The Application of Artificial Intelligence-Based Bone Marrow Cell Analysis System in Pediatric Hematological Diseases
Funding: The work was supported by the Health Commission of Heilongjiang Province (20231111000188).
ABSTRACT
Introduction
The clinical diagnosis of hematological diseases depends on the differential count of nucleated cells on the bone marrow (BM) smears, and an artificial intelligence (AI)-based system was applied to automatically classify BM nucleated cells in pediatric hematological disease samples in this study.
Methods
The BM aspirate smears were collected from 213 pediatric patients (under 18 years old) at Harbin Medical University Affiliated Sixth Hospital from October 2023 to June 2024. The entire smear of BM was scanned by a ×40 objective lens to obtain complete digital images using an automated analysis method named Morphogo. Next, Morphogo was used to capture nucleated cells in an area of BM smears that was selected by hematopathologists, with a magnification of ×100 objective lens.
Results
Morphogo demonstrated a high overall accuracy (> 87.8%) in pre-classifying nucleated cells in BM aspirate smears obtained from masked pediatric patients. In addition, the average values of sensitivity and accuracy in Morphogo cell classification were remarkably high. Moreover, Morphogo could reduce the time costs on classifying BM nucleated cells. Besides, there were positive correlations between Morphogo and manual categorization for immunologic thrombocytopenic purpura, BM failure, hyperplastic anemia, acute leukemia, chronic myeloid leukemia, and other hematological diseases.
Conclusion
This research demonstrated the clinical potential of the Morphogo in early screening of pediatric hematological diseases and its reliability as an automated tool for differential counting and analysis of BM nucleated cells.
1 Introduction
Pediatric hematological diseases are a complex disease group, mainly including anemia, hemorrhagic diseases, leukemia, bone marrow failure (BMF), and so forth, which pose a serious threat to the physical and mental health as well as the safety of children's lives [1, 2]. According to the cancer statistics in 2022, the high incidence age of childhood cancer in China is between 1 and 4 years old, among which the rate of childhood leukemia was reported to be 32.89%, ranking first [3]. Moreover, acute leukemia (AL) is the most common leukemia in childhood, and it was reported that approximately 15,000 children in China are diagnosed with acute lymphoblastic leukemia (ALL) or acute myeloid leukemia (AML) each year [3]. Although both ALL and AML are highly aggressive tumors characterized by rapid onset and progression, many children in the early stages of these diseases have a high probability of cure [4, 5]. Therefore, the early diagnosis and treatment are of great help and significance to the prognosis of pediatric leukemia and other hematological diseases.
With the progress of current clinical medical examination technology, although immunological examination, cytogenetic examination, and other methods play an important role in the clinical diagnosis of hematological diseases, the cellular morphological examination such as peripheral blood and bone marrow (BM) smears is still the basis of the morphology, immunology, cytogenetics, and molecular biology (MICM) diagnostic system [6, 7]. In clinic, the hematological BM smears require experienced hematopathologists to examine, while the experience and professional levels of hematopathologists vary greatly, and the accuracy of examination will be difficult to guarantee, which leads to some patients being unable to be detected at an early stage and receive suitable treatment in a timely manner [8]. Traditional methods are time-consuming, laborious, and subjective due to the large scale of histopathological images, which makes them prone to human error and affects the final diagnosis [9]. Thus, designing a fast, accurate, and reliable BM smear detection method for the investigation of hematological diseases is an essential prerequisite for early intervention as well as subsequent treatment. Currently, many studies have applied computer-aided diagnosis technology to clinical medical diagnosis due to the rapid development of artificial intelligence (AI) and computer vision [10, 11]. Jie Liu et al. applied a deep learning method and device for BM imaging cell detection and found that the response speed of the diagnostic system is faster than that of trained hematopathologists [8], which indicates the feasibility of AI and deep learning in the identification of BM nucleated cells.
The Morphogo is a cell morphology analysis system that contains hardware and software, which can automatically scan BM smears and generate fully digital images, then locate, preclassify, and count nucleated cells based on AI [12]. Subsequently, hematopathologists can review the pre-classification results and edit diagnostic reports [12], which seems to reduce time costs and improve work efficiency. In order to analyze whether the Morphogo has significant advantages over traditional manual methods, this research selected 213 pediatric patients admitted from Harbin Medical University Affiliated Sixth Hospital as the research subject.
2 Materials and Methods
2.1 Sample Collection
The BM aspirate smears from 213 retrospective pediatric patients (with an average age of 17 ± 0.7 years) at Harbin Medical University Affiliated Sixth Hospital from October 2023 to June 2024 were masked, and three patients were excluded due to incomplete data, leading to a final sample size of 210 patients included in the study. The pediatric hematological cases were divided into six groups: immunologic thrombocytopenic purpura (ITP, 56 cases), hyperplastic anemia (33 cases), BMF (28 cases), AL (39 cases), chronic myeloid leukemia (CML, 5 cases), and others (49 cases). Among the hyperplastic anemia group, there were 20 cases of iron deficiency anemia (IDA), 5 cases of megaloblastic anemia (MA), and 8 cases of hemolytic anemia (HA). In the AL group, 25 cases were ALL and 14 cases were AML. Additionally, in the BMF group, 19 cases were aplastic anemia (AA), 5 cases were myelodysplastic syndromes (MDS), and 4 cases were reduced BM hyperplasia caused by autoimmune diseases. Other cases encompassed leukopenia/leukocytosis, lymphoma without BM invasion, thrombocytosis, etc. This study was approved by the Ethical Committee of Harbin Medical University Affiliated Sixth Hospital (LC2024-089).
The staining of BM aspirate smears was performed using the Wright-Giemsa protocol in accordance with the requirements of the National Guide to Clinical Laboratory Procedures (NGCLP, fourth edition) or the recommendations of the International Council for Standardization in Haematology (ICSH) [13].
2.2 Instrument
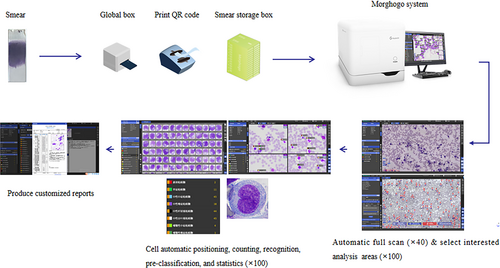
The convolutional neural network-based automated AI system (Morphogo) was developed by Hangzhou Zhiwei Information Technology Co. Ltd. (China), which makes use of neural networks with 27 layers. The system can identify over 35 different types of BM nucleated cells, which have been trained on over 3000 BM samples. This method uses a ×40 objective lens to scan the whole BM smear, and then turns to a ×100 objective lens to obtain dozens of detailed cell pictures in an interested area. The AI algorithms of the device are capable of counting and typing the nucleated cells set on each smear.
2.3 Digital Workflow
First, the area for smear analysis was set up, and the smears were loaded into the instrument in batches to obtain clear cell images through autofocusing and to standardize the image color. Next, BM nucleated cells on the digital images were pinpointed, segmented, and identified. The hematopathologists can review the pre-classification results in the device. Lastly, the count of cell classification was autocalculated, whose results were available for reference, modification, and reporting by hematopathologists.
Digitalized smears were acquired from BM smears routinely examined, and an investigator manually chose a suitable analysis region beneath the ×40 objective lens before ×100 objective scanning. The following criteria were used to choose nucleated cell analysis areas: (1) relatively even dispersion of mature erythrocytes and nucleated cells; (2) the smear's body-tail junction, where nucleated cells were located, would be generally independent but not too separated; (3) the smear was colored more standardly with distinctive red-pink and purple-blue [14]. The instrument was operated by trained investigators with the necessary training and credentials following the instrument's standard operating procedure. The entire workflow was illustrated in Figure 1.
2.4 Cell Classification
First, the device automatically processed and reported the pre-classification of cells that were obtained from the chosen region; the data were examined by a hematopathologist, and it was rectified for different cell types that the system had wrongly or unidentifiably designated. With the aim of assessing the instrument's efficacy in pre-classifying nucleated cells within BM aspirate smears, traditional manual microscopic cell counting was adopted as the gold standard (control). The results from manual microscopy were reviewed by two hematopathologists: one for the initial review and the other for the final review. If inconsistent results are found between two hematologists, a third hematopathologist will be added to discuss and reach a consensus conclusion. Subsequently, linear regression was performed to assess the correlation between pre-classification and manual results of various pediatric hematological disease types. Moreover, the results which were pre-classified by Morphogo were also reviewed by the initial hematopathologist and checked and reported by the final hematopathologist. A confusion matrix for cell categorization was performed to compute the consistency between pre-classification and audited data in the device.
2.5 Statistical Analysis
The numerical variables were shown as mean ± standard deviation (SD), and the Pearson correlation test was applied to calculate the correlation coefficient. The p value less than 0.05 indicated the presence of a statistically significant difference. The software used for all the analyses was Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA, USA), Python 3.6.5 (Python Software Foundation), and Pycm 2.1 libraries (IBM, Chicago, IL, USA).
3 Results
3.1 Performance of BM Nucleated Cell Classification
Table 1 displays the cell classification confusion matrix that was produced by Morphogo and manual review. The columns represent the AI-predicted outcome, while the rows reflect the actual categorization. Correctly and wrongly categorized observations are separately represented by diagonal and off-diagonal cells. The morphological classification results of nucleated cells, which had been meticulously reviewed by hematopathologists, were adopted as the final standard results for calculating the consistency of the pre-classification results of BM nucleated cells. Overall, the device's morphological pre-classification accuracy of blasts, promyelocytes, neutrophilic myelocytes, neutrophilic metamyelocytes, neutrophilic granulocyte band forms, neutrophilic granulocyte segmented forms, eosinophilic myelocytes, basophilic myelocytes, erythrocytoblasts, lymphocytes, monocytes, plasma cells, and other cells in BM aspirate smears was over 87.8% (Table 1).
| Morphogo pre-classification | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Blasts | Promyelocytes | Neutrophilic myelocytes | Neutrophilic metamyelocytes | Neutrophilic granulocyte band forms | Neutrophilic granulocyte segmented forms | Eosinophilic myelocytes | ||||
| Manual review | Total | 10 360 | 6470 | 5266 | 5808 | 7348 | 10 133 | 1246 | ||
| Blasts | 0 | 16 191 | 9878 | 3022 | 25 | 15 | 3 | 0 | 3 | |
| Promyelocytes | 1 | 3058 | 146 | 2794 | 46 | 0 | 0 | 0 | 23 | |
| Neutrophilic myelocytes | 2 | 4824 | 3 | 95 | 4652 | 11 | 1 | 0 | 20 | |
| Neutrophilic metamyelocytes | 3 | 5600 | 1 | 11 | 329 | 5138 | 9 | 0 | 35 | |
| Neutrophilic granulocyte band forms | 4 | 7836 | 0 | 9 | 10 | 445 | 7242 | 11 | 69 | |
| Neutrophilic granulocyte segmented forms | 5 | 10 184 | 2 | 9 | 0 | 2 | 15 | 10 025 | 15 | |
| Eosinophilic myelocytes | 6 | 1038 | 0 | 4 | 2 | 0 | 0 | 1 | 1029 | |
| Basophilic myelocytes | 7 | 119 | 0 | 4 | 0 | 0 | 0 | 0 | 1 | |
| Erythrocytoblasts | 8 | 16 326 | 83 | 63 | 13 | 17 | 0 | 3 | 3 | |
| Lymphocytes | 9 | 18 068 | 148 | 172 | 45 | 37 | 0 | 1 | 2 | |
| Monocytes | 10 | 3058 | 45 | 231 | 84 | 49 | 5 | 5 | 29 | |
| Plasma cells | 11 | 619 | 0 | 4 | 0 | 2 | 0 | 0 | 0 | |
| Other cells | 12 | 12 179 | 54 | 52 | 60 | 92 | 73 | 87 | 17 | |
| Morphogo pre-classification | Pre-classification accurate quantity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Basophilic myelocytes | Erythrocytoblasts | Lymphocytes | Monocytes | Plasma cells | Other cells | |||||
| Manual review | Total | 190 | 17 359 | 19 295 | 2796 | 1463 | 11 366 | |||
| Blasts | 0 | 16 191 | 3 | 234 | 2419 | 90 | 139 | 360 | 9878 | |
| Promyelocytes | 1 | 3058 | 1 | 16 | 0 | 2 | 29 | 1 | 2794 | |
| Neutrophilic myelocytes | 2 | 4824 | 3 | 1 | 0 | 27 | 8 | 3 | 4652 | |
| Neutrophilic metamyelocytes | 3 | 5600 | 4 | 0 | 0 | 68 | 2 | 3 | 5138 | |
| Neutrophilic granulocyte band forms | 4 | 7836 | 6 | 5 | 0 | 35 | 1 | 3 | 7242 | |
| Neutrophilic granulocyte segmented forms | 5 | 10 184 | 3 | 13 | 1 | 3 | 1 | 95 | 10 025 | |
| Eosinophilic myelocytes | 6 | 1038 | 1 | 0 | 0 | 0 | 0 | 1 | 1029 | |
| Basophilic myelocytes | 7 | 119 | 114 | 0 | 0 | 0 | 0 | 0 | 114 | |
| Erythrocytoblasts | 8 | 16 326 | 0 | 15 944 | 1 | 2 | 130 | 67 | 15 944 | |
| Lymphocytes | 9 | 18 068 | 11 | 941 | 16 315 | 69 | 284 | 43 | 16 315 | |
| Monocytes | 10 | 3058 | 7 | 33 | 3 | 2484 | 77 | 6 | 2484 | |
| Plasma cells | 11 | 619 | 0 | 2 | 0 | 1 | 610 | 0 | 610 | |
| Other cells | 12 | 12 179 | 37 | 170 | 556 | 15 | 182 | 10 784 | 10 784 | |
| 0.877991927 | ||||||||||
- Note: The green highlighted numbers (shading) represent the situation where the pre-classification results are consistent with manual review.
Furthermore, a detailed illustration of Morphogo's performance in classifying BM nucleated cells was shown in Table 2. Notably, the accuracy of blasts was the lowest, reaching only 61.8%, which clearly demonstrated a certain disparity between Morphogo's classification of blasts and that of manual review. In contrast, several cell types exhibited an accuracy rate exceeding 95%, namely neutrophilic myelocytes (96.3%), neutrophilic granulocyte segmented forms (98.3%), eosinophilic myelocytes (99.2%), basophilic myelocytes (96.5%), erythrocytoblasts (97.5%), and plasma cells (98.2%), which indicated that Morphogo's recognition of these cell types was highly comparable to the outcomes of manual review.
| Class | Sensitivity, % | Accuracy, % | Specificity, % | False positive rate, % | False negative rate, % |
|---|---|---|---|---|---|
| Blasts | 87.4 | 61.8 | 81.1 | 7.2 | 12.6 |
| Promyelocytes | 42.5 | 86.4 | 10.4 | 0.5 | 57.5 |
| Neutrophilic myelocytes | 88.2 | 96.3 | 22.5 | 0.2 | 11.8 |
| Neutrophilic metamyelocytes | 88.8 | 91.6 | 42.2 | 0.5 | 11.2 |
| Neutrophilic granulocyte band forms | 98.6 | 92.7 | 84.3 | 0.6 | 1.4 |
| Neutrophilic granulocyte segmented forms | 98.9 | 98.3 | 61.9 | 0.2 | 1.1 |
| Eosinophilic myelocytes | 81.2 | 99.2 | 3.5 | 0.0 | 18.8 |
| Basophilic myelocytes | 59.9 | 96.5 | 5.2 | 0.0 | 40.1 |
| Erythrocytoblasts | 91.9 | 97.5 | 22.4 | 0.5 | 8.1 |
| Lymphocytes | 84.9 | 87.0 | 45.5 | 3.0 | 15.1 |
| Monocytes | 88.6 | 80.9 | 64.9 | 0.6 | 11.4 |
| Plasma cells | 40.5 | 98.2 | 1.2 | 0.1 | 59.5 |
| Other cells | 95.1 | 86.9 | 74.8 | 1.7 | 4.9 |
| Macroaverage | 80.5 | 90.2 | 40.0 | 1.2 | 19.5 |
In addition, the sensitivities of all classes of BM nucleated cells were above 40.5%, with an average of 80.5% and a range spanning from 40.5% to 98.9%. The specificity, however, varied significantly among different cell types, ranging from a mere 1.2% for plasma cells to 84.3% for neutrophilic granulocyte band forms, with an average of 40.0%. The average false positive rate was 1.2%, fluctuating between 0.00% and 7.2%. The false negative rate also showed substantial variation depending on the cell type, ranging from 1.1% for neutrophilic granulocyte segmented forms to 59.5% for plasma cells (Table 2).
3.2 Comparison Between Morphogo and Manual Classification of BM Nucleated Cells in Pediatric Hematological Diseases
In order to more intuitively demonstrate the advantages of Morphogo in reducing time costs and improving work efficiency, a comparison was made between Morphogo and manual microscopy regarding the time needed for the classification of BM nucleated cells in different disease types (Table 3). The results revealed that the total time consumed by Morphogo in classifying BM nucleated cells was remarkably shorter than that of manual microscopy (p < 0.001, Table 3), which evidently demonstrated that Morphogo was capable of significantly enhancing the classification efficiency of nucleated cells in BM smears.
| Manual microscopy | Morphogo | p | |||||
|---|---|---|---|---|---|---|---|
| Disease type | Initial inspection time (min) | Final review time (min) | Total time (min) | Pre-classification time (min) | Final review time (min) | Total time (min) | Total time |
| Immunologic thrombocytopenic purpura | 30.3 ± 4.0 | 11.3 ± 1.2 | 41.6 ± 4.6 | 10.5 ± 1.2 | 7.1 ± 0.9 | 17.6 ± 1.5 | < 0.001 |
| Hyperplastic anemia | 29.5 ± 4.3 | 10.4 ± 1.2 | 40.0 ± 4.9 | 10.2 ± 1.3 | 6.7 ± 1.1 | 16.9 ± 1.9 | < 0.001 |
| Acute leukemia | 44.3 ± 7.6 | 11.6 ± 1.5 | 55.9 ± 8.3 | 10.6 ± 1.3 | 8.3 ± 0.8 | 18.6 ± 1.8 | < 0.001 |
| Bone marrow failure | 46.4 ± 7.8 | 11.2 ± 1.3 | 57.6 ± 8.6 | 10.8 ± 1.5 | 8.3 ± 1.2 | 19.1 ± 2.1 | < 0.001 |
| Chronic myeloid leukemia | 43.7 ± 7.5 | 11.7 ± 1.8 | 55.4 ± 8.3 | 10.7 ± 1.2 | 7.9 ± 0.7 | 18.6 ± 1.8 | < 0.001 |
| Others | 45.7 ± 8.0 | 10.4 ± 1.3 | 56.1 ± 8.8 | 10.8 ± 1.2 | 8.3 ± 0.8 | 19.0 ± 1.8 | < 0.001 |
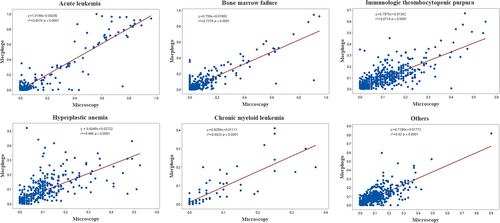
Besides, correlation analysis was used to evaluate the degree of matching between Morphogo and manual proofreading for the classification of BM nucleated cells in pediatric hematological disease samples. As displayed in Figure 2, the results indicated positive correlations between the two approaches for ITP (r2 = 0.6714, p < 0.0001), BMF (r2 = 0.7378, p < 0.0001), hyperplastic anemia (r2 = 0.486, p < 0.0001), AL (r2 = 0.8576, p < 0.0001), CML (r2 = 0.6623, p < 0.0001), and others (r2 = 0.62, p < 0.0001). Linear regression equations are listed below: ITP y = 0.7875x + 0.01362, BMF y = 0.759x + 0.01965, hyperplastic anemia y = 0.6048x + 0.02722, AL y = 0.10198x −0.00206, CML y = 0.8289x + 0.01111, and others y = 0.7186x + 0.01773 (Figure 2).
4 Discussion
The traditional morphological diagnosis method for pediatric hematological diseases requires manual counting and sorting by professional hematopathologists under a microscope, which are labor-intensive, time-consuming, and experience-dependent [15]. Deep learning technology has enormous potential in medical diagnosis, as it can improve the quality, reproducibility, and speed of diagnosis, thereby reducing the workload of hematopathologists [16]. In the field of pathology, the performance of deep learning algorithms in certain tasks has been comparable to that of hematopathologists in diagnosis, providing an effective theoretical basis for the development of hematological disease detection systems [17]. Morphogo, a deep learning-based BM nucleated cell analysis system, was used to compare the traditional manual method to detect the efficiency in pediatric hematological diseases in this research.
Since children are in the stage of growth, their hematopoietic function, BM nucleated cell composition, and physiological changes are significantly different from adults [18]. Clinically, children exhibit a high level of nucleated cell proliferation in their BM, and the granulocyte proportion decreases as the age of the child becomes younger [19]. Due to the unstable hematopoietic function during childhood, excessive proliferation of BM nucleated cells is prone to occur under external stimuli [20]. Accordingly, some hematological diseases are more commonly found in children, such as ALL, juvenile myelomonocytic leukemia, ITP, and neuroblastoma BM metastasis [20, 21]. Therefore, the use of Morphogo seems to be of great significance in the detection of pediatric hematological diseases.
BM nucleated cells mainly comprise erythrocyte lines, granulocyte lines, lymphocyte lines, monocyte lines, plasma cell lines, and megakaryocyte lines [22]. Each cell line has different morphological characteristics at different stages of development [23], thus Morphogo can recognize and pre-classify different types of BM nucleated cells, based on different morphological features after data accumulation and algorithm training [24]. As the results displayed, Morphogo had a high overall accuracy in pre-classifying blasts, promyelocytes, neutrophilic myelocytes, neutrophilic metamyelocytes, neutrophilic granulocyte band forms, neutrophilic granulocyte segmented forms, eosinophilic myelocytes, basophilic myelocytes, erythrocytoblasts, lymphocytes, monocytes, plasma cells, and other cells, and had a high degree of consistency with manual proofreading results (> 87.8%) in 210 pediatric patients' BM aspirate smears. In addition, the average accuracy, sensitivity, and specificity of Morphogo for 13 types of BM nucleated cells were 90.2%, 80.5%, and 40.0%, respectively. It has been reported that CellaVision DM96 automatic digital image analyzer can also perform subtyping of leukocytes, but it is unreliable in identifying granulocytes and plasma cells [25]. In this research, Morphogo demonstrated a high accuracy in identifying normal BM nucleated cells, proving its effectiveness and feasibility in recognizing nucleated cells in BM smears.
It is noteworthy that the pre-classification accuracy of blasts in this study was 61.8%, far lower than that of other BM nucleated cell types. This discrepancy stems mainly from the morphological complexity and cellular heterogeneity of blasts, plus subjectivity in data annotation [26]. First, the morphological features of various blast types exhibit high variability, including but not limited to differences in nuclear morphology (round, indented, folded) among subtypes, variations in chromatin structure (fine or coarse) and nucleoli (prominent or indistinct), and cytoplasmic characteristics. These variations pose challenges for blast cell classification [27]. Second, hematopathologists may disagree on blast definitions (e.g., the divergence between blasts and abnormal promyelocytes) [28], and inconsistent training data annotation can lead models to learn flawed features, amplifying discrepancies of Morphogo in BM nucleated cell classification. Therefore, future research should optimize algorithms to improve classification accuracy through multimodal AI, such as integrating morphological, flow cytometry, and molecular genetics data. Additionally, it is also necessary to optimize human-machine collaboration workflows. For example, Morphogo should output confidence scores, with cases of low confidence automatically referred for manual review.
Furthermore, the morphological characteristics of BM nucleated cells are crucial in the morphological diagnosis [29], and the cell morphological characteristics vary among different types of hematological diseases and age of patients [30]. By analyzing the morphological characteristics of BM nucleated cells, hematopathologists can make a preliminary judgment on whether patients may have hematological diseases, providing a basis for further examination and diagnosis. The results of our study proved that Morphogo could reduce the time spent on classifying BM nucleated cells, and there were positive correlations between the Morphogo automatic classification and manual categorization for ITP, BMF, hyperplastic anemia, AL, CML, and other hematological diseases. The coefficient of determination r2 is a measure of the goodness of fit of a regression model, ranging from 0 to 1 [31]. The closer r2 is to 1, the better the model fits, meaning that a high r2 value indicates a small difference between the predicted and actual observed values [32]. Specifically, the r2 value of hyperplastic anemia was less than 0.5, which implied a significant difference between the predicted results of the model and the results of manual examination. Besides, the r2 value of ITP, BMF, hyperplastic anemia, CML, and other diseases is between 0.6 and 0.8, indicating that the difference between Morphogo and manual examination results is relatively small in these diseases. In addition, the r2 value of AL is greater than 0.8, denoting that the system's classification of AL is very close to the results of manual examination. According to the results of linear regression and confusion matrix, we found that these two parts of the results are mutually consistent. The above results suggested that the application of Morphogo can provide necessary assistance for the classification and subtyping of BM nucleated cells in pediatric hematological diseases.
According to the results, the consistency of different pediatric hematological diseases varies. Specifically, the consistency of hyperplastic anemia is lower than that of the other diseases, which may be due to the diversity of anemia types and the significant differences in anemia cells. For example, MA is a type of large cell anemia, and all three lineages of the BM show obvious megaloblastic changes [33]. These differences have led to a decrease in pre-classification accuracy. For the purpose of improving the accuracy of detecting atypical cells in anemia and related diseases, a larger number of anemia samples will be collected in future studies.
ITP refers to a hemorrhagic disease characterized by acquired, immune, isolated thrombocytopenia [34]. In the absence of obvious IDA, the cell morphology and the proportion of each cell lineage in ITP are generally normal, leading to relatively high consistency [34]. However, since there are more types of nucleated cells in ITP than in AL samples, its consistency is lower than that of AL. In addition, this study is currently confined to the research of BM nucleated cells in pediatric hematological diseases, and megakaryocytes were not included in the statistical results of this study. In fact, the morphology of megakaryocytes is vital for the diagnosis of various diseases, such as ITP, MDS, and so forth. Therefore, in future research, classification and morphological analysis of megakaryocytes will be added to make the AI recognition system more comprehensive and the results more reliable.
BMF is a pathological condition in which the BM's hematopoietic function is severely compromised, with a morphological manifestation of decreased hematopoietic cells [35]. In this study, BMF pertains to autoimmune abnormalities mediated by AA, immune-associated pancytopenia, and MDS cases. When the number of hematopoietic stem cells and precursor cells is significantly diminished, resulting in overall hematopoietic reduction, as seen in the decreased erythroid, granulocyte, and megakaryocyte lineages, the type and number of BM nucleated cells also decline [36]. Concurrently, the varieties, quantities, and complexity of nucleated cells are markedly diminished, and the proportion of non-hematopoietic cells such as mature lymphocytes and plasma cells is relatively elevated [37]. Due to the low count and relatively normal cellular morphology of nucleated cells in BM, the AI recognition of BMF demonstrates good performance and high consistency, as evidenced by the current study's results. To obtain more reliable findings, more samples should be collected and analyzed in future research. Additionally, the scanning area could be adjusted and expanded to increase the number of nucleated cells scanned at 100× magnification in BMF patients. However, in some children with MDS-related BMF, the quantity of BM nucleated cells does not decrease, yet morphological dysplasias are observed across all cell lineages [26]. In the granulocyte lineage, a reduced or abnormally enlarged cytosol, nuclear hyposegmentation (Pelger-Huet-like aberration), or pseudo Chediak-Higashi granules and Auer vesicles are readily detectable. In the megakaryocyte lineage, nuclear hyposegmentation, multinucleated megakaryocytes, and small-sized megakaryocytes frequently appear in BM aspirate smears [26]. According to the World Health Organization's quantitative criterion, a cell lineage is considered dysplastic when the proportion of cells with morphological abnormalities exceeds 10%. However, the AI appears to lack relevant experience. Therefore, it is crucial to increase the MDS sample size in subsequent studies to validate the Morphogo's effectiveness for BMF. This will enable the AI to identify dysplastic cells more accurately and quantify them, thus enhancing clinical work efficiency.
Moreover, remarkable concordance between Morphogo and manual results was achieved in AL cases, which holds significant importance for the early detection and treatment of childhood tumors. Additionally, the number of CML cases is relatively limited compared to other disease cases, and as this disease predominantly afflicts the middle-aged and elderly population, with a low incidence rate among children [38]. Owing to the restricted sample size of CML in this particular study, we will enlarge the sample number in the forthcoming research endeavors, thereby aiming to enhance and refine the study to a greater extent.
Other diseases pertain to non-hematological disorders with peripheral hemogram alterations, mainly including leukopenia or leukocytosis, lymphadenopathy, and febrile compensation [39, 40]. In most instances, BM hyperplasia is normal or active, and morphological abnormalities are predominantly due to infections and develop in the short term, such as the presence of toxic granules and vacuoles in neutrophils, an increase in atypical lymphocytes, and the appearance of hemophagocytes [41, 42]. Given that all cell lineages are present in significant numbers in the BM of these diseases, along with diverse morphological changes simultaneously, their consistency is lower than that of other disease types, except for hyperplastic anemia. Future system enhancements will concentrate on strengthening recognition training for atypical lymphocytes, cellular toxicity changes, and other atypical cells.
To conclude, the Morphogo system is capable of accurately and rapidly classifying different types of BM nucleated cells, and its results demonstrate a high degree of concordance with those of hematopathologists. Therefore, it holds the potential to offer early screening for pediatric hematological diseases, with the objective of enhancing the diagnostic efficiency in pediatric hematology. Moreover, large-scale multi-center validation studies that are now underway will offer further details to support the system's clinical usefulness.
Author Contributions
Conceptualization: Xin He and Haiyan Gao. Methodology: Fei He and Mingrui Yu. Data curation: Yan Wang, Yu Liu, and Xiaopeng Gao. Writing – original draft: Xin He. Writing – review and editing: Xin He and Haiyan Gao.
Ethics Statement
The BM aspirate smears from 213 retrospective pediatric patients (with an average age of 17 ± 0.7 years) at Harbin Medical University Affiliated Sixth Hospital from October 2023 to June 2024 were masked, and this study was approved by the Ethical Committee of Harbin Medical University Affiliated Sixth Hospital (LC2024-089).
Consent
All patients who participated signed a written informed consent form.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.