Significance of Myelodysplasia-Related Mutations and the Genetic Landscape of Acute Leukemias of Ambiguous Lineage
Funding: The authors received no specific funding for this work.
ABSTRACT
The recent fifth edition WHO classification and ICC classification systems have moved further toward genetically defined classifications of acute leukemias. Both now recognize myelodysplasia-related (MR) mutations as defining of MDS-related AML (AML-MR). Acute leukemias of ambiguous lineage (ALAL) are a heterogenous group of acute leukemias characterized by leukemic blasts that either express markers of multiple lineages, mixed phenotype acute leukemia (MPAL), or too few to be assigned a definitive lineage, acute undifferentiated leukemia (AUL). However, the recent classifications are unclear on how ALALs should be categorized in the presence of MR mutations. In short, the current recommendations are to classify cases that are immunophenotypically consistent with ALAL but harbor MR cytogenetics or mutations as AML-MR. Due to their rarity, investigations into the genetic basis of ALAL are limited but show great heterogeneity in their mutational landscapes. Data on the frequencies and significance of MR mutations in ALAL is particularly scant. Our comprehensive review of the literature reporting on the genetic landscapes of MPAL and AUL shows that a significant proportion of MPAL and AUL cases, ~32% and ~59% on average respectively, may harbor one or more mutations in MR genes, with mutations in RUNX1 and ASXL1 among the most common. Additional research is needed into the clinical, immunophenotypic, and genetic characteristics of ALAL to aid in refining classification and to support therapeutic decision making.
1 Introduction
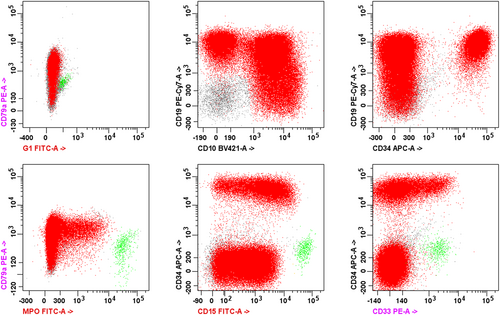
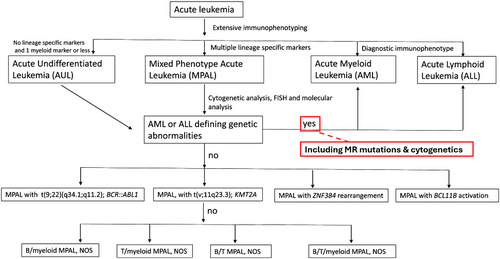
Acute myeloid leukemia (AML) and acute leukemia of ambiguous lineage (ALAL) comprise a diverse variety of acute leukemias that are defined by their morphologic, immunophenotypic, and genetic features. ALALs are leukemias composed of blast populations that do not show differentiation along a single lineage. These include leukemias that do not show any clear lineage, known as acute undifferentiated leukemia (AUL), and those that show differentiation along more than one lineage, known as mixed phenotype acute leukemias (MPAL). ALALs are rare, comprising approximately 4% of all acute leukemias, with the majority of those being MPAL [1]. A diagnosis of MPAL requires ≥ 20% blasts in the peripheral blood or bone marrow that meet defined immunophenotypic criteria consistent with differentiation along two or more lineages (Table 1) [2, 3]. The blast population may consist of a single population of blasts that express markers of two or more lineages, referred to as biphenotypic, or two separate blast populations each expressing markers of a single lineage, referred to as bilineal. Lineage assignment is best assessed by flow cytometry (Figure 1). However, leukemias that meet the criteria for other defined categories, such as AML with defining genetic abnormalities, are excluded from MPAL (Figure 2).
| Lineage | Criterion |
|---|---|
| B lineage | |
| CD19 stronga | One or more of the following also strongly expressed: CD10, CD22, CD79ac |
| OR | |
| CD19 weakb | Two or more of the following also strongly expressed: CD10, CD22, CD79ac |
| T lineage | |
| CD3 (cytoplasmic or surface)d |
Intensity in part exceeds 50% of that of mature T cells by flow cytometry OR Immunocytochemistry positive with non–ζ-chain reagent |
| Myeloid lineage | |
| Myeloperoxidase | Intensity in part exceeds 50% of mature neutrophil level |
| OR | |
| Monocytic differentiation | Two or more of the following expressed: nonspecific esterase, CD11c, CD14, CD64, lysozyme |
- Note: This table delineates the specific criteria required to establish lineage differentiation when considering a diagnosis of MPAL by flow cytometry. Rendering a diagnosis of MPAL requires identifying aberrant blast populations that meet criteria for two or more lineages. Adapted from Khoury et al. [2].
- a CD19 intensity in part exceeds 50% of that of normal B-cell progenitor by flow cytometry.
- b CD19 intensity does not exceed 50% of that of normal B-cell progenitor by flow cytometry.
- c Provided T lineage is not under consideration; if it is, CD79a cannot be used.
- d Using CD3 epsilon-chain antibody.
AML is the most common acute leukemia in the adult population and is characterized by clonal expansion of immature hematopoietic precursors of myeloid lineage, known as myeloblasts (or blast equivalents), in the peripheral blood and bone marrow [5]. AML may arise de novo, evolve from prior myelodysplastic syndromes (MDS) or myelodysplastic/myeloproliferative neoplasms (MDS/MPN), or therapy-related AML, which arises after exposure to cytotoxic and/or radiation therapy [6, 7]. The more recent classifications of the World Health Organization (WHO), most recently the fifth edition classification of Haematolymphoid Tumours, and the International Consensus Classification of Myeloid and Lymphoid Neoplasms (ICC) both allow for the classification of cases of AML that arose from, or are otherwise related to, MDS as distinct entities, if diagnostic and exclusionary criteria are met [2, 3]. These subcategories include AML, myelodysplasia related in the WHO fifth edition and AML with myelodysplasia-related gene mutations or AML with myelodysplasia-related cytogenetic abnormalities in the ICC classification.
Both the WHO and ICC classifications have moved toward genetically defined classifications of acute leukemias. Both classifications now recognize MDS-associated mutations as defining of MDS-related AML (AML-MR), a disease with poor prognosis [8, 9]. AML-MR comprises approximately 24%–35% of all AML cases [2]. Among the cytogenetic and molecular features that define AML-MR are a category of mutations known as “secondary-type” or “myelodysplasia-related (MR)” mutations. In the context of AML, they are highly specific for AML arising from preceding MDS, MPN, or therapy-related clonal aberrations [10]. The presence of mutations in SRSF2, SF3B1, U2AF1, ZRSR2, ASXL1, EZH2, BCOR, or STAG2 genes is > 95% specific for these myelodysplasia-related or secondary AMLs [11]. Somatic mutations in RUNX1 are also commonly associated with mutations in other genes characterizing AML-MR and are included in this category in the ICC classification but not in the fifth edition of WHO classification [12]. AML with mutated RUNX1 is a provisional entity in the WHO fourth edition classification [13].
2 Cytogenetics of ALAL
Most cases of MPAL, up to 86%, show an abnormal karyotype [14]. The most common recurrent alterations include BCR::ABL1, seen in approximately 20%–30% of MPALs, and KMT2A rearrangements, seen in approximately 10% of MPALs [15, 16]. MPALs with these cytogenetic findings are classified as distinct entities in the most recent WHO and ICC classifications and often show a B/M phenotype. B/M MPALs also frequently show del(1)(p32), trisomy 4, del(6q), 12p11.2 aberrancies, and near tetraploidy [15]. Cytogenetic abnormalities commonly associated with T/M MPAL include t(10;11)(p13;q21) which results in PICALM::MLLT10 rearrangement and is seen in 10%–15% of cases, but may also show B-lineage differentiation [14, 15, 17]. Other cytogenetic aberrations commonly seen in MPAL include monosomy 5, monosomy 7, polysomy 21, trisomy 8, and others [18]. A complex karyotype, defined as the presence of three or more unrelated cytogenetic aberrations, is among the most common cytogenetic findings in immunophenotypic MPAL, seen in approximately 33% of cases [14, 19]. However, as a complex karyotype is a MR-defining abnormality, these cases would be classified as myelodysplasia-related AML by current WHO and ICC criteria (Figure 1).
Karyotypic abnormalities are also common in AUL, seen in approximately 40% of cases [20]. They frequently show alterations involving chromosomes 12 and 13 as well as 5q [21]. Similar to MPALs, they may show BCR::ABL1 and KMT2A rearrangements [22]. Other gene fusions such as SET::NUP214 may be seen [15, 23]. Complex karyotypes have also been reported in cases that meet immunophenotypic criteria for AUL. Cases of AUL reclassified as AML-MR based on the presence of a complex karyotype or other myelodysplasia-related cytogenetics may show a worsened prognosis, similar to other cases of AML-MR, compared to conventional AULs [20].
3 Molecular Landscapes of MPAL
Due to its rarity, large-scale investigations into the genetic basis of ALAL are limited. The few large, published studies on the genetic basis of ALAL have shown great heterogeneity in its genetic landscape. MPAL-associated gene mutations are a mixture of those commonly seen in AML and ALL as well as others associated with neither. Yan et al. demonstrated the presence of at least one ALL-related mutation in 12 of 31 (39%) of MPAL cases [14]. One of the largest and most comprehensive genomic analyses comparing 115 cases of pediatric ALAL performed by Alexander et al. provided insights into the genomic relationships between immunophenotypically defined subtypes of acute leukemia [15]. They demonstrated an ALL-like genomic landscape among cases of B/M MPAL as well as similar genetic alterations between B/M MPAL with ZNF384r alterations and B-ALL, suggesting a biologic continuum between these entities. They also showed genomic and epigenomic similarities between T/M MPAL compared to T-ALL and ETP-ALL, another acute leukemia that characteristically demonstrates both T and myeloid lineage markers. Cases categorized as ALAL-other, including MPAL with KMT2A rearrangement, AUL, and MPAL NOS showed a wide range of alterations, primarily clustering between the AML and B-ALL clusters [15]. Takahashi et al. compared the molecular profiles of 31 MPALs to AML, B-ALL, and T-ALL which also demonstrated the presence of both ALL and AML-type mutations in MPAL. They also showed distinct mutational and methylation profiles between B/M MPAL and T/M MPAL [16]. Similarly, Mumme et al. performed single cell RNA sequencing on pediatric MPAL cases and showed that the transcriptomes of B/M MPAL overlapped with those of B-ALL and AML while the transcriptomes of T/M MPAL overlapped with T-ALL and AML. T/M MPAL showed even greater overlap with ETP-ALL compared near-ETP and non-ETP T-ALL [24].
Thus, the different immunophenotypic subtypes of MPAL seem to show distinct mutational landscapes. Anomalies associated with B/M MPAL include alterations in genes commonly associated with ALL, such as IKZF1, PAX5, and ZNF384, seen in nearly half of childhood B/M MPAL cases [16]. Mutations commonly associated with AML that are commonly seen in B/M MPAL include RUNX1, TET2, EZH2, and ASXL1 [14]. T/M MPAL may show a higher mutational burden compared to B/M MPAL and display genetic overlap with ALL, MPAL, and ETP-ALL, including mutations involving WT1, RUNX1, ETV6, DNMT3A, PHF6, and NOTCH1 [16, 17, 25, 26]. Alterations leading to deregulation of BCL11B are seen in 10%–15% of MPALs overall and up to one-third of T/M MPALs [27]. Notably, MPAL with ZNF384 rearrangement and ALAL with BCL11B rearrangement are now distinct genetically defined subtypes of MPAL in the WHO and ICC classifications.
The limited data on B/T MPAL has demonstrated a mutational profile similar to T-ALL and ETP-ALL, another entity that characteristically displays immunophenotypic markers of T and myeloid lineages [28]. Mutations described in B/T MPAL include those involving PHF6, DNMT3A, NOTCH1, WT1, FLT3, EZH2, JAK3, MED12, CTCF, IL7R, SF3B1, PTPN11, and TP53 [17, 25, 28]. However, a recent case series of three B/T MPALs suggests that the genetic similarity to T-ALL only occurs in cases with a T-lineage predominance, while B/T co-dominant cases lack that signature [29]. Thus, in addition to genetic variation between immunophenotypic subtypes of MPAL, there may also be genetic heterogeneity within immunophenotypic subtypes. However, the genetic landscape in MPAL does not reliably correlate with immunophenotype, and while MPAL shows worse overall survival compared to ALL and AML overall, the immunophenotype does not appear to correlate with overall survival [30]. Thus, the biologic connection between genetics and immunophenotypes in MPAL remains unknown.
Not only does the genetic and immunophenotypic heterogeneity in MPAL pose a diagnostic challenge, but it also poses a challenge to therapeutic decision making. Current first-line chemotherapy regimens used in the treatment of acute leukemias are often directed against either myeloid lineages or lymphoid lineages. While current studies suggest that most cases of MPAL respond more favorably to an ALL-directed regimen compared to an AML-directed regimen, the optimal treatment regimen for patients with MPAL remains controversial. Attempts to subclassify MPAL based on immunophenotypic and/or genomic features are ongoing in hopes of tailoring treatment. Takahashi et al. compared genome-wide mutational and methylation signatures between MPAL, AML, T-ALL, and B-ALL and subclassified MPALs as “AML-type” and “ALL-type.” They showed that using therapies lineage-matched to these subtypes was associated with better clinical response [16]. Wang et al. integrated NGS and RNA sequencing data of 176 adult patients with MPAL and identified eight molecular subgroups with distinct mutational and gene expression characteristics, with some groups showing disease signatures similar to hematopoietic stem cells, myeloid progenitors, or lymphoid progenitors [26]. Peretz et al. performed multiomic single cell profiling of 14 newly diagnosed adult MPAL patients, showing that MPAL blasts express a shared stem cell-like transcriptional profile indicative of high differentiation potential [30]. They conclude that MPAL is a distinct leukemia with stem cell-like features rather than one that lies on a continuum with other acute leukemias. These studies highlight the great clinical, immunophenotypic, and genetic heterogeneity of MPAL and the ongoing efforts to refine our understanding and classification of these entities as well as help facilitate potential precision therapies.
4 Molecular Landscape of AUL
There is very limited data on the genetic landscape of AUL. The few published studies show approximately 40% of cases may show mutations in PHF6, RUNX1, and SRSF2, with approximately 25% showing BCOR mutations [15, 20, 31]. The enrichment of RUNX1 and ASXL1 mutations may suggest that AUL is more genetically related to AML compared to ALL [15, 31]. However, the increased frequency of PHF6 mutations suggests a biological continuum with T/M MPAL and ETP-ALL [17].
5 Myelodysplasia-Related Mutations in ALAL
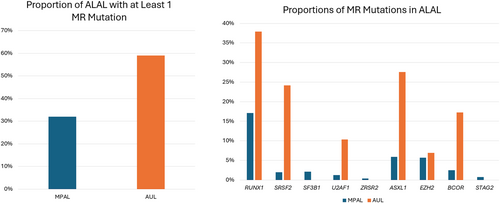
Few studies have evaluated the prevalence and significance of MR-mutations in ALAL. Review of the published literature yielded 18 publications with genetic analysis of multiple cases of MPAL, all of which showed a mutation in one or more secondary or myelodysplasia-related genes in at least one case (Table 2). Overall, approximately 32% (177/560) of cases (range: 12%–100%) with available genetic data on average showed at least one MR-mutation, with many cases showing more than one. The most commonly mutated MR gene was RUNX1, seen in 17% (96/560) of cases on average and accounting for approximately 45% of total MR mutations seen in MPAL. The second and third most commonly mutated MR genes were ASXL1 and EZH2, each seen in ~6% of cases and comprising 16% and 15% of total MR mutations seen in MPAL, respectively. The least common was ZRSR2, seen in < 1% of cases.
| # of cases with mutation in each respective MR gene | # of cases with one or more MR mutation (% of cohort) | Total # of MPAL cases in cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RUNX1 | SRSF2 | SF3B1 | U2AF1 | ZRSR2 | ASXL1 | EZH2 | BCOR | STAG2 | |||
| Alexander [15] | 15 | 7 | 12 | 30 (33%) | 90 | ||||||
| Di Giacomo [27] | 3 | 1 | 1 | 2 | 7 (39%) | 18 | |||||
| Eckstein [25] | 2 | 2 | 4 (17%) | 23 | |||||||
| Hennawi [32] | 6 | 1 | 2 | 7 (39%) | 18 | ||||||
| Kern [33] | 1 | 1 | 1 | 3 | 6 (33%) | 18 | |||||
| Kresch [34] | 10 | 5 | 4 | 3 | 1 | 7 | 1 | 2 | 3 | 18 (69%) | 26 |
| Lazzarotto [35] | a | a | Up to 8 (12%)a | 66 | |||||||
| Merati [31] | 1 | 1 | 1 | 2 (40%) | 5 | ||||||
| Mi [28] | 1 | 2 | 1 | 1 | 4 (44%) | 9 | |||||
| Montefiori [36] | 16 | 2 | 3 | 20 (33%) | 61 | ||||||
| Mumme [24] | 1 | 1 | 1 (33%) | 3 | |||||||
| Peretz [30] | 3 | 2 | 2 | 1 | 7 (50%) | 14 | |||||
| Quesada [37] | 2 | 2 | 3 (21%) | 14 | |||||||
| Takahashi [16] | 8 | 5 | 1 | 5 | 1 | 1 | 1 | 12 (39%) | 31 | ||
| Wang [26] | 20 | 7 | 5 | 32 (31%) | 104 | ||||||
| Xiao [17] | 4 | 1 | 3 | 6 (23%) | 26 | ||||||
| Yan [14] | 1 | 2 | 3 | 1 | 7 (23%) | 31 | |||||
| Zheng [29] | 2 | 1 | 3 (100%) | 3 | |||||||
- Note: This table summarizes the cases with MR mutations among reported cohorts of MPAL. Many cases reportedly had multiple MR mutations.
- a Lazarotto et al. grouped the molecular results of eight patients together in a category that included mutations in JAK2, FLT3-TKD, RUNX1, EZH2, DNTM3A, CDKN2A, and RUNX1-RUNX1T1. Mutation frequencies for individual genes in this category were not reported.
Only three publications contained genetic data on AUL for evaluation that showed MR-mutations (Table 3). These studies do show a high frequency of MR-mutations in AUL, with approximately 59% (17/29) of cases on average showing one or more MR-mutations. Similar to MPAL, multiple cases show more than one MR-mutation. Also like MPAL, the most commonly mutated MR gene was RUNX1, seen in ~38% (11/29) of cases on average and accounting for ~31% (11/36) of total MR mutations seen in AUL, with AXSL1 as the second most common, seen in ~28% (8/29) of cases on average and accounting for ~22% (8/36) of total MR mutations seen in AUL.
| # of cases with mutation in each respective MR gene | # of cases with one or more MR mutation (% of cohort) | Total # of AUL cases in cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RUNX1 | SRSF2 | SF3B1 | U2AF1 | ZRSR2 | ASXL1 | EZH2 | BCOR | STAG2 | |||
| Alexander [15] | 2 | 2 (40) | 5 | ||||||||
| Merati [31] | 3 | 1 | 2 | 3 | 2 | 1 | 5 (100) | 5 | |||
| Weinberg [20] | 6 | 6 | 1 | 5 | 4 | 10 (53) | 19 | ||||
- Note: This table summarizes the cases with MR mutations among reported cohorts of AUL. Many cases reportedly had multiple MR mutations.
Overlapping clinical and genetic features between ALAL and genetically defined AML-MR pose a diagnostic challenge. In regard to evaluating the significance of MR mutations, changes in the classification systems resulting in some cases of immunophenotypic AUL or MPAL being reclassified as AML-MR can complicate retrospective analyses, especially considering the rarity of these entities.
Review of the cohorts in many of these studies shows that a significant proportion of MPAL and AUL cases may harbor one or more MR mutations (Figure 3). Despite this, the significance of these mutations in ALALs remains unclear. Mutations in RUNX1 and SRSF2 were significantly associated with an AML-like pattern of mutation and methylation signatures, suggesting an overlap with AML [16]. RUNX1 mutation shows a particularly high incidence in B/M MPAL. Some data suggest that RUNX1 and SRSF2 may predict a more favorable outcome in MPAL in contrast to AML, and that ASXL1 mutations in AML-MR may show improved overall survival after adjusting for cytogenetic risk [34]. Mutations in MR genes are among the common recurrent genetic abnormalities in AUL; however, the limited data suggest that AUL shows distinct characteristics and outcomes compared to AML-MR [20].
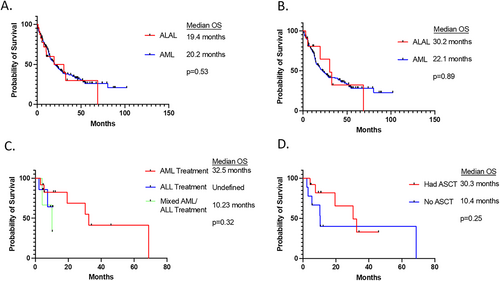
Clinically, the increased frequencies of MR mutations in ALAL may have important implications therapeutically and prognostically. As a result of their rarity, there is limited data evaluating outcomes in ALALs, particularly among the subset of cases with MR mutations. The subset of AML with MR mutations are associated with a particularly poor prognosis and refractoriness to many standard chemotherapy regimens used to treat AML [8, 9]. This appears to hold true among the subset of ALAL with MR mutations as well. A recent analysis comparing outcomes of ALAL with MR mutations to AML with MR mutations shows similarly poor overall survival and relapse-free survival between the two groups (Figure 4) [38]. Among cases of ALAL with MR mutations, the type of chemotherapy regimen used (ALL-directed vs. AML-directed vs. combination) as well as receiving allogeneic hematopoietic stem cell transplant (AHSCT) did not show an improved outcome compared to the other regimens. These conclusions also hold true when comparing MPAL with MR mutations to AUL with MR mutations. This study suggests that the presence of MR mutations in acute leukemias confers a worse prognosis and refractoriness to therapy, regardless of the immunophenotypic lineage(s) expressed by the malignant blasts.
6 Conclusion
The current WHO and ICC classifications are not clear on how acute leukemias with ambiguous lineage should be classified in the presence of MR mutations. They currently suggest excluding leukemias with ambiguous lineage phenotypes that harbor MDS-associated cytogenetic abnormalities and/or MDS-associated gene mutations from classification as AUL or MPAL. Recent studies comparing the outcomes of MPAL with complex karyotypes and ALAL with MR mutations both show similarly poor outcomes compared to AML-MR, favoring inclusion of these ALALs within AML-MR [38, 39].
Clinically, MR mutations are relatively common in AUL and MPAL, and their presence appears to confer resistance to standard therapies used in acute leukemias. This presents a therapeutic dilemma, as current classification frameworks do not clearly guide treatment selection for these patients. Ongoing research into novel therapeutic approaches is essential to improve outcomes for this biologically and clinically challenging subset of acute leukemias. Several types of ongoing or future studies would be particularly valuable, including investigations into the clonal evolution and the impact of MR mutations on lineage commitment and the correlation of mutation profiles with response to different treatments. Prospective cohort studies would be helpful in evaluating long-term outcomes and treatment responses in MPAL/AUL patients with MR mutations.
Author Contributions
All authors contributed meaningfully to the concept and design, collection of data, analysis of data, interpretation of results, and writing and reviewing the manuscript. All authors reviewed and agreed on the final revision of the manuscript.
Ethics Statement
The referenced studies were approved by the respective Institutional Review Boards (IRBs) governing each study. All cases included were fully anonymized.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.