Multicentre verification of haematology laboratory blood collection tubes during a global blood collection tube shortage
Helena Vreede and Marizna Korf are the Joint last authors.
Abstract
Introduction
Verification of blood collection tubes is essential for clinical laboratories. The aim of this study was to assess performance of candidate tubes from four alternative suppliers for routine diagnostic haematology testing during an impending global shortage of blood collection tubes.
Methods
A multicentre verification study was performed in Cape Town, South Africa. Blood from 300 healthy volunteers was collected into K2EDTA and sodium citrate tubes of BD Vacutainer® comparator tubes and one of four candidate tubes (Vacucare, Vacuette®, V-TUBE™ and Vacutest®). A technical verification was performed, which included tube physical properties and safety. Routine haematology testing was performed for clinical verification.
Results
Vacucare tubes did not have a fill-line indicator, Vacuette® tubes had external blood contamination on the caps post-venesection and Vacutest® tubes had hard rubber stoppers. K2EDTA tubes of Vacuette®, Vacucare and Vacutest® performed similarly to the comparator. Unacceptable constant bias was seen for PT in Vacucare (95% CI −2.38 to −0.10), Vacutest® (95% CI −1.91 to −0.49) and Vacuette® (95% CI 0.10–1.84) tubes and for aPTT in Vacuette® (95% CI 0.22–2.00) and V-TUBE™ (95% CI −2.88 to −0.44). Unacceptable %bias was seen for aPTT in Vacucare (95% CI 2.78–4.59) and Vacutest® tubes (95% CI 2.53–3.82; desirable ±2.30), and in V-TUBE™ for mean cell volume (95% CI 1.15–1.47, desirable ±0.95%) and mean cell haemoglobin concentration (95% CI −1.65 to −0.93, desirable ±0.43%).
Conclusion
Blood collection tubes introduce variability to routine haematology results. We recommend that laboratories use one brand of tube. Verification of new candidate tubes should be performed to ensure consistency and reliable reporting of results.
1 INTRODUCTION
Accurate and reliable laboratory test reporting is crucial for clinical decision-making related to admission and discharge of patients from hospital as well as administration of medications and/or blood products.1 The quality of the reported test result is directly linked to the quality of the specimen, therefore, unsuitable samples can compromise the accuracy of reported results.2 Errors may occur in the pre-analytical, analytical and post-analytical phases of testing. Technological advances have reduced the errors in the analytical and post-analytical phases.3 Control of errors within the pre-analytical phase, where the majority of errors occur, is difficult as the pre-analytical phase often involves non-laboratory personnel and also escapes quality control and proficiency testing programmes.3, 4 An important pre-analytical consideration is that blood collection tubes are important sources of laboratory variability.5, 6 Thus, verification or validation studies are required prior to the use of new brands of blood collection tubes.7 Limited studies have been published that assess the differences in the performance of different blood collection tube brands within the haematology context.
To our knowledge, only one study has assessed different brands of dipotassium ethylenediaminetetraacetic acid (K2EDTA) blood collection tubes as a source of pre-analytical variability.8 The full blood count (FBC) and differential white cell count (Diff) parameters of BD Vacutainer® (Becton Dickinson, Rutherford, New Jersey, USA), Greiner Bio-One Vacuette® (Greiner Bio-One GmbH, Kremsmünster, Austria) and Venosafe™ (Terumo Europe, Leuven, Belgium) K2EDTA blood collection tubes were assessed on the Advia 2120i (Siemens Healthcare Diagnostics, Deerfield IL, USA). Haematocrit (Hct), mean cell volume (MCV) and platelet distribution width (PDW) varied significantly between the different manufacturers.
A few studies have assessed different brands of sodium citrate blood collection tubes as a source of pre-analytical variability. Adequate filling of citrate tubes to maintain the 9:1 ratio of blood to buffered citrate is pertinent to the accuracy of clotting times.5, 6 Filling accuracy was found to be acceptable for sodium citrate tubes (3.2%) of BD Vacutainer®, Greiner Bio-One Vacuette® and Vacutest® Kima (Vacutest Kima SRL, Arzegrande, Italy), but there was considerable between-lot bias, approaching 13% in the BD Vacutainer® tubes.9 A study assessing sodium citrate (2.8%) blood collection tubes by measuring clotting times of recalcified blood and plasma samples using free oscillation rheometry, showed that BD Vacutainer® and Greiner Bio-One Vacuette® tubes had shorter clotting times than S-Monovette® tubes (Sarstedt, Nümbrecht, Germany), which was not due to the anticoagulant solution.10 Another study, which assessed prothrombin time (PT), activated partial thromboplastin time (aPTT) and fibrinogen on the ACL TOP coagulation analyser (Instrumentation Laboratory, Milan, Italy), showed significant differences in PT and aPTT, but not fibrinogen, between sodium citrate (3.2%) blood collection tubes of Venosafe™, Greiner Bio-one Vacuette®, BD Vacutainer®, S-Monovette® and LABOR IMPORT (Shandong Weigao Group Medical Polymer, People's Republic of China).11 The variation in results for different blood collection tube brands reported in the above studies supports the need blood tube verification in the haematology laboratory.
Early in the COVID-19 pandemic, blood collection tube manufacturers experienced transportation delays, shortage of production inputs and increased cost of raw materials. This led to impending shortages of the widely used BD Vacutainer® blood collection tubes.12 The aim of this study was therefore to verify alternative K2EDTA and sodium citrate tubes for routine haematology testing, in accordance with published recommendations.13, 14
2 MATERIALS AND METHODS
2.1 Study design
A multicentre blood collection tube verification study was performed to assess technical and clinical aspects of disposable vacuum blood collection tubes of four manufacturers. The suppliers provided K2EDTA and sodium citrate (3.2%) tubes with similar anticoagulant concentrations to the in-use BD Vacutainer® comparator tubes, as per recommendation.15 Serum tubes with gel separator, lithium heparin tubes, sodium fluoride tubes and plasma tubes with gel separator were also assessed for each volunteer as part of the broader study. These findings will be reported separately.
The study was conducted between September 2021 and December 2021 at the National Health Laboratory Service (NHLS) branches of two large academic hospitals in Cape Town, South Africa, namely Groote Schuur Hospital (GSH) and Tygerberg Hospital (TBH) and a regional laboratory in Green Point (GPL). All three laboratories are accredited according to international standards by the South African National Accreditation System (SANAS). This verification was performed in accordance with NHLS standard operating procedures, the Clinical and Laboratory Standards Institute (CLSI) (GP41 for sample collection; EP09c for assessment of bias) and published literature.16, 17
A total of 300 healthy adult volunteers, both male and female, were recruited for the study. Volunteers were not eligible for the study if they had clinical conditions or were on therapies known to affect haematology test results such as cytopenias, coagulation disorders, anticoagulation therapy and/or pregnancy. The study was approved by the Stellenbosch University Health Research Ethics Committee (N21/09/095) in accordance with the World Medical Association (WMA) Declaration of Geneva and Declaration of Helsinki. All volunteers signed informed consent. Volunteer confidentiality was maintained by de-identification of the samples at the time of collection with only the principal investigator retaining the identification key.
2.2 Collection of blood samples
The collection of blood samples was performed by NHLS trained phlebotomists, according to the recommendations of the Clinical Laboratory Standard Institute (CLSI).17 The four candidate tube manufacturers that were assessed are presented in Table 1.
| Tube | Code | K2EDTA | Sodium citrate (3.2%) | ||||
|---|---|---|---|---|---|---|---|
| Volume (mL) | Lot numbers | Study sites | Volume (mL) | Lot numbers | Study sites | ||
| Vacucare (EREZlabmed, tubes manufactured by Zhejiang Gongdong Medical Technology Co Ltd, Huangyan Taizhou, China) | EV | 4 | 210505 210 608 | GSH TBH GPLa | 4.5 | 210312 | GSH TBH |
| Vacuette® (Greiner Bio-One GmbH, Kremsmünster, Austria) | LG | 4 | A21013NY | GSH TBH GPLa | 3 | A21083A3 A2103473 | GSH TBH |
| V-TUBE™ (AB Medical Inc, Seoul, South Korea) | VT | 4 | 2519002 | GSH TBH GPLb | 2.7 | 1619006 | GSH TBH |
| Vacutest® (Vacutest Kima SRL, Arzergrande, Italy) | KV | 4 | WZ1321 WZ2501 | GSH TBH GPLb | 3.5 | A0461 A2781 A1721 | GSH TBH |
- a Clinical verification only.
- b Technical verification only.
Sodium citrate (3.2%) and K2EDTA candidate tubes were tested in parallel with the comparator BD Vacutainer® sodium citrate (3.2%) and K2EDTA tubes. Blood was collected from 25 volunteers for each of the four candidate tube manufacturers at each study site. Blood was collected from the anterior cubital fossae of each arm into the comparator and candidate tubes respectively. A 21- or 23-gauge needle was used, which was connected directly to the blood collection tubes via a BD Vacutainer® holder. In volunteers with difficult venous access a butterfly needle and/or dorsa of the hands were utilized to acquire samples. The order in which the comparator and the candidate tubes were drawn was alternated to prevent bias, in keeping with guidelines.18
2.3 Technical verification
Prior to blood collection, all candidate and comparator tubes were assessed for physical defects of manufacturing. Each comparator and candidate tube pack received a unique number, which was recorded during phlebotomy. During phlebotomy, fitting and difficulty with insertion of blood tubes into the collection device, defective vacuum, external blood contamination and any difficulty with phlebotomy which may affect the clinical verification were noted by phlebotomists on a phlebotomy checklist. K2EDTA samples used for the clinical verification of chemistry analytes, published separately, were included in the technical verification results for the quality indicators evaluated during phlebotomy.
The remainder of the technical verification was performed during the pre-analytical phase in the laboratory. All samples were assessed for undue clotting. The filling distance of each tube was determined by measuring the distance between the top of the fill line and the bottom of the tube as an indication of the filling volume. Under-filling and over-filling was defined as a fill level 10% below and above the top of the fill line, respectively.13
Sodium citrate tubes were centrifuged at 4000 rpm for 15 min. Thereafter, the integrity of the tubes was assessed for breakage and spillage, and the samples were assessed visually for haemolysis. An additional check for the presence of blood clots was conducted post-analysis.
Blood films were prepared from K2EDTA samples. At GSH, blood films were made and stained by an automated process on the Sysmex XN9000 slide-making/staining module (SP-10) (Sysmex Corporation, Kobe, Japan). At TBH, blood films were made manually and stained on the HemaTek (Paderborn, Nordrhein-Westfalen, Germany) flat-bed stainer within 2 h of blood collection. The Wright-Giemsa stained blood films were assessed for staining quality, specifically assessing for adequate staining of cellular components such as nuclear chromatin and granules and EDTA-related changes.
2.4 Clinical verification
All reagents and controls were stored and handled appropriately as per standard laboratory practice and manufacturer's specifications. The K2EDTA tubes of the comparator and candidate tubes were assessed for an automated full blood count (FBC), differential count and reticulocyte count on the Sysmex XN2000 (Sysmex Corporation, Kobe, Japan) at GSH and on the Siemens Advia 2020i (Siemens HealthCare Diagnostics, Erlangen, Germany) at TBH. At GPL, FBC parameters for the EV and LG studies were analysed on the Sysmex XN2000 (Table 1).
The sodium citrate tubes of the comparator and candidate blood tubes were assessed for an automated prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen and D-dimers on the ACL TOP 500 CTS (Werfen Group, Barcelona, Spain) at GSH and the Sysmex CS-2100i (Sysmex Corporation, Kobe, Japan) at TBH. Sodium citrate tubes were not analysed at GPL.
2.5 Statistical analysis
Data analysis was performed using Microsoft Excel® (Microsoft Corp., Redmond, Washington, USA) and EP Evaluator™ (Data Innovations LLC, Colchester, Vermont, USA). Clotted samples and sodium citrate samples that were haemolysed or under-filled were excluded from the statistical analysis of the clinical verification, as these samples would be rejected in routine clinical practice.
For comparison of tubes, Passing-Bablok regression was performed and the confidence intervals from the slope and intercept were assessed for each of the parameters. The slope 95% confidence interval (95% CI) was deemed acceptable if it included 1 and the intercept 95% CI was deemed acceptable if it included 0.16
Normality of the differences was assessed by histograms provided in EP Evaluator. Bland–Altman plots were evaluated and mean percentage bias was determined for parameters with normal distribution. For parameters with non-normal distribution, pairwise Walsh averages of the differences were calculated in a trial version of Minitab® (Minitab, LCC, Pennsylvania, USA) with subsequent determination of the Hodges-Lehmann point estimator (median) and Tukey two-sided confidence intervals.16 The average percentage bias was considered acceptable if it was less than the desirable bias or if the 95% CI of the mean bias included the desirable bias.16 Desirable bias was based on either the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) biological variation database or Ricos criteria.19, 20 The correlation coefficient (R) was assessed to determine whether the range of results was appropriate.
3 RESULTS
3.1 Technical verification
All K2EDTA tubes had purple caps. All sodium citrate tubes had light blue caps. Cap lengths of the tubes was 19 mm for all except the LG tubes, which were 13 mm in length. On all tube labels, a CE marking, tube draw volume in mL, lot number and expiry date were displayed. The EV tubes did not have the anticoagulant concentration displayed on the tube labels. Additionally, there was no fill-line indicator on the EV tubes and the bottom of the cap was used as the indicator line, as per instruction from the distributor (EREZlabmed, Gauteng, South Africa). The fill lines of VT tubes were 6 mm thick, which may result in uncertainty in the determination of under-filling. As outlined in the methods, the top of the fill line was used to report filling for this study. Fill-line indicators on other tubes were acceptable.
No major problems were identified during phlebotomy for BD, EV and VT tubes (Table 2). The LG tubes had more angular sideways movement in the blood collection barrel because of the shorter cap. Additionally, the LG tubes had a small amount of blood leaking from the tube caps where the rubber had been pierced. The rubber of the KV tube caps was hard, which made it more difficult to pierce than other tubes and created more resistance to tube removal. The force required to insert and remove tubes into and from the blood collection barrel occasionally displaced the needle during phlebotomy. Under-filling was seen in less than 10% each of the comparator tubes. Stain quality was acceptable for slides prepared from samples in all candidate tubes and the comparator tubes with adequate staining of cellular components such as nuclear chromatin and granules, and no EDTA-related changes seen. The percentage of errors was less than 5% for all tubes (Table 2).
| Tube comparison | K2EDTA | Sodium citrate | ||||
|---|---|---|---|---|---|---|
| n | BD | Candidate tube | n | BD | Candidate tube | |
| EV study | ||||||
| 1. Tubes with physical defects of manufacturing | 75 | 0 | 0 | 50 | 0 | 0 |
| 2. Tubes with no vacuum/fail to form a vacuum | 75 | 0 | 0 | 50 | 0 | 0 |
| 3. Tubes not properly fitting into blood collection device | 75 | 0 | 0 | 50 | 0 | 0 |
| 4. Tubes underfilled | 75 | 0 | 0 | 50 | 2 | 2 |
| 5. Leaking from tube caps where rubber pierced | 75 | 0 | 0 | 50 | 0 | 0 |
| 6. External surface contamination after venepuncture | 75 | 0 | 0 | 50 | 0 | 0 |
| 7. Haemolysed specimens after centrifugation | n/a | – | – | 50 | 1 | 1 |
| 8. Specimen clotted | 50 | 0 | 0 | 50 | 0 | 0 |
| 9. Poor Romanowsky stain quality | 50 | 0 | 0 | n/a | – | – |
| Total observations (percentage errors) | 550 | 0 (0) | 0 (0) | 400 | 3 (0.75) | 3 (0.75) |
| LG study | ||||||
| 1. Tubes with physical defects of manufacturing | 75 | 0 | 0 | 50 | 0 | 0 |
| 2. Tubes with no vacuum/fail to form a vacuum | 75 | 0 | 0 | 50 | 0 | 0 |
| 3. Tubes not properly fitting into blood collection device | 75 | 0 | 0 | 50 | 0 | 0 |
| 4. Tubes underfilled | 75 | 0 | 7 | 50 | 1 | 1 |
| 5. Leaking from tube caps where rubber pierced | 75 | 0 | 18 | 50 | 0 | 6 |
| 6. External surface contamination after venepuncture | 75 | 0 | 0 | 50 | 0 | 0 |
| 7. Haemolysed specimens after centrifugation | n/a | – | – | 50 | 0 | 0 |
| 8. Specimen clotted | 50 | 0 | 0 | 50 | 0 | 0 |
| 9. Poor Romanowsky stain quality | 50 | 0 | 0 | n/a | – | – |
| Total observations (percentage errors) | 550 | 0 (0) | 25 (4.55) | 400 | 1 (0.25) | 7 (1.75) |
| VT study | ||||||
| 1. Tubes with physical defects of manufacturing | 100 | 0 | 0 | 50 | 0 | 0 |
| 2. Tubes with no vacuum/fail to form a vacuum | 100 | 0 | 0 | 50 | 0 | 0 |
| 3. Tubes not properly fitting into blood collection device | 100 | 0 | 0 | 50 | 0 | 0 |
| 4. Tubes underfilled | 74 | 5 | 0 | 50 | 3 | 0 |
| 5. Leaking from tube caps where rubber pierced | 100 | 0 | 0 | 50 | 0 | 0 |
| 6. External surface contamination after venepuncture | 100 | 0 | 0 | 50 | 0 | 0 |
| 7. Haemolysed specimens after centrifugation | n/a | – | – | 50 | 0 | 0 |
| 8. Specimen clotted | 50 | 0 | 0 | 50 | 0 | 0 |
| 9. Poor Romanowsky stain quality | 50 | 0 | 0 | n/a | – | – |
| Total observations (percentage errors) | 674 | 5 (0.74) | 0 (0) | 400 | 3 (0.75) | 0 (0) |
| KV tubes | ||||||
| 1. Tubes with physical defects of manufacturing | 100 | 0 | 0 | 50 | 0 | 0 |
| 2. Tubes with no vacuum/fail to form a vacuum | 96 | 0 | 0 | 48 | 0 | 0 |
| 3. Tubes not properly fitting into blood collection device | 96 | 0 | 0 | 48 | 0 | 0 |
| 4. Tubes underfilled | 71 | 5 | 4 | 47 | 4 | 6 |
| 5. Leaking from tube caps where rubber pierced | 96 | 1 | 1 | 48 | 0 | 0 |
| 6. External surface contamination after venepuncture | 96 | 0 | 0 | 48 | 0 | 0 |
| 7. Haemolysed specimens after centrifugation | n/a | – | – | 48 | 0 | 0 |
| 8. Specimen clotted | 50 | 0 | 0 | 48 | 0 | 0 |
| 9. Poor Romanowsky stain quality | 50 | 0 | 0 | n/a | – | – |
| Total observations (percentage errors) | 655 | 6 (0.91) | 5 (0.76) | 385 | 4 (1.04) | 6 (1.56) |
- Abbreviations: BD, Becton Dickinson Vacutainer® tubes; EV, Zhejiang Gongdong Medical Technology Co. Vacucare tubes; KV, Kima Vacutest® tubes; K2-EDTA, dipotassium ethylenediaminetetraacetic acid; LG, Greiner Bio-One Vacuette® tubes; n/a, not applicable; VT, AB Medical Inc V-TUBE™ tubes.
3.2 Clinical verification: K2EDTA blood collection tubes
EV, LG and KV K2EDTA tubes showed acceptable results across all parameters assessed (Table 3).
| Parameters | Tubea | N | Slope (95% CI) | Intercept (95% CI) | R | Obtained %bias (95% CI) | Desirable %bias (source References [19, 20] |
|---|---|---|---|---|---|---|---|
| Full blood count parameters | |||||||
| White cell count (WCC) | EV | 71 | 0.966 (0.989–1.06) | 0.048 (−0.525 to 0.397) | 0.969 | −2.65 (−4.37 to 0.92) | 4.91 (EFLM) |
| LG | 68 | 1.000 (0.951–1.042) | −0.500 (−0.285 to 0.294) | 0.991 | −1.01 (−2.07 to 0.04) | ||
| VT | 50 | 0.987 (0.937–1.038) | −0.091 (−0.400 to 0.200) | 0.990 | −2.16 (−3.24 to 1.08) | ||
| KV | 50 | 0.996 (0.938–1.056) | −0.123 (−0.464 to 0.222) | 0.980 | −2.29 (−3.75 to 0.83) | ||
| Red cell count (RCC) | EV | 71 | 0.964 (0.895–1.045) | 0.163 (−0.299 to 0.489) | 0.972 | −0.13 (−0.79 to 0.53) | 1.80 (Ricos) |
| LG | 68 | 1.000 (0.951–1.042) | −0.050 (−0.285 to 0.294) | 0.991 | −1.01 (−0.98 to 0.42) | ||
| VT | 50 | 1.018 (0.968–1.073) | −0.83 (−0.344 to 0.153) | 0.985 | −0.09 (−0.56 to 0.39) | ||
| KV | 50 | 1.000 (0.929–1.100) | −0.005 (−0.493 to 0.345) | 0.953 | −0.26 (−0.88 to 0.37) | ||
| Haemoglobin (Hb) | EV | 71 | 1.000 (0.929–1.059) | 0.000 (−0.840 to 0.990) | 0.976 | −0.25 (−0.81 to 0.31) | 1.62 (EFLM) |
| LG | 68 | 1.000 (0.941–1.082) | 0.000 (−1.190 to 0.780) | 0.965 | 0.20 (−0.48 to 0.87) | ||
| VT | 50 | 1.000 (0.964–1.056) | 0.000 (−0.770 to 0.490) | 0.988 | −0.06 (−0.52 to 0.40) | ||
| KV | 50 | 1.000 (0.941–1.053) | −0.100 (−0.790 to 0.760) | 0.973 | −0.22 (−0.79 to 0.34) | ||
| Haematocrit (Hct) | EV | 71 | 0.966 (0.877–1.081) | 0.014 (−0.036 to 0.054) | 0.945 | 0.09 (−0.62 to 0.81) | 1.54 (EFLM) |
| LG | 68 | 1.043 (0.964–1.143) | −0.019 (−0.061 to 0.016) | 0.933 | 0.08 (−0.68 to 0.83) | ||
| VT | 50 | 1.028 (0.980–1.083) | −0.006 (−0.030 to 0.014) | 0.983 | 1.24 (0.77 to 1.71) | ||
| KV | 50 | 1.000 (0.916–1.112) | 0.000 (−0.046 to 0.036) | 0.951 | 0.14 (−0.51 to 0.79) | ||
| Mean cell volume (MCV) | EV | 71 | 1.007 (0.977–1.044) | −0.470 (−3.820 to 2.270) | 0.993 | 0.21 (0.03–0.39) | 0.95 (EFLM) |
| LG | 68 | 0.987 (0.965–1.008) | 1.400 (−0.490–3.430) | 0.996 | 0.35 (0.20–0.50) | ||
| VT | 50 | 1.007 (0.993–1.030) | 0.500 (−1.560 to 1.710) | 0.997 | 1.31 (1.15–1.47) | ||
| KV | 50 | 1.000 (0.959–1.028) | 0.500 (−2.000 to 3.990) | 0.993 | 0.40 (0.26–0.58)b | ||
| Mean cell haemoglobin (MCH) | EV | 71 | 1.000 (0.925–1.053) | −0.100 (−0.600 to 2.140) | 0.972 | −0.05 (−0.46 to 0.35) | 1.12 (EFLM) |
| LG | 68 | 1.000 (0.964–1.068) | 0.100 (−1.790 to 1.150) | 0.986 | 0.45 (0.07–0.84) | ||
| VT | 50 | 1.000 (0.971–1.063) | 0.000 (−1.830 to 0.860) | 0.990 | 0.07 (−0.31 to 0.46) | ||
| KV | 50 | 0.952 (0.889–1.000) | 1.370 (−0.100 to 3.280) | 0.978 | −0.32 (−0.71 to 0.32) | ||
| Mean cell haemoglobin concentration (MHCH) | EV | 71 | 0.931 (0.773–1.071) | 2.720 (−2.400 to 7.240) | 0.850 | −0.27 (−0.71 to 0.17) | 0.43 (EFLM) |
| LG | 68 | 1.143 (1.000–1.333) | −4.680 (−10.870 to 0.00) | 0.917 | 0.07 (−0.37 to 0.52) | ||
| VT | 50 | 1.000 (0.885–1.167) | −0.300 (−5.800 to 3.550) | 0.956 | −1.29 (−1.65 to 0.93) | ||
| KV | 50 | 0.951 (0.813–1.077) | 1.430 (−2.69 to 6.020) | 0.874 | −0.35 (−0.79 to 0.10) | ||
| Platelet count | EV | 71 | 1.000 (0.946–1.053) | −6.500 (−20.20 to 9.600) | 0.976 | −1.70 (−1.01 to 0.52) | 5.90 (Ricos) |
| LG | 68 | 0.955 (0.902–1.009) | 10.70 (−2.800 to 25.20) | 0.984 | 0.20 (−1.00 to 1.50) | ||
| VT | 50 | 0.994 (0.958–1.035) | −0.700 (−11.80 to 11.40) | 0.990 | −0.70 (−1.80 to 0.40) | ||
| KV | 50 | 0.984 (0.938–1.045) | 4.200 (−12.60 to 15.70) | 0.984 | −0.40 (−1.50 to 0.70) | ||
| Differential count parameters | |||||||
| Neutrophil count | EV | 50 | 0.972 (0.914–1.035) | −0.008 (−0.158 to 0.147) | 0.983 | −2.26 (−3.94 to 0.58) | 6.86 (EFLM) |
| LG | 50 | 0.983 (0.941–1.027) | 0.01 (−0.136 to 0.179) | 0.992 | −1.22 (−2.35 to 0.90) | ||
| VT | 50 | 0.952 (0.912–0.994) | 0.071 (−0.070 to 0.206) | 0.992 | −2.40 (−3.70 to 1.10) | ||
| KV | 50 | 0.988 (0.941–1.040) | −0.008 (−0.186 to 0.134) | 0.986 | −2.22 (−3.70 to 0.74) | ||
| Lymphocyte count | EV | 50 | 0.995 (0.906–1.088) | −0.083 (−0.273 to 0.117) | 0.974 | −3.16 (−5.59 to 1.03)b | 6.28 (EFLM) |
| LG | 50 | 1.009 (0.938–1.067) | −0.18 (−0.153 to 0.320) | 0.980 | −0.43 (−2.16 to 1.31) | ||
| VT | 50 | 1.000 (0.947–1.072) | −0.025 (−0.154 to 0.069) | 0.978 | −0.90 (−2.64 to 0.84) | ||
| KV | 50 | 0.971 (0.920–1.035) | 0.009 (−0.114 to 0.106) | 0.978 | −2.04 (−4.02 to 0.06) | ||
| Monocyte count | EV | 50 | 1.082 (0.923–1.130) | −0.024 (−0.074 to 0.012) | 0.951 | −4.99 (−8.70 to 1.27) | 6.47 (EFLM) |
| LG | 50 | 0.961 (0.875–1.034) | 0.004 (−0.025 to 0.044) | 0.987 | −1.48 (−4.64 to 1.67) | ||
| VT | 50 | 0.941 (0.875–1.000) | 0.009 (−0.010 to 0.038) | 0.968 | −3.57 (−6.27 to 0.88) | ||
| KV | 50 | 1.048 (0.957–1.167) | −0.034 (−0.078 to 0.010) | 0.981 | −2.95 (−6.31 to 0.41) | ||
| Eosinophil count | EV | 50 | 1.000 (0.941–1.050) | 0.000 (−0.010 to 0.004) | 0.981 | −2.86 (−7.69 to 0.48)b | 16.77 (EFLM) |
| LG | 50 | 1.000 (0.929–1.000) | 0.000 (0.000 to 0.004) | 0.984 | −2.86 (−7.37 to 1.69)b | ||
| VT | 50 | 1.000 (0.935–1.016) | −0.010 (−0.011 to 0.002) | 0.997 | −6.24 (−11.74 to 0.75) | ||
| KV | 50 | 1.000 (0.947–1.021) | −0.005 (−0.010 to 0.003) | 0.989 | −3.98 (−8.20 to 0.00)b | ||
| Basophil count | EV | 50 | 1.000 (1.000–1.500) | 0.000 (−0.020 to 0.000) | 0.774 | −4.58 (−12.43 to 3.27) | 7.27 (EFLM) |
| LG | 50 | 1.000 (1.000–1.000) | 0.000 (0.000 to 0.000) | 0.875 | −1.65 (−10.98 to 7.68) | ||
| VT | 50 | 1.000 (0.833–1.000) | 0.000 (0.000 to 0.007) | 0.850 | 5.57 (−4.42 to 15.57) | ||
| KV | 50 | 1.000 (0.750–1.000) | 0.000 (0.000 to 0.007) | 0.862 | −1.83 (−10.5 to 6.84) | ||
| Reticulocyte count | EV | 50 | 0.964 (0.895–1.045) | −0.048 (−0.525 to 0.397) | 0.970 | −2.65 (−1.08 to 4.8) | 7.20 (EFLM) |
| LG | 50 | 1.000 (0.951–1.042) | −0.002 (−0.009 to 0.004) | 0.961 | −0.61 (−2.76 to 1.55) | ||
| VT | 50 | 1.100 (0.978–1.217) | −0.007 (−0.015 to 0.001) | 0.924 | −2.16 (−2.07 to 4.68) | ||
| KV | 50 | 1.105 (1.000–1.226) | −0.007 (−0.017 to 0.001) | 0.938 | 1.47 (−1.56 to 4.50) | ||
| Coagulation parameters | |||||||
| Prothrombin Time (PT) | EV | 45 | 1.100 (1.000–1.200) | −1.20 (−2.38 to 0.10) | 0.965 | −0.34 (−1.12 to 0.43) | 2.00 (Ricos) |
| LG | 49 | 0.929 (0.857–1.000) | 0.99 (0.10–1.84) | 0.976 | 1.41 (0.93–1.88) | ||
| VT | 47 | 0.933 (0.842–1.000) | 0.67 (−0.10 to 1.71) | 0.940 | −0.97 (−1.80 to 0.00)b | ||
| KV | 39 | 1.120 (1.059–1.182) | −1.19 (−1.91 to 0.49) | 0.989 | 1.16 (0.55 to 1.77) | ||
| Activated partial thromboplastin time (aPTT) | EV | 45 | 0.988 (0.916–1.057) | 1.49 (−0.41 to 3.53) | 0.977 | 3.69 (2.78–4.59) | 2.30 (Ricos) |
| LG | 49 | 0.987 (0.953–1.015) | 1.00 (0.22–2.00) | 0.994 | 2.42 (1.89–2.95) | ||
| VT | 47 | 1.038 (0.992–1.078) | −1.65 (−2.88 to 0.44) | 0.977 | −2.35 (−3.25 to 1.45) | ||
| KV | 39 | 1.006 (0.976–1.039) | 0.79 (−0.17 to 1.54) | 0.996 | 3.31 (2.53–3.82)b | ||
| Fibrinogen | EV | 45 | 1.000 (0.929–1.067) | 0.00 (−0.19 to 0.21) | 0.976 | 0.31 (−1.19 to 1.82) | 4.80 (Ricos) |
| LG | 49 | 1.000 (0.941–1.000) | 0.00 (0.00–0.20) | 0.989 | 0.38 (−0.61 to 1.37) | ||
| VT | 47 | 1.000 (0.923–1.000) | −0.10 (−0.10 to 0.15) | 0.991 | −2.50 (−3.79 to 1.20) | ||
| KV | 39 | 1.000 (0.900–1.000) | 0.00 (0.00–0.35) | 0.976 | 0.14 (−1.15 to 1.42) | ||
- Abbreviations: 95% CI, 95% confidence interval; BD, Becton Dickinson Vacutainer® tubes; EFLM, European Federation of Clinical Chemistry and Laboratory; EV, Zhejiang Gongdong Medical Technology Co. Vacucare tubes; K2-EDTA, dipotassium ethylenediaminetetraacetic acid; KV, Kima Vacutest® tubes; LG, Greiner Bio-One Vacuette® tubes; VT, AB Medical Inc V-TUBE™ tubes.
- a Compared to the comparator BD blood collection tube. Results that are out of acceptable range are highlighted in bold.
- b Median %bias calculated.
For VT K2EDTA tubes, there was a significant proportional bias for neutrophil count (95% CI 0.912–0.994). Average %bias was acceptable for all parameters, except for mean cell haemoglobin concentration (MCHC) where a negative %bias was noted (95% CI −1.65 to −0.93, desirable %bias ±0.43%) and MCV where a positive %bias was noted (95% CI 1.15–1.47, desirable %bias ±0.95%) (Table 3). Sub-analysis of MCHC and MCV at individual centres showed that the bias was seen on both the Sysmex analysers (GSH and GPL) and the Advia analyser (TBH).
The range of values obtained from basophil count was suboptimal in all candidate tubes, while for Hct and MCHC was suboptimal in EV and LG tubes, and for reticulocyte count it was suboptimal in VT and KV tubes (Table 3).
3.3 Clinical verification: sodium citrate blood collection tubes
D-dimers of all volunteers had analyser readings of <0.25 mg/L (normal level: <0.25 mg/L). As such, no further analysis could be performed for D-dimers. Fibrinogen showed acceptable results across all candidate tubes (Table 3).
In EV sodium citrate tubes, PT had a negative constant bias (95% CI −2.38 to −0.10, n = 45). A positive average %bias was noted for aPTT (95% CI 2.87–4.59; desirable %bias ±2.30%, n = 45) (Table 3).
In LG sodium citrate tubes, PT and aPTT showed a positive constant bias (PT: 95% CI 0.10–1.84, aPTT: 95% CI 0.22–2.00, n = 49). The average %bias was acceptable for all parameters (n = 49) (Table 3).
VT sodium citrate tubes showed a negative constant bias for PTT (95% CI −2.88 to −0.44) (n = 47). The average %bias was acceptable for all parameters (n = 47). The full range of values was not present for PT (R = 0.940) (Table 3).
In KV sodium citrate tubes, PT showed a significant proportional bias (95% CI 1.059–1.182) and constant bias (95% CI −1.91 to −0.49) (n = 39). The average %bias was acceptable for all parameters (n = 39) (Table 3).
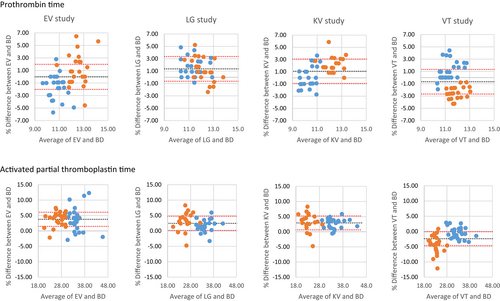
A sub-analysis of Bland–Altman plots of PT and aPTT at GSH and TBH was performed. There was a difference in the range of results obtained for PT and aPTT at each of the centres (x-axis, Figure 1). A similar average %bias was seen at TBH and GSH for PT in the LG tubes (y-axis, Figure 1). However, %bias was negative at TBH in EV and KV tubes and positive in VT tubes, while the opposite was seen in these tubes at GSH. Similar average %bias was seen at TBH and GSH for aPTT in EV, LG and KV tubes. In the VT tubes, the %bias was more negative at GSH (Figure 1).
4 DISCUSSION
Blood collection tubes are an often overlooked cause of variability in the pre-analytic phase of laboratory testing.21 This study showed that the selected tube brand influences the results obtained in routine haematology testing.
For the clinical verification of K2EDTA tubes, the EV, LG and KV tubes had acceptable results in comparison to BD tubes. VT K2EDTA tubes did not meet the standard for clinical verification, as the MCV and MCHC had unacceptable bias. Since MCHC is calculated from the measured MCV, the bias in the MCV is likely responsible for the bias in the MCHC. A previous study has also demonstrated differences in MCV between different tubes, though VT tubes were not included in their study.8
For the clinical verification of sodium citrate tubes, none of the tubes had acceptable results in comparison to BD tubes. There was significant bias seen for PT and PTT, though results were acceptable for fibrinogen. Differences in PT and aPTT across different brands of sodium citrate blood collection tubes was also found in an Italian study performed on the ACL TOP analyser.11
It should be noted that for the aPTT of all the candidate tubes, the mean %bias was greater than the desirable %bias. However, for the LG and VT tubes the 95% CI of the %bias crossed the desirable %bias limit and were therefore considered acceptable in accordance with the guidelines.16 The degree of %bias is nonetheless of concern and if one of these candidate blood collection tubes were to be used, tube specific verification of reference ranges would be needed.
The reason for performing a multicentre verification study was to ensure that the results obtained for the candidate tubes were translatable across different platforms. While the pre-analytical factors were controlled as far as possible, including the same phlebotomists performing venesection at each of the centres, it should be noted that there were different analysers for both the FBC/Diff and haemostasis testing at the different participating centres. While the results of the FBC and Diff were similar between the centres, there were differences in the results obtained for the haemostasis test results. In this study we have shown that the analyser performing the PT and aPTT also contributes to the variability of the obtained results and that different tubes may have varying bias on different analysers. While there were differences in the range of results obtained for PT and aPTT at each of the centres, attributable to different instruments and reagents, the bias could not be attributed to differences in instrument alone. These differences were more pronounced for PT than for PTT. For PT, a positive bias was observed at GSH in the EV and KV studies, while a negative bias was observed at GSH in the VT study and the opposite was observed at TBH (Figure 1). Thus, there is also an interaction between tube and instrument, noted in the differing bias obtained. A similar bias between Sysmex and ACL TOP analysers has previously been demonstrated using tubes of KABE Labortechnik GmbH (Nümbrecht, Germany).22
These findings highlight that verification of blood collection tubes should ideally be performed within each laboratory that is using the blood collection tubes. Additionally, this highlights the importance of using the same brand of tube for monitoring of blood result trends over time, ideally for all laboratory tests, but especially for coagulation parameters. Differences in PT or aPTT results due to blood collection tube variability may erroneously be attributed to clinical improvement or deterioration of the patient's condition or unnecessary anticoagulation dosage adjustments. Furthermore, blood collection tubes are not assessed in EQA programmes and thus it is a challenging aspect of laboratory quality.
While the clinical aspects of verification are important, the user-friendliness and safety of the blood collection tubes should not be overlooked. The bottom of the cap was used as the fill indicator of the EV tubes making it difficult to exclude over-filling. The difficulty that the phlebotomists had with removal of KV tubes from the blood collection barrel was noted as a concern. Since this was a problem for experienced phlebotomists in a controlled environment with healthy volunteers, the difficulty would likely be amplified in pressurised clinical situations. While the angular movement of LG tubes within the BD barrel was not a problem for the phlebotomists in this study, it may lead to difficulties in users with less expertise. Additionally, the LG tubes had external blood contamination from the rubber stopper at the site of piercing which is of concern from a health and safety perspective. It should be noted that a BD collection device was used for all candidate tubes, as the purpose of this verification study was to find an interim tube replacement for an impending BD blood collection tube shortage. Thus we cannot confirm whether these technical concerns are valid if collection devices specific to the candidate tubes were to be used.
The limitations of the study include that only healthy volunteers were included and as such, for some of the parameters the correlation coefficient was less than 0.95 indicating that an insufficient range of values was obtained. Inclusion of individuals with elevated or reduced haematological parameters would allow for the full range of values to be obtained and assessed. Similarly, we were unable to analyse data for D-dimers results because a value of <0.25 mg/L across all volunteers could not be compared statistically. Inclusion of individuals with D-dimer levels above the lower limit of reporting would be required for statistical analysis. A further limitation to note is that when results of the KV citrate tubes are from an assessed 39 samples, which is less than the 40 samples required for the verification. Unfortunately, five KV tubes and six BD tubes were under-filled in that study and could not be assessed for coagulation parameters.
The findings of this study highlight the importance of considering all parts of the pre-analytical process. While BD, EV and KV K2EDTA tubes could be used interchangeably without affecting results, there is considerable variation in PT and aPTT across candidate sodium citrate tubes. For laboratories experiencing tube shortages, we recommend a verification of candidate tubes and if unacceptable results are obtained, a full validation and establishing of new reference ranges would be required.
AUTHOR CONTRIBUTIONS
Erica-Mari Nell partook in data collection and data analysis, and was the primary author responsible for writing the manuscript. Jenique Bailly, Diana Oelofse, and Michael Linström partook in data collection and data analysis and contributed to writing the manuscript. Jessica Opie and Zivanai Cuthbert Chapanduka were involved in oversight of the study and contributed to writing the manuscript. Marizna Korf co-designed the study, partook in data collection, was the primary author performing the data analysis, assisted in critical appraisal of the study and contributed to the manuscript. Helena Vreede co-designed the study, advised with data analysis, assisted in critical appraisal of the study and contributed to writing of the manuscript.
ACKNOWLEDGMENTS
The authors acknowledged ILEX South Africa (Pty) Ltd (Gauteng, South Africa) for supplying V-TUBE™, LASEC (Cape Town, South Africa) for supplying VACUETTE®, Reliable Diagnostic Supplies (Pty) Ltd (Gauteng, South Africa) for supplying Vacutest® and Synbiolab (Gauteng, South Africa) for supplying Vacucare blood collection tubes for the verification; NHLS for funding analysis and supply of BD Vacutainer® blood collection tubes for the verification. Phlebotomists: Aqeela Abrahams, Jehaan Brown, Nokuzola Gemi, Rayaan Isaacs, Marcellino Johannes, Asiphe Ngqandu, Boraro Sehoshe. Assistants: Gadija Abdullah, Shanaaz Arendse, Morné Bezuidenhout, Wendy Blose, Sylvester Chabunya, Benito Dekela, Cameron Francis, Thando Gcingca, Kulsoem Kasker, Thobile Khanyile, Daleen Kriel, Gail Lawrence, Mariam Mahomed, Lwando Mampunye, Abugyer Mosavel, Thembi Mthembu, Igsaan Noordien, David Richardson, Eva Rienhart, Kagiso Seakamela, Bianca Southon, Ashuelita Thompson, Dieter van der Westhuizen, Sigrid Vollmer, Laetitia Walters, Owen Wiese.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to disclose.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.