculated as weight divided by height squared (kg/m2).13 Waist circumference (WC), considered a parameter of abdominal adiposity, was measured in an upright position with a relaxed abdomen and feet together using a non-elastic tape (SECA 200) with a precision of 0.1 cm, in the mid-point between the iliac crest and the lowest rib, to the nearest 0.1 cm.14
2.3 Genotyping and quality control
Standardized procedures were followed to perform the DNA extraction from saliva or blood samples. Children were genotyped on the UK Biobank Axiom array (Santa Clara, USA) using 2 different batches (2015 and 2017, respectively). The initial genotyping sample was 3515 children. After sampling and genotyping quality control measures were put in place,10 a final sub-sample of 3098 participants resulted in 3 424 677 genotypes after imputation. To control for population stratification, the previously calculated first 5 principal components (PCs) of ancestry were used to adjust the individual data based on the genetic variability between individuals. Hence, we believe that the potential ethnic differences encountered among the individual' genomes should not have interfered with the prediction of genetic risk to a certain phenotype.15
2.4 Polygenic risk score calculation
The calculation of BMI-PRS was based on genome-wide summary statistics for BMI from populations of European ancestry proposed by Khera et al.,9 as published elsewhere.10 The Khera et al. PRS comprises a total of 2 100 302 SNPs, derived from summary statistics obtained in the first large-scale genome-wide association study (GWAS) assessing BMI (~300 000 samples).6 The PRS was constructed and validated using the R package LDPred,16 a computational algorithm using a Bayesian approach to estimate posterior mean effects for all variants using external weights, followed by shrinkage considering linkage disequilibrium. Using LDPred, each considered variant was rescaled based on prior GWAS.6
2.5 Dietary intake assessment and NCI correction
Short-term dietary patterns were assessed by 24-h dietary recalls, using the computer-based SACINA tool (‘Self-Administered Children and Infant Nutrition Assessment’)17 in baseline and FU1, and the online SACANA tool (‘Self-Administered Children, Adolescents, and Adult Nutrition Assessment’) in FU2 (extended version from the HELENA study18). The long-term dietary patterns were collected with food frequency questionnaires (FFQs), which included different food items clustered in 14 food groups according to the nutritional profile, allowing comparisons with the 24-h dietary recalls.19 A total of four 24-h dietary recall surveys collected at each measurement point were considered for the present analysis. Incomplete 24-h dietary recalls were excluded from the analysis. Total energy intake was assessed by the 24-HDR in kilocalories per day (kcal/day), and participants who reported <500 kcal/day were excluded. Daily intakes of all food groups required to assess the MD, recorded in grams per day (g/day), were calculated. Six food groups, based on the MD pattern, were created: vegetables and legumes, fruits and nuts, cereal grains and potatoes, fish products, meat products, and dairy products.20 Eggs were considered meat products. The cereal grains and potatoes group excluded fried potatoes and chips. Meat and fish fried products' intake was collected in the 24 h recalls, and their frequency of consumption was documented in the FFQ questionnaire. To utilize the whole sample with genetic information, random forest imputation was performed using the MICE R package for each food group to impute multiple missing 24-HDR values based on chained equations.21 Individual usual daily energy intake (EI, kcal/day) and individual usual intakes of the food groups (g/day) were estimated based on the U.S. National Cancer Institute Method (NCI).22 This method integrates food frequency information, accounts for different intakes on weekends vs. weekdays, and corrects for the variance caused by the daily changes in diet.23, 24 The NCI analyses were performed using the statistical software package SAS (version 9.3; SAS Institute, Cary, NC, USA). Usual intake estimation for children's data can reduce bias when dietary patterns are derived subsequently.25
2.6 Mediterranean diet score development
A Mediterranean Diet Score (MDS) was based on a previously developed MDS using the 24-HDR SACINA data.26 The MDS included the 6 Mediterranean food groups previously described. In addition, a ratio of unsaturated (mono- and polyunsaturated fats) to saturated fats was also integrated into the score; thus, the final MDS included a 7-item scale. The fat ratio was integrated due to the fact that polyunsaturated fatty acids, rather than monounsaturated fatty acids alone, are the main unsaturated fats in non-Mediterranean diets.27 Food intake for all food groups was standardized to 1000 kcal. Medians were calculated separately for sex and age categories. Children were categorized as preschool (<6 years old) and school age (⩾6 years old) for baseline and FU1, and prepubertal (<11 years old) and pubertal (⩾11 years old) for FU2. One point was given for participant intakes above the sex and age-adjusted median for vegetables and legumes, fruit and nuts, cereal grains and potatoes, and dairy products. For the unsaturated-to-saturated fat ratio, one point was given when participants were above the median. On the contrary, for meat products, one point was given when participants were below the median. Finally, since fish was scarcely consumed throughout all time points, one point was assigned to consumers. Furthermore, one point was assigned for dairy product consumption above the median, since they are recommended during periods of growth and development,28 aligning with previous MD scores used in cohorts of European children, which suggest the inclusion of dairy products as a positive item due to the high consumption of goat and sheep yogurt and cheese in the MD pattern.29 The sum of the 7 item scores as a continuous value represented the total MD adherence in the present analysis, with higher scores indicating high adherence and lower scores representing low adherence.
2.7 Physical activity and screen time assessment
The parental questionnaire was used to collect information regarding the participants' physical activity and sedentary screen time. Playing outdoors and weekly participation in sports club activities were used as a proxy for physical activity in hours per day (h/day). The total number of hours played was derived from hours playing outdoors on weekdays and hours playing outdoors on weekend days using the formula: (weekday*5 + weekend*2)/7. Screen time in hours per week was estimated by summing up the total screen time spent on audiovisual media, considering TV, video, DVD, computer, and game console, on weekdays and weekend days using the formula: weekday*5 + weekend*2. Missing values were imputed using random forest imputation as described above.
2.8 Sociodemographic assessment
The educational level of both parents was used as a proxy indicator for the socio-economic status (SES) of the family, according to the International Standard Classification of Education (ISCED).30 ISCED categories were as follows: low (levels 1–2), medium (levels 3–4) and high (levels 5–6).
2.9 Statistical analyses
Multiple linear regressions were used to evaluate the associations of MDS (and their individual food groups) with adiposity, both cross-sectionally and longitudinally. Cross-sectional models were adjusted by age, sex, parental education, region of residence, sedentary screen time, physical activity, and the first 5 principal components of ancestry, as well as adding the effect of the BMI-PRS alone (20). We further evaluated gene-diet interactions cross-sectionally at all time points and longitudinally by assessing MD- and adiposity-change using multiple linear regression, considering the BMI-PRS as exposure, the effect of changes in the MDS as an interaction term, and BMI and WC as outcome variables. All models were adjusted by the same set of covariates mentioned above. In the longitudinal design, changes in MD were defined as the difference between FU2 and baseline scores. The effect of changes in adiposity was measured as the difference between BMI or WC in FU2 and baseline. The longitudinal models were adjusted for baseline age, sex, and the first 5 principal components of ancestry, also adding the independent effect of the BMI-PRS. Survey methods considering cluster variance estimators were used to account for the impact of family clustering, using standard error calculations. Finally, to limit the proportion of false positives due to multiple testing, a false discovery rate (FDR) correction was applied at q = 0.05, obtaining an attenuating effect; the results with FDR-adjusted p-values showed a significant restrictive threshold in all models, thus not applicable for the present analysis.
We used R version 3.6.1 for the statistical analysis in the present manuscript. The significance level was set to 5%, i.e. p-values < 0.05 were considered statistically significant. Confidence intervals were calculated at the 95% confidence level.
3 RESULTS
3.1 Descriptive analyses
The study sample included a total of 5538 measurements from three time points (baseline = 1982; FU1 = 1649; FU2 = 1907) of children aged 2–16 years (Table 1). At all time points, approximately half of the participants were females, and the majority of them were from medium to high socioeconomic status. Across all time points, we observed a sustained intermediate MD adherence. Similar energy intakes were observed from baseline to FU2 after 6 years of follow-up.
| Baseline | FU1 | FU2 | |
|---|---|---|---|
| N | 1982 | 1649 | 1907 |
| Age (years) | |||
| Mean (SD) | 6.23 (1.8) | 8.60 (1.7) | 11.71 (1.8) |
| Median (IQR) | 6.60 (3.2) | 8.19 (3.1) | 11.80 (3.3) |
| Range | 2.1–9.7 | 4–11.2 | 7.8–15.5 |
| Sex | |||
| Male (N, %) | 995 (50.2) | 824 (49.9%) | 957 (50.2%) |
| Female (N, %) | 987 (49.8%) | 825 (50.1%) | 950 (49.8%) |
| Parental education categories | |||
| Low (N, %) | 147 (7.4%) | 126 (7.6%) | 111 (5.9%) |
| Medium (N, %) | 982 (49.5%) | 778 (47.2%) | 842 (44.1%) |
| High (N, %) | 853 (43.1%) | 745 (45.2%) | 954 (50.0%) |
| European region categoriesa | |||
| North (N, %) | 392 (19.8%) | 312 (18.9%) | 573 (30.0%) |
| Central (N, %) | 875 (44.1%) | 484 (29.3%) | 643 (33.7%) |
| South (N, %) | 715 (36.1%) | 853 (51.8%) | 691 (36.3%) |
| MDS (score units)b | |||
| Mean (SD) | 4 (1) | 4 (1) | 4 (1) |
| Median (IQR) | 4 (1) | 5 (1) | 4 (1) |
| Range | 0–7 | 1–7 | 1–7 |
| Vegetables and legumes (g/1000 kcal) | |||
| Mean (SD) | 51.4 (18.2) | 52.4 (17.1) | 57.5 (22.1) |
| Median (IQR) | 49.8 (24.1) | 48.9 (21.6) | 53.5 (26.0) |
| Range | 38.2–62.3 | 40.0–61.5 | 42.1–68.1 |
| Cereals and potatoes (g/1000 kcal) | |||
| Mean (SD) | 89.4 (23.8) | 80.0 (20.2) | 101.6 (25.0) |
| Median (IQR) | 89.7 (28.1) | 82.4 (18.8) | 104.5 (24.7) |
| Range | 75.8–104.0 | 72.7–91.6 | 91.2–116.0 |
| Fruits and nuts (g/1000 kcal) | |||
| Mean (SD) | 60.9 (30.3) | 67.9 (37.2) | 50.8 (28.3) |
| Median (IQR) | 56.1 (41.2) | 63.1 (55.0) | 44.3 (37.4) |
| Range | 37.3–78.5 | 36.2–91.3 | 29.1–66.4 |
| Dairy products (g/1000 kcal) | |||
| Mean (SD) | 198.3 (84.7) | 164.4 (68.5) | 153.6 (72.8) |
| Median (IQR) | 194.5 (101.7) | 162.3 (75.2) | 140.3 (93.0) |
| Range | 145.7–247.4 | 127.0–202.2 | 100.8–193.8 |
| Fish (g/1000 kcal) | |||
| Mean (SD) | 11.8 (20.4) | 15.5 (31.8) | 15.9 (32.5) |
| Median (IQR) | 1.5 (1.7) | 1.4 (2.3) | 1.5 (1.9) |
| Range | 1.1–2.8 | 1.1–3.4 | 1.1–3.6 |
| Meat (g/1000 kcal) | |||
| Mean (SD) | 44.0 (14.7) | 45.7 (22.2) | 47.5 (14.4) |
| Median (IQR) | 47.3 (15.0) | 52.2 (20.6) | 45.6 (16.2) |
| Range | 38.4–53.4 | 40.0–60.4 | 38.1–54.4 |
| Energy intake (kcal/day) | |||
| Mean (SD) | 1591 (214) | 1654 (220) | 1622 (275) |
| Median (IQR) | 1589 (287) | 1666 (278) | 1607 (360) |
| Range | 972–2588 | 991–2647 | 780–2741 |
| Physical activity (h/day) | |||
| Mean (SD) | 2.52 (1.4) | 2.39 (1.4) | 1.90 (1.2) |
| Median (IQR) | 2.28 (1.6) | 2.14 (1.4) | 1.57 (1.6) |
| Range | 0–13.7 | 0–10.9 | 0–8.6 |
| Sedentary screen time (h/week) | |||
| Mean (SD) | 11.55 (7.3) | 13.78 (8.2) | 16.6 (10.3) |
| Median (IQR) | 10.50 (8.5) | 12.50 (9.7) | 14.4 (12.1) |
| Range | 0–56 | 0–56 | 0–56 |
| BMI (kg/m2) | |||
| Mean (SD) | 16.64 (2.7) | 17.71 (3.5) | 19.53 (4.0) |
| Median (IQR) | 15.90 (2.7) | 16.70 (4.3) | 18.70 (5.0) |
| Range | 10.70–32.90 | 10.20–40.40 | 12.60–38.70 |
| BMI categories | |||
| Underweight (N, %) | 205 (10.3%) | 148 (9.0%) | 142 (7.4%) |
| Normo-weight (N, %) | 1337 (67.4%) | 986 (59.8%) | 1246 (65.3%) |
| OW/OB (N, %) | 440 (22.3%) | 515 (31.2%) | 529 (27.3%) |
| WC (cm) | |||
| Mean (SD) | 54.58 (7.4) | 60.13 (9.2) | 66.86 (10.4) |
| Median (IQR) | 53.00 (7.7) | 57.70 (11.5) | 64.90 (13.3) |
| Range | 28.80–91.50 | 40–99.20 | 40.90–110.50 |
| BMI-PRSc | |||
| Mean (SD) | 0.07 (1.0) | 0.08 (1.0) | −0.01 (1.0) |
| Median (IQR) | 0.07 (1.3) | 0.08 (1.3) | −0.01 (1.4) |
| Range | −3.28 to 3.21 | −3.02 to 3.21 | −3.02 to 3.21 |
- Abbreviations: BMI, body mass index; BMI-PRS, body mass index-polygenic risk score; FU1, follow-up 1; FU2, follow-up 2; IQR, interquartile range; MDS, Mediterranean diet score; OW/OB, overweight/obesity; SD, standard deviation; WC, waist circumference.
- a Although the region of residence was displayed in Table 1, the models considered the country of residence (individual level) as an adjusting variable.
- b Mediterranean diet score (MDS) resulting from the sum of 7 food subgroups compliance. Score ranging from 0 to 7 points.
- c Polygenic risk score based on the estimation of a weighted score, multiplying the risk alleles for each estimated coefficient.
3.2 Association between mediterranean diet and gene associations with adiposity indicators
Table 2 shows the crude and adjusted association models for both cross-sectional and longitudinal analyses. Higher MDS was significantly associated with lower adiposity estimates at baseline (WC, β = −0.40 CI [−0.72, −0.09]) and at FU1 (BMI, β = −0.18 CI [−0.36, −0.01]; WC, β = −0.54 CI [−1.00, −0.07]) in cross-sectional analyses, but not in FU2 or in longitudinal analyses. The adjusted models showed no significant relationships between MDS and adiposity estimates. The BMI-PRS was positively and significantly associated with adiposity estimates at baseline and FU1 in cross-sectional analyses as well as in longitudinal analyses in both the non-adjusted and adjusted models. No BMI-PRS – adiposity estimates association was observed at FU2, except for the WC in the adjusted model (β = 0.55 CI [0.03, 1.07]). No MDS-obesity markers association was observed in the longitudinal approach (Table 2).
| Cross-sectional | ||||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | FU1 | FU2 | Longitudinal | |||||
| BMI | WC | BMI | WC | BMI | WC | BMI | WC | |
| β [95% CI] | β [95% CI] | β [95% CI] | β [95% CI] | β [95% CI] | β [95% CI] | β [95% CI] | β [95% CI] | |
| Crude models | ||||||||
| MDS | −0.11 [−0.22,0.01] | −0.40 [−0.72, −0.09] | −0.18 [−0.36, −0.01] | −0.54 [−1.00, −0.07] | 0.05 [−0.12, 0.24] | 0.07 [−0.37, 0.52] | −0.01 [−0.21, 0.18] | 0.01 [−0.51, 0.54] |
| BMI-PRS | 0.77 [0.61, 0.92] | 1.76 [1.35, 2.17] | 1.01 [0.80, 1.22] | 2.40 [1.86, 2.94] | 0.11 [−0.07, 0.30] | 0.42 [−0.04, 0.89] | 0.33 [0.07, 0.59] | 0.97 [0.27, 1.66] |
| Adjusted models | ||||||||
| MDS | −0.07 [−0.11, 0.09] | 0.01 [−0.25, 0.28] | −0.01 [−0.16, 0.13] | −0.11 [−0.52, 0.28] | 0.07 [−0.11, 0.26] | 0.13 [−0.33, 0.61] | 0.03 [−0.16, 0.23] | 0.05 [−0.46, 0.57] |
| BMI-PRS | 0.62 [0.50, 0.74] | 1.40 [1.07, 1.74] | 0.82 [0.65, 0.99] | 2.00 [1.58, 2.42] | 0.20 [−0.02, 0.40] | 0.55 [0.03, 1.07] | 0.42 [0.13, 0.70] | 1.08 [0.32, 1.81] |
- Note: Cross-sectional adjusted models were corrected by age, sex, country of residence, ISCED, physical activity, sedentary screen time, and the first 5 principal components of genetic ancestry. Longitudinal adjusted model was corrected by baseline age and sex, and the first 5 principal components of genetic ancestry. Associations at p < 0.05 are shown in bold.
- Abbreviations: BMI, body mass index; BMI-PRS, body mass index-polygenic risk score; FU1, follow-up 1; FU2, follow-up 2; MDS, Mediterranean diet score; and WC, waist circumference.
3.3 Gene × mediterranean diet effect on adiposity indicators
Table 3 shows the interaction effects between BMI-PRS and MDS on adiposity. At baseline, MDS exacerbated the association between PRS-BMI and BMI (β = 0.12, CI [0.01, 0.24]). The interaction effects described at baseline are displayed according to different genetic risk levels in Figure 2. No BMI-PRS × MDS effect was observed for WC at baseline. At FU2, MDS attenuated the association between BMI-PRS and BMI (β = −0.19, CI [−0.38, −0.02]). Interaction plots at FU2 for BMI are shown in Figure 2B. Finally, no BMI-PRS × MDS interaction effects were observed in the longitudinal approach for BMI or WC.
| BMI | Cross-sectional | |||
|---|---|---|---|---|
| Baseline | FU1 | FU2 | Longitudinal | |
| β [95% CI] | β [95% CI] | β [95% CI] | β [95% CI] | |
| MDS | −0.01 [−0.11, 0.08] | −0.02 [−0.17, 0.12] | 0.08 [−0.10, 0.27] | 0.03 [−0.17, 0.23] |
| BMI-PRS | 0.09 [−0.44, 0.63] | 0.60 [−0.20, 1.41] | 1.01 [0.15, 1.88] | 0.42 [0.13, 0.70] |
| BMI-PRS × MDS | 0.12 [0.01, 0.24] | 0.04 [−0.12, 0.22] | −0.19 [−0.38, −0.01] | −0.01 [−0.18, 0.18] |
| WC | Baseline | FU1 | FU2 | Longitudinal |
|---|---|---|---|---|
| p-value | p-value | p-value | p-value | |
| MDS | −0.01 [−0.26, 0.26] | −0.11 [−0.51, 0.28] | 0.12 [−0.35, 0.59] | 0.06 [−0.46, 0.59] |
| BMI-PRS | 0.46 [−0.80, 1.72] | 2.19 [0.25, 4.12] | 2.40 [0.38, 4.43] | 1.10 [0.36, 1.84] |
| BMI-PRS × MDS | 0.21 [−0.06, 0.50] | −0.04 [−0.45, 0.37] | −0.43 [−0.88, 0.01] | 0.08 [−0.37, 0.54] |
- Note: Cross-sectional models at baseline (N = 1982), FU1 (N = 1649) and FU2 (N = 1907) additionally adjusted by age, sex, country of residence, ISCED, the first 5 principal components of genetic ancestry, sedentary screen time, and physical activity. Longitudinal models adjusted by baseline age, sex, and the first 5 principal components of genetic ancestry. Associations at p < 0.05 are shown in bold.
- Abbreviations: BMI, body mass index; MDS, Mediterranean diet score; PRS, polygenic risk score; and WC, waist circumference.

3.4 Main effect associations and interaction effects of BMI-PRS × MD food groups on adiposity measures
At FU2, a higher intake of vegetables and legumes, standardized to g/1000 kcal units, was associated with a lower BMI (β = −0.01; CI [−0.02, 0.00]) and WC (β = −0.02; CI [−0.03, 0.00]). This association was confirmed despite adding the BMI-PRS × vegetables and legumes effect, which was not significant (Table 4). No further associations or interactions were observed for the other food groups. Main effects and interaction effects at baseline and FU1 for each MD food group are displayed in the Tables S1 and S2.
| FU2 | Interaction effect: BMI-PRS × MD food group | |
|---|---|---|
| BMI | WC | |
| β (95% CI) | β (95% CI) | |
| Vegetables and legumes | −0.01 (−0.02, 0.00) | −0.02 (−0.03, 0.00) |
| BMI-PRS | −0.02 (−0.50, 0.46) | 0.37 (−1.21, 1.31) |
| V&L × BMI-PRS | 0.00 (0.00, 0.01) | 0.00 (−0.01, 0.02) |
| Cereals and potatoes | 0.00 (0.00, 0.00) | 0.00 (−0.02, 0.01) |
| BMI-PRS | 0.36 (−0.41, 1.13) | 2.33 (−0.22, 4.43) |
| C&P × BMI-PRS | 0.00 (0.00, 0.00) | 0.00 (−0.03, 0.01) |
| Fruits and nuts | 0.00 (0.00, 0.00) | 0.00 (−0.02, 0.00) |
| BMI-PRS | 0.21 (−0.18, 0.62) | 0.01 (−0.62, 1.46) |
| F&N × BMI-PRS | 0.00 (0.00, 0.00) | 0.00 (−0–01, 0.01) |
| Dairy products | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) |
| BMI-PRS | −0.02 (−0.41, 0.50) | 0.04 (−0.94, 1.35) |
| Dairy products × BMI-PRS | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) |
| Fish | 0.04 (−0.43, 0.51) | 0.63 (−0.82, 1.56) |
| BMI-PRS | 0.37 (0.00, 0.47) | 0.66 (0.06, 1.27) |
| Fish × BMI-PRS | −0.18 (−0.64, 0.28) | −0.53 (−1.68, 0.61) |
| Meat | 0.00 (0.00, 0.00) | 0.00 (−0.04, 0.02) |
| BMI-PRS | −0.10 (−0.64, 0.85) | 0.44 (−1.40, 2.29) |
| Meat × BMI-PRS | 0.00 (−0.01, 0.01) | 0.01 (−0.03, 0.04) |
- Note: All food group units are as per (g/1000 kcal). Results are presented as beta-coefficients and their respective 95% CIs. Models were adjusted by age, sex, country of residence, ISCED, the first 5 principal components of genetic ancestry, sedentary screen time, and physical activity. Associations at p < 0.05 are shown in bold.
- Abbreviations: 95% CI, 95% confidence interval; BMI, body mass index; C&P, cereals and potatoes; FU2, follow-up 2; F&N, fruits and nuts; MD, Mediterranean diet; PRS, polygenic risk score; V&L, vegetables and legumes; WC, waist circumference.
4 DISCUSSION
The main findings of the present study showed that MDS adherence moderated the genetic susceptibility to obesity in European early adolescents (FU2). Furthermore, a higher consumption of vegetables and legumes was inversely associated with adiposity measures, irrespective of genetic susceptibility to obesity. However, the observed effect sizes were small. In early adolescents (FU2), the strength of the association between the BMI-PRS and the studied adiposity indicators appeared to be modulated by the MDS, even though the MDS was not initially associated with either BMI or WC. Thus, early adolescent participants with a high MDS had a BMI that is less determined by their genetic susceptibility, as the environment is more likely to play a greater role in these cases compared with those early adolescents with a low MDS. Finally, no relevant findings were observed in FU1.
We observed an exacerbating interaction for BMI-PRS × MDS on adiposity at baseline, which was later (at FU2) attenuated when we categorized children according to their genetic risk for obesity. Nevertheless, it seems to represent a positive change in the modulation effect of the MDS, initially strengthening the genetic susceptibility to obesity and later acting as an attenuating factor in the pre-adolescence years.31 Our findings at baseline, in younger children, do not agree with a previous study carried out in Finnish school children, as those children genetically prone to adiposity showed a stronger association of unhealthy foods with BMI than those with lower genetic susceptibility to adiposity.32 However, this study is similar to our results in early adolescents, as the MD attenuated the genetic susceptibility to obesity. Similar findings were observed in the HELENA study, indicating that European adolescents with higher MD adherence may attenuate their genetic risk of obesity (12). These observed changes of the MD effect between time points could be partly explained by the differences in gene expression between adolescence and childhood years, showing a closer relationship to the diet-attenuating effect on adiposity in early adolescent years.33 In addition, the BMI-PRS used was based on adult summary statistics; thus, it could be the case that a BMI-PRS based on children could explain more BMI variance, and perhaps we could find a different diet effect when evaluating interaction effects. Nevertheless, the BMI-PRS suggested by Khera et al.9 outperformed other PRSs previously suggested in the literature when testing gene × diet effects in adiposity indicators.34 In addition, the BMI-PRS was not significantly associated with WC or BMI at baseline. However, the BMI-PRS estimate effect was statistically significant in the model including interactions in terms of BMI. This means that the BMI-PRS did not seem to be associated with BMI (a priori), but it seems to modulate the strength of association between MD and BMI. Thus, it might be the case that some genes linked to BMI might contribute to the MD and BMI association. However, in our case, when investigating changes in BMI over time, no statistically significant MD or BMI-PRS × MD effects were observed.
The gene × diet effects in young populations are scarcely studied. Comparatively, more research has been performed in adults. An interventional study on MD showed the beneficial effects of a higher MD adherence in terms of adiposity indicators, even if the genetic risk to obesity, measured through a GRS, was high.35 Another study showed a favourable effect in individuals following a healthy diet with higher BMI-PRS in adiposity indicators. However, this gene × diet effect was not applicable for MD.36 On the contrary, a study carried out in the Swiss population showed an association between diet – WC, independent of the genetic risk of obesity.37
Moreover, in addition to analysing the MD as a dietary adherence and its associations with health outcomes in the three measurement timepoints, we also intended to reflect the effect of changes in MD over time in the longitudinal approach, in an attempt to reflect how changes in diet quality could be associated with obesity markers. However, the results obtained did not show MDS-PRS interactions or MDS-WC associations over time. These findings align with some of the longitudinal analyses studied lately in recent literature.38 The observed associations in different directions in other studies emphasize the need for performing more high-quality longitudinal studies targeting European children. Overall, our study suggests that it is unclear, within our study design, to what extent changes in MD during childhood may allow for improved obesity marker values in a span of a few years during childhood. This suggests that ensuring high MDS in children at an early age may remain the best strategy to prevent children from developing obesity at an early age, where obesity is known to persist across age groups, from childhood to adulthood.
When assessing the MD food groups adherence, we observed that the vegetables and legumes group had a strong association with BMI or WC. According to the results obtained, the BMI-PRS effect is captured by vegetable and legume intake. This unexpected finding is not a priori biased due to changes in the sample or supported by previous literature.39 Thus, these results should be interpreted with caution due to the small size effect obtained and the possible overreporting of vegetable and legume consumption. In contrast to these results, another study showed that genetic susceptibility to obesity could be attenuated by increased vegetable and legume intake, showing a more pronounced effect in those individuals at higher genetic risk of obesity.40
Several aspects were considered in the present analysis when interpreting the multifactorial nature of obesity and the exposure to different dietary factors. We are currently trying to understand the underlying mechanisms which could explain the transitioning role of MD adherence from childhood to early adolescence years. First, both baseline and FU2 time points showed moderate MD adherence. Hence, the level of adherence should not be an attributable factor. However, the BMI-PRS replicating power differed between 6 and 12 years old, being less predictive in the older ones. In line with this, a study performed on a large sample of European adults revealed different responses testing for environmental × BMI analysis, depending on the genetic risk among individuals. Despite showing unclear results in the interaction effects between obesity susceptibility and some lifestyle characteristics, these findings support that the MD effect could be partly explained by the genotypic profile of individuals.41 Thus, the implementation of more comprehensive genomic approaches will help to understand individual responses to environmental exposure and its association with adiposity.42 Further health determinants could partly explain the MD influence over time on the genetic susceptibility to obesity, such as potential changes in eating behaviours,43 or emotional eating (the genetic effect on weight loss44), yet to be studied in interaction analysis.
The strengths of the present study include a detailed and repeated phenotyping of children of the IDEFICS/I.Family cohort, which comprehends thousands of participants from diverse countries around Europe, and the longitudinal approach during important periods of development (16). Also, the standardized dietary assessment methods using validated 24 h dietary recalls, which provided detailed information of children's dietary intake, together with the FFQ information considered in the NCI correction, are strengths of this study. Moreover, the use of Khera et al. BMI-PRS,9 one of the most relevant genome-wide PRS for adiposity to date, is also considered to be a good genetic predictor associated with other adiposity risk indicators such as WC. Finally, the integration of a longitudinal approach, despite not obtaining relevant findings in the present cohort, allowed us to investigate whether changes in diet quality could potentially improve obesity marker values over time.
As a main limitation, the acknowledgement of reporting-related errors concerning lifestyle behaviours. Nevertheless, measurement error in environmental exposure usually biases the interaction effect towards the null, which should not increase the risk of false-positive occurrence. Since the current common variants known from GWAS to date explain a small proportion of the BMI variation,9 it is likely that other loci from rarer variants could emerge to explain the polygenic architecture of adiposity in larger sample sizes.45 Although the sample size was sufficient to report representative findings of the studied population, the availability of participants with complete genotyping information was limited as compared with the total sample recruited within the IDEFICS/I.Family consortium. Finally, the established comparisons with other findings in the literature should be interpreted with caution since similar studies included in this discussion did not report the used dietary assessment method.
In the studied European youth, higher MDS was not associated with lower adiposity in all cross-sectional analyses. Changes in MD over time were not associated with obesity markers or interacted with the genetic susceptibility to obesity. However, it was observed that the MD modulated the association between the BMI-PRS and adiposity indicators during childhood and early adolescent periods, having an exacerbating effect in children measured during infancy years (baseline) and an attenuating effect in early adolescent years (FU2). In addition, a higher intake of vegetables and legumes was associated with lower adiposity, irrespective of the obesity genetic risk of the studied European early adolescents at FU2.
AUTHOR CONTRIBUTIONS
The Core Management Group of this study is based on the IDEFICS/I.Family cohort formed by authors: SDH, LL, DM, VP, MT, TV, and LM; Study design: MSC, GM, and LM; Data analysis: MSC, GD, GM, and TI; Data interpretation: MSC, GM, and GD; Supervision: LB, PDE, IL, and LM; Writing the original draft: MSC and GD; Writing reviews & editing: GD, GM, LB, SDH, RF, TI, LL, DM, RN, CP, PR, GT, MT, TV, MR, PDE, IL, and LM. All authors were involved in writing and editing the paper and had final approval of the submitted and published versions.
ACKNOWLEDGEMENTS
We express gratitude for the support extended by school boards, headmasters, teachers, school staff, and communities, as well as acknowledge the dedication of all study nurses and our data managers.
FUNDING INFORMATION
This work was done as part of the IDEFICS (http://www.idefics.eu) and I.Family studies (http://www.ifamilystudy.eu/). We gratefully acknowledge the financial support of the European Union within the Sixth RTD Framework Programme Contract No. 016181 (FOOD) and the Seventh RTD Framework Programme Contract No. 266044. Participating partners also allocated their own resources towards the genotyping of children.
CONFLICT OF INTEREST STATEMENT
No conflict of interest was declared.
Open Research
DATA AVAILABILITY STATEMENT
The data from the IDEFICS and I.Family studies, which underpin the findings of this research, are not publicly accessible, as they contain crucial information for maintaining study quality. Nevertheless, the data sharing committee is open to receiving requests. Researchers interested in accessing the data can reach out to the IDEFICS and I.Family consortia (http://www.ideficsstudy.eu/Idefics/ and http://www.ifamilystudy.eu/) to explore potential requests for data access.




