Paediatric Reference Intervals and Curves for Haemoglobin Estimated Using Direct Methods: A Systematic Review and Meta-Analysis
Funding: This work was supported by Natural Sciences and Engineering Research Council of Canada, Government of Canada (grant number: RGPIN-2018-06693).
ABSTRACT
Introduction
Haemoglobin is a commonly ordered laboratory test, used to assess both individual and population-level health. To interpret test results, laboratories provide reference intervals (RIs) with lower (2.5th%) and upper (97.5th%) limits according to age and sex. Reference curves (RCs) treat age as a continuous variable. The objectives were to synthesise evidence on Paediatric haemoglobin RIs/RCs and investigate possible sources of heterogeneity. We placed our findings in the context of the age- and sex-based haemoglobin thresholds to define anaemia, recommended for international use by WHO.
Methods
We conducted a systematic review of studies publishing Paediatric haemoglobin RIs/RCs (PROSPERO: CRD42023399802). EMBASE, MEDLINE, SCOPUS and The Cochrane electronic libraries were searched from inception to July 31, 2023. Studies involving unhealthy children, lacking males and females RIs/RCs, or limited to cord-blood RIs/RCs were excluded. Studies adhering to guidelines for RIs development from the Clinical Laboratory Standards Institute (CLSI) and RCs studies reporting confidence intervals (CIs) were included in the meta-analysis. Lower and upper males and females RI limits were pooled for age groups with heterogeneity I2 < 75%. All studies meeting eligibility criteria were included in the narrative synthesis. Sources of heterogeneity were analyzed using heatmaps, forest plots and Shiny app.
Results
Of 9123 studies screened, 177 were retained for full-text review. We identified 48 eligible studies (63 529 male and 59 969 female participants) from 25 countries (4 continents) published 1938–2023. There was inconsistency in age partitioning and length of age intervals. Meta-analysis was conducted on 13 studies reporting RIs and 2 studies reporting RCs. Pooled estimates for the 0–3 months age group could not be generated for males or females due to paucity of data. For children aged 3 months or older, both lower and upper RI limits generally increased with age, from approximately 100 to 130 g/L and from approximately 130 to 150 g/L, respectively. For visualisation of our narrative synthesis of all 48 studies, we created a novel web-based computational tool using Shiny-app. Sources of heterogeneity included child age, sex, analyser type and country. For many studies, the lower RIs were substantially different from WHO anaemia thresholds. Study limitations include a small sample size for younger age groups, potentially impacting heterogeneity estimates, reliance on CLSI guidelines due to the lack of a suitable quality assessment tool for RIs/RCs and restriction to English-language studies.
Conclusion
Evidence synthesis of locally developed Paediatric haemoglobin RIs/RCs revealed substantial heterogeneity, suggesting the need for more rigorously developed estimates that may be used globally along with WHO thresholds to define anaemia. Future research is needed on RIs for the youngest children. Percentile curves should be explored to provide continuous haemoglobin charts.
1 Introduction
Haemoglobin is one of the most commonly ordered laboratory tests used to assess individual and population health status globally [1]. Decreased haemoglobin levels may indicate anaemia, a prevalent condition with numerous aetiologies that, if untreated, may lead to poor short-term and long-term health outcomes [2].
Laboratory tests play a crucial role in clinical decision-making for individual patients as well as in population-based surveillance and policy for public health practitioners. Meaningful interpretation of laboratory test results requires an accompanying range of values, calculated from a healthy reference population. These values are commonly referred to as reference intervals (RIs) [3]. It is essential to establish accurate and reliable RIs, which often involves providing specific RIs for different sub-groups (termed partitions), such as age and sex, when appropriate [4]. Interpretation of laboratory results to determine the presence of high-risk or disease requires an accompanying single threshold, known as a clinical decision limit (CDL) [3]. CDLs may be derived from clinical outcome studies, guidelines and expert consensus.
The International Federation of Clinical Chemistry and Laboratory Medicine Committee on Reference Intervals and Decision Limits has developed the concepts of RIs and CDLs in laboratory medicine [3]. The Clinical Laboratory Standards Institute (CLSI) develops laboratory standards [4].
CLSI provides guidelines for establishing RIs, including the selection of an apparently healthy reference population and minimum sample size requirements [4]. Two RI limits (lower and upper) are calculated using recommended statistical methods (parametric, non-parametric or robust methods). The lower limit represents the 2.5th percentile, and the upper limit represents the 97.5th percentile of the distribution (with corresponding 90% confidence intervals) in a healthy reference population. The choice of appropriate statistical methods for estimating RIs is crucial to the accuracy and precision of RIs, and it is important that researchers carefully examine the distribution of the analytes [4]. In addition to the CLSI guideline, recommendations for choosing appropriate methods are available in the literature [5]. Reference curves (RCs), which treat age as a continuous variable, avoid the need for extensive age partitioning and address problems arising from insufficient sample sizes and dynamic physiological changes [5]. Nevertheless, their practical application in laboratory and clinical settings is limited. To our knowledge, there is no clinical or methodological guideline (similar to RIs) available for estimating RCs.
Developing RIs for paediatric populations is particularly challenging because many biomarkers show dynamic changes as children grow from birth through adolescence; thus, narrower sex-specific age partitions may be needed to reflect their age, physiological changes and developmental stages [5]. Many studies struggle to achieve sufficient sample sizes for precise RIs in paediatric populations, especially considering the difficulty of acquiring blood from younger children [5]. Despite efforts over the last several decades to establish high-quality RIs for paediatric biomarkers, they remain highly inconsistent [6].
RIs for haemoglobin should be derived from apparently healthy populations [4]. However, unrecognised nutritional and genetic factors may cause geographic variation, especially affecting the lower reference limit used for anaemia assessment [7]. The World Health Organization (WHO) recently updated haemoglobin thresholds to define anaemia based on the 5th percentile of the haemoglobin distribution from a pooled international healthy reference sample [1, 8]. This single threshold represents a CDL. The WHO views this as “an opportunity for global harmonisation of haemoglobin thresholds to define anaemia across countries, clinical guidelines and diagnostic laboratories” [8]. This presents a dilemma for clinicians and laboratories: should they use the lower RI limit from a local reference population or the single WHO threshold? Understanding the global variation in RIs for haemoglobin may help to address this dilemma.
In this study, we conducted a systematic review and meta-analysis of available evidence to identify and synthesise (where possible) RIs and RCs for haemoglobin in healthy paediatric populations. We also examined heterogeneity across studies and identified potential sources of variation in RIs and RCs.
2 Methods
The protocol for our systematic review has been published and registered with PROSPERO (CRD42023399802) [9]. We reported the results according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10].
2.1 Search, Screening and Data Extraction
The search strategy was developed in consultation with a librarian. We searched major medical databases, MEDLINE, EMBASE, SCOPUS and the Cochrane Library from inception to search date (July 31, 2023). We also searched the reference lists of included studies. We included studies that involved a healthy paediatric population (< 18 years), provided RIs or/and RCs for haemoglobin and were published in English. We excluded studies that did not report separately male or female RIs or RCs, studies reporting only cord-blood RIs or RCs and studies using indirect methods. When multiple studies used the same database, only the study with the largest sample size, or the most recent study if sample sizes were identical, was included in the systematic review. The full search strategy is available in Supporting Information (search strategies.docx).
Two reviewers (V.B., M.B.) independently performed the first and second stages of screening; discrepancies were resolved by discussion. Two team members (J.L., M.P.) extracted data, which was verified by V.B. Study details (e.g., year and country), information on age partitioning and other methods (e.g., the analyser used, the statistical methods) and the reported lower and upper RI limits for each partition with their corresponding confidence intervals (CIs) were extracted from each study. CIs for the parametric RI method, when not provided, were calculated using the formula provided by Solberg [11]. When the equations representing the RCs were provided, the mid-point of pre-specified age partitions was used to calculate RIs (from the RCs).
2.2 Risk of Bias Assessment
There is no established risk of bias (RoB) tool for RIs/RCs; therefore, we were unable to perform a formal risk of bias assessment. Instead, we examined whether or not studies adhered to the CLSI guidelines, which are considered a gold standard for estimating RIs. Two reviewers (V.B., M.B.) independently reviewed each study using CLSI criteria for developing RI (outlier detection, calculations/reporting of CIs for RIs, use of the recommended statistical methods for estimation of RIs and data partitioning). It is worth mentioning that CLSI guidelines were first published in 2008 using Solberg (1987)-approved recommendations on RIs [11]. Publication bias, which is concerned about the possibility of negative or null results not being published, is not applicable to this systematic review, which synthesised studies estimating RIs and RCs.
2.3 Evidence Synthesis
Data was synthesised using descriptive statistics and meta-analysis. Only RIs from studies that adhered to the CLSI guidelines and RCs that provided CIs were included in the meta-analysis. To overcome the challenges associated with different age partitions being used by different studies, we employed a novel strategy whereby we created standardised age partitions. We used 3-month age ranges for children below 3 years and 1-year age ranges for children above 3 years. We defined terms “within range” (study age partition corresponded to or was fully embedded within our standardised age partitions) and “out of range” (study age partition was wider than standardised age partitions) to indicate the extent to which a study's age partition corresponded to our standardised age partition. We used this information to study age partitioning as a source of heterogeneity in the evidence synthesis of haemoglobin RIs and RCs. For each age partition, we performed meta-analyses separately for males and females and for lower and upper RI limits. Heterogeneity was studied graphically using forest plots, using the I2 statistic and displayed on a heatmap. Pooled estimates of haemoglobin RIs were calculated only when I2 ≤ 75. Random effects meta-analysis was used to account for heterogeneity.
Due to high heterogeneity in methods and results across studies, to complement and extend the meta-analysis, we produced a narrative synthesis of all reviewed studies, inclusive of those for which meta-analysis was not possible. We examined the distribution (using the 10th and 90th percentile) of the lower and upper RI limits across all included studies and provided comparative evaluations of the lower RI limits in relation to the recently published age-specific WHO thresholds for haemoglobin [1]. To facilitate visual evaluation of heterogeneity with respect to the many factors our systematic review revealed, including regional and methodological differences, and to overcome the challenge we faced in having too many partitions, we developed a novel web-based graphical and computational tool (Paediatric Reference Intervals and Curves Evidence Synthesis—Haemoglobin [PRINCES-H] tool). The web-based tool allows investigation of heterogeneity of RIs by selecting specific age groups either using WHO or standardised age partitions, choosing different geographic regions (Asia, Africa, North America, Europe) or choosing if the study adhered to CLSI guidelines. It provides dynamic visualisations, stratified by age, sex, region and adherence to the CLSI guidelines, together with detailed numerical summaries, including medians, interquartile ranges (IQRs) and CIs. The tool highlights sex-specific differences, particularly during critical growth periods such as adolescence, and enables comparisons of RIs across multiple studies to assess variability in reporting. Additionally, users can input specific haemoglobin values for benchmarking against reported RIs. This tool serves as a practical resource for visualising and analysing heterogeneity of haemoglobin RIs, facilitating understandings of regional and methodological differences.
All analyses were performed using the R statistical software version 4.3.1; the metafor package and the R Shiny App were used for meta-analysis and developing the web-based tool [12].
2.4 Patient and Public Involvement
No patients or members of the public were involved in this systematic review.
2.5 Deviations From the Published Protocol
In the development of our protocol, we planned to use the Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies to assess RoB. However, after further evaluation, the tool was deemed not suitable for RoB assessment of RIs and RCs. As per our protocol, we considered using both fixed and random effects meta-analysis. However, we encountered a high level of heterogeneity across the studies; hence, we decided to present (and interpret) the pooled estimates from the random effects models, as it accounts for heterogeneity. Nevertheless, in the forest plots, we still provided pooled estimates from both the fixed effects and the random effects models, mainly to abide by our protocol for completeness of reporting.
3 Results
The search yielded 14 897 citations, of which 5774 were duplicates. A total of 9123 titles and abstracts were screened at the first stage, 177 studies were retained for full-text review and 48 studies [13-60] met all eligibility criteria and were included in our final systematic review (Figure 1, Table S1).
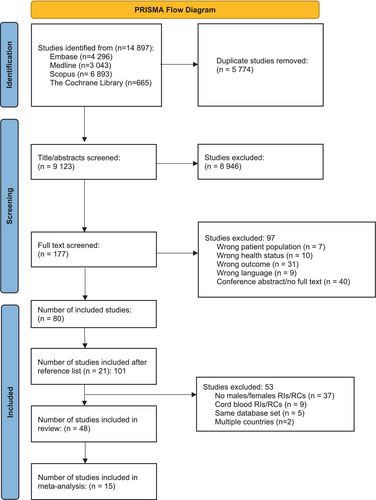
Estimates for the lower and upper limits for RIs from 13 studies [13-15, 18, 24, 25, 27, 29, 32, 33, 36, 38, 39] that adhered to CLSI guidelines and 2 studies reporting RCs [16, 22] that provided CIs for lower and upper limits were considered in the meta-analysis (Table S2) and pooled when appropriate. All 48 studies were included in the narrative synthesis as well as in the graphical examination of heterogeneity.
The publication year for the included studies ranged from 1938 to 2023. The geographic distribution covered Africa (n = 14, 29.2%), Asia (n = 12, 25.0%), Europe (n = 9, 18.8%) and North America (n = 13, 27.1%) (Table 1). The most commonly used analysers were the Sysmex, Beckman–Coulter and Abbott models. Forty-four (91.7%) studies provided RIs; of these, 21 (47.7%) used the non-parametric method for estimating RIs, 18 (40.9%) used the parametric method and 5 (11.4%) used the robust method. Four of the 48 studies (8.3%) provided RCs, of which 3 used the Lambda Mu and Sigma method and one used quantile regression. Additional summary statistics are provided in Table S1.
| Characteristicsa | Number of studies, n (%)a | Number of children (sample size) |
|---|---|---|
| Total | 48 | 123 498 |
| Sex | ||
| Male | 48 (100) | 63 529 (51.4) |
| Female | 47 (97.9) | 59 969 (48.6) |
| Regions | ||
| Africa | 14 (29.2) | 5843 (4.7) |
| Asia | 12 (25.0) | 66 038 (53.5) |
| Europe | 9 (18.8) | 6410 (5.2) |
| North America | 13 (27.1) | 45 207 (36.6) |
| Interval type | ||
| Discrete reference interval | 44 (91.7) | 68 361 (55.4) |
| Reference curve | 4 (8.3) | 55 137 (44.6) |
| Estimation method | ||
| Reference intervals | ||
| Non-parametric | 21 (47.7) | 28 555 (41.8) |
| Parametric | 18 (40.9) | 15 269 (22.3) |
| Robust | 5 (11.4) | 668 (1.0) |
| Other | 5 (11.4) | 23 869 (34.9) |
| Reference curves | ||
| Lambda Mu and Sigma method | 3 (75.0) | 54 641 (99.1) |
| Quantile regression | 1 (25.0) | 496 (0.9) |
| Analyzer models | ||
| Abbott | 3 (6.2) | 1643 (1.3) |
| Beckman Coulter | 12 (25.0) | 40 812 (33.0) |
| Horiba | 2 (4.2) | 1091 (0.9) |
| Mindray | 2 (4.2) | 780 (0.6) |
| Ortho | 1 (2.1) | 404 (0.3) |
| Sysmex | 20 (41.7) | 31 535 (25.5) |
| Other | 4 (8.3) | 4063 (3.3) |
| Not provided | 5 (10.4) | 43 170 (35.0) |
| Adhered to CLSI guidelinesb | 13 (29.5) | 36 160 (52.9) |
| Outlier detection | 22 (50.0) | 40 881 (59.8) |
| Partitioning | 42 (95.5) | 68 108 (99.6) |
| Method for RIs estimation | 32 (72.7) | 59 772 (87.4) |
| Estimation of CIs for RIs | 32 (72.7) | 60 968 (89.2) |
- a Does not add up to 100%.
- b Only applicable to RIs (n = 44 studies).
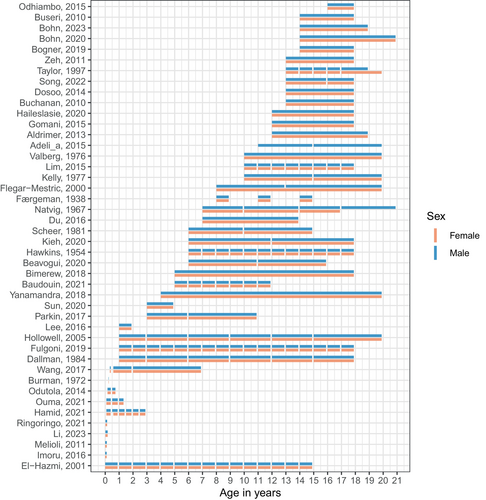
We observed inconsistencies in age partitioning across studies, for both males and females (Figure 2). The length of age intervals also varied considerably, ranging from less than one month (e.g., birth to 4 days) to as wide as 16 years (e.g., 4 years to 20 years) for both sexes.
3.1 Meta-Analysis
Sixteen of the 48 (33.3%) studies were included in the meta-analysis. Fourteen RI studies followed all four key recommendations from the CLSI guidelines, and two RC studies provided CI for lower and upper limits [4]. The most common reasons for considering a study to be non-adherent to CLSI guidelines were lack of adherence to recommendations related to outlier detection (n = 22, 50%) and calculations/reporting of CIs for RIs (n = 12, 27.3%), followed by use of other than the recommended statistical methods for estimation of RIs (n = 11, 25%) and data partitioning (n = 2, 4.5%). One study [36] with missing CIs reported using the parametric method; hence, we were able to calculate CIs for this study (Tables 1 and S2). The pooled estimates for the lower and upper limits by age and sex partition, with the corresponding 90% CIs, are provided in Table 2. The corresponding forest plots are provided in Figures S1–S24.
| Age partitionsa | Pooled lower RI limit (90% CI) | Pooled upper RI limit (90% CI) | ||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| 0–3 months | b | b | b | b |
| 3–6 months | b | 102.3 (98.2–106.4) | 133.1 (123.0–143.1) | 132.9 (122.8–143.1) |
| 6–9 months | 98.6 (94.8–102.4) | b | 132.3 (123.6–141.0) | b |
| 9–12 months | 100.7 (95.3–106.1) | 99.0 (93.5–104.5) | 133.1 (120.8–145.3) | b |
| 12–15 months | b | b | b | b |
| 15–18 months | b | b | b | b |
| 18–21 months | 103.7 (99.9–107.4) | 104.6 (101.2–108.0) | b | b |
| 21–24 months | 104.9 (100.7–109.1) | b | b | b |
| 24–27 months | b | b | b | b |
| 27–30 months | b | b | b | b |
| 30–33 months | b | b | b | b |
| 33–36 months | b | b | b | b |
| 3–4 years | b | b | b | b |
| 4–5 years | b | b | b | b |
| 5–6 years | b | b | b | b |
| 6–7 years | 113.4 (111.4–115.4) | b | b | b |
| 7–8 years | b | b | b | b |
| 8–9 years | 114.4 (112.1–116.7) | b | b | b |
| 9–10 years | b | b | b | b |
| 10–11 years | b | b | b | b |
| 11–12 years | b | 116.5 (112.9–120.1) | b | b |
| 12–13 years | 122.0 (120.5–123.5) | b | b | b |
| 13–14 years | 124.1 (122.8–125.4) | b | b | b |
| 14–15 years | 126.0 (124.8–127.3) | b | b | b |
| 15–16 years | 128.5 (127.1–130.0) | b | b | b |
| 16–17 years | 130.7 (129.1–132.2) | b | b | 152.2 (150.6–153.7) |
| 17–18 years | 131.7 (130.2–133.3) | b | b | 152.2 (150.6–153.8) |
- a Represents the standardised age partitions we used in our systematic review.
- b Pooling was not possible because of highly significant heterogeneity I2 > 75%.
As shown in Table 2, Figures 3 and S1–S24, we were able to pool the RI estimates for only a limited number of partitions. This was mainly because of the high level of heterogeneity (I2 > 75), which is displayed using a heatmap presented in Figure 3. There was a paucity of data for children aged 0–3 months (Figures S1–S4), and a pooled estimate could not be generated for males or females in this subgroup. Lower limits of the RIs increased with age from 3 months onwards (from approximately 100 to 130 g/L) (Table 2). Upper limits also increased with age from 3 months onwards (from approximately 130 to 150 g/L).
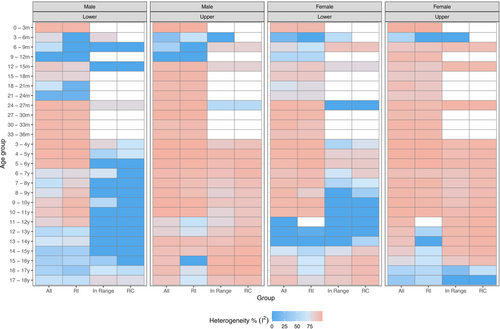
In general, lower heterogeneity (I2 ≤ 75%, dark and light blue on the heatmap) was found for males (compared with females), older age partitions with 1-year intervals (compared with younger age partitions with 3-month intervals), narrower age partitions (compared with wider age partitions), “within range” age partitions (compared with “out of range” age partitions) and lower RI limits (compared with upper RI limits) (Figure 3). When heterogeneity could not be calculated, due to an insufficient number of studies (n < 2), cells were left blank on the heatmap.
3.2 Narrative Synthesis
Detailed descriptions of the full set of 48 studies are provided in Table S1. The distributions of the RIs for all age partitions, separately for males and females, are presented in the forest plots and descriptive statistics provided in Figures S1–S24, Table S3, where regional differences are also highlighted. A more comprehensive visualisation of the RIs is provided using our novel web-based PRINCES-H tool (https://svetric.shinyapps.io/princesH/) where the user can specify age group, region and adherence to the CLSI guidelines. The age-specific haemoglobin thresholds published by WHO as well as the 10th and 90th percentiles of RIs across the studies are displayed in figures generated by our tool. The tool also provides a summary narrative synthesis based on the user-specified features. An illustrative example based on one scenario is provided in Figure S25, Table S3.
Based on the set of 48 studies, for children aged 0–6 months, the lower RI limits ranged from 85 g/L (males) and 86 g/L (females) [19] to 147 g/L (males) and 142 g/L (females) [45]. The upper RI limits in this age partition ranged from 117.4 g/L (males) and 116.6 g/L (females) [57] to 224 g/L (males) and 227 g/L (females) [45] (Figures S26 and S27, Table S3).
For children aged 6–23 months, the lower RI limits ranged from 66 g/L (males) and 69 g/L (females) [19] to 112.5 g/L (males) and 122.2 g/L (females) [50]. In this age partition, the upper RI limits ranged from 117.3 g/L (males) and 120.9 g/L (females) [50] to 141 g/L (males) [36] and 145.8 g/L (females) [21] (Figures S28 and S29, Table S3).
For children aged 24–59 months, the lower RI limits ranged from 91.1 g/L (males) and 93 g/L (females) [21] to 150.8 g/L (males) and 138 g/L (females) [31]. The upper RI limits in this age group ranged from 123.5 g/L (males) and 126.2 g/L (females) [50] to 159 g/L (males) and 157 g/L (females) [27] (Figures S30 and S31, Table S3).
For children aged 5–12 years, the lower RI limits ranged from 80.9 g/L (males) and 85.9 g/L (females) [23] to 150.8 g/L (males) and 138 g/L (females) [31]. The upper RI limits ranged from 117.9 g/L (males) and 117 g/L (females) [17] to 196 g/L (males) [30] and 162 g/L (females) [56] (Figures S32 and S33, Table S3).
For children aged 12–15 years, lower RI limits ranged from 90 g/L (males) [23] and 81 g/L (females) [46] to 150.8 g/L (males) [31] and 140.5 g/L (females) [58]. The upper RI limits ranged from 133.2 g/L (males) and 133.2 g/L (females) [58] to 196 g/L (males) [30] and 162 g/L (females) [56] (Figures S34 and S35, Table S3).
For children aged 15–18 years, lower RI limits ranged from 90 g/L (males) [23] and 75 g/L (females) [41] to 154 g/L (males) and 140.5 g/L (females) [58]. The upper RI limits ranged from 140.5 g/L (males) [23] and 131.5 g/L (females) [59] to 196 g/L (males) [30] and 162 g/L (females) [56] (Figures S36 and S37, Table S3).
The Figure S38 highlights the heterogeneity of haemoglobin reference intervals across age and between sexes and the complexity involved in the visual presentation of all the RIs. Since the age ranges for RIs reported in the studies spanned months or years, the RI limits were displayed on the graphs as lines, while the corresponding CIs were displayed as shaded boxes spanning each age range as reported in the corresponding studies. Significant variability was observed, particularly during adolescence, where males and females showed distinct variation in their reference intervals. Based on the visual presentation of the studies using the PRINCES-H tool, we identified substantial variability related to study characteristics, including child age and sex, analyser type and country. There also appeared to be significant variation by countries within continental regions. For many studies, the lower RI was substantially different (lower or higher) than the WHO threshold to define anaemia.
4 Discussion
We conducted a systematic review of paediatric RIs/RCs for haemoglobin (lower and upper limits) in males and females under 18 years of age. We identified 48 studies from 25 countries spanning 4 continents published between 1938 and 2023. There was inconsistency in age partitioning and length of age intervals across studies. Fourteen studies reporting RIs adhered to CLSI guidelines and were included in the meta-analysis, along with 2 studies that reported RCs. RI estimates were pooled for a limited number of age partitions due to high heterogeneity. A pooled estimate for 0–3 months could not be generated due to a paucity of data. Lower RI limits increased with age from 3 months onwards from approximately 100 to 130 g/L. Upper limits increased from 3 months onwards from approximately 130 to 150 g/L. We also conducted a narrative synthesis of all 48 studies accompanied by a comprehensive visualisation of the RIs using our novel web-based PRINCES-H tool, according to our standardised age partitions as well as the WHO age groups. We identified substantial heterogeneity in lower and upper RI limits related to study characteristics, including child age and sex, analyser type and country.
To our knowledge, this is the first systematic review on paediatric haemoglobin RIs, yet it has several limitations. First, we did not use a formal RoB or quality assessment because there is no tool applicable for RIs/RCs; instead, we used adherence to the CLSI guideline as a criterion to pool RI estimates. Second, our study was limited to publications in English, which may have led to the exclusion of important studies published in other languages. Third, there was a small number of studies for some of the age groups, especially the younger age groups, which could affect heterogeneity estimates.
While our review focused on RIs/RCs (lower and upper limits), there is another body of literature focused on a single haemoglobin threshold to define anaemia (a type of clinical decision limit). Jorgensen et al., commissioned by WHO, conducted a narrative review of 60 studies (1975–2018) reporting haemoglobin thresholds across the life cycle in males and females [7]. Recognising the substantial heterogeneity in study results and inclusion of individuals with iron deficiency or inflammation, WHO supported a subsequent study by Braat et al., which pooled international data sources and excluded individuals with clinical or laboratory evidence of conditions that might reduce haemoglobin (i.e., creating a healthy reference population) [1]. The authors estimated haemoglobin thresholds at the 5th percentile for five age groups (6 months to 65 years), noting similar thresholds for males and females under 12 years of age [1]. Commenting on the WHO guidelines, Pasricha et al. suggest there is “an opportunity for global harmonisation of haemoglobin thresholds to define anaemia across countries, clinical guidelines and diagnostic laboratories” [8]. Our finding of substantial heterogeneity of locally developed RIs/RCs suggests that global harmonisation of RIs is not yet possible, and it remains uncertain whether local or global RIs will lead to improved clinical decision-making. More research on the rigorous development of RIs is warranted. In addition, a discussion of the usefulness and precision of local versus global paediatric RIs is warranted. The question related to the risk of erroneous diagnosis of anaemia using global limits for some populations should be investigated in future studies.
Community- or hospital-based data, using indirect methods, play important roles in establishing RIs and RCs. Indirect methods have been explored in several studies as alternatives to direct approaches and have the potential to influence clinical practice, particularly in vulnerable populations such as the paediatric population, where acquiring sufficient sample sizes from healthy children is often limited or not possible [61-64]. Studies utilising indirect methods estimate RIs using large databases from hospital information systems and/or community-based datasets, leveraging traditional and emerging statistical techniques including the Hoffmann method and methods involving the mixture distribution [61, 65-68]. The CLSI guidelines, which are currently considered the gold standard for RI estimation, acknowledge the use of indirect techniques. However, the guidelines also highlight reservations, and the recommendations often focus on RIs and RCs established using healthy populations. Considering this and the ongoing debate around direct versus indirect methods, and the anticipated heterogeneity and scale of data in this systematic review, we focused on RIs/RCs derived exclusively from “healthy populations” and only those that followed the CLSI guidelines were combined quantitatively to provide pooled estimates. By identifying and highlighting areas where the estimation of precise RIs is not yet possible, our review provides support for the use of indirect methods using community-based data and hospital samples, as a provisional solution in these areas. At the same time, our findings emphasize the critical importance of ongoing and future paediatric data collection efforts for gathering sufficient samples from healthy children. Our systematic review and meta-analysis employed novel strategies, such as standardised age partitioning, to advance evidence synthesis methodologies specific to RIs/RCs. By limiting the scope of this analysis to “healthy populations” we presented this work as a proof-of-concept for future studies in the field. The methods employed and the analysis strategies used, including our novel web-based tool computational and graphical, can be adapted and used in evidence synthesis involving RIs and RCs for other biomarkers of health, including RIs and RCs obtained using indirect methods.
There are several implications for practice, policy and future research. First, our evidence synthesis of locally developed RIs/RCs revealed substantial heterogeneity, suggesting the need for more rigorously developed estimates that may be used globally, similar to the approach by Braat et al. for the development of haemoglobin thresholds to define anaemia. Second, standardised age partitions are needed for RIs, and RCs may eliminate the need for age partitioning. Third, future research is needed on RIs for the youngest children, using very narrow age partitions, where haemoglobin changes significantly from birth to 6 months of age. Fourth, we support the aspirational goal of global harmonisation of RIs and RCs (lower and upper limits) and haemoglobin thresholds to define anaemia (single CDL), which will provide clinicians, public health practitioners and laboratories with a comprehensive set of values to assess the health of individuals and populations. Use of percentile curves should be explored to provide continuous haemoglobin charts (similar to WHO growth charts), which will eliminate the need for age partitioning. Fifth, at the same time, we also advocate for regional RIs and RCs to understand the range of values of the population.
Author Contributions
V.B., J.S.H., P.C.P. and B.K.P. conceptualised and designed the study. V.B., J.S.H., P.C.P., B.K.P., F.M. and M.L. contributed to the development of the published study protocol. V.B. and M.B. conducted the literature search with the help of a librarian, independently screened the articles for inclusion and exclusion and in consultation with J.S.H., P.C.P. and B.K.P. V.B., M.B., M.P. and J.L. conducted data extraction. V.B. conducted statistical analysis and drafted the initial manuscript. J.S.H., P.C.P., B.K.P., M.B., F.M. and M.L. contributed to drafting the manuscript. All authors critically reviewed, provided feedback towards its improvement and approved the final manuscript.
Acknowledgements
The authors acknowledge the support from the University of Ottawa in the library search. This research is supported by JSH's grant from the Natural Sciences and Engineering Research Council of Canada, Government of Canada (Grant number: RGPIN-2018-06693). The funder had no role in the design and development of this protocol.
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Study data used for this analysis are publicly available through Shiny app (https://svetric.shinyapps.io/princesH/).