The diagnostic accuracy of Sysmex XN for identification of pseudothrombocytopenia using various thresholds for definition of platelet aggregation
Abstract
Introduction
The aim of this study was to investigate the diagnostic accuracy of the flag PLT-Clumps from the WNR/WDF and the PLT-F channels from Sysmex XN and to study how different cut-offs for investigation for pseudothrombocytopenia (PTCP) and the definition of platelet aggregation affected the diagnostic accuracy.
Methods
A smear review was performed for samples with platelet count <150 × 109/L and samples flagged for platelet aggregation by Sysmex XN-20. The samples were investigated for platelet aggregation in 30 fields using 40× objective. Findings were classified by size and quantity using two definitions of aggregation: the Norwegian quality improvement of laboratory investigations (Noklus) and the Groupe Francophone de'Hematologie Cellulaire (GFHC). The Q-values for the PLT-Clumps flag from the WNR/WFD channel and the PLT-F channel were compared with smear findings.
Results
ROC analysis showed that the diagnostic accuracy of the PLT-clumps flags increased with increasing stringency of the definition of platelet aggregation and when only samples with thrombocytopenia were investigated. With the most stringent PTCP definitions, the diagnostic accuracy of the PLT-Clumps flag from PLT-F was very high (AUC 0.97–0.98) and markedly better than for WNR/WDF.
Conclusion
The diagnostic accuracy of PLT-F from Sysmex XN-20 for identification of PTCP was very good and superior to the WNDR/WDF channel in samples with platelet count <150 × 109/L and a moderate to high number of aggregates in the smear. There is a need for a more precise definition of platelet aggregation.
1 INTRODUCTION
In vitro aggregation of platelets can cause pseudothrombocytopenia (PTCP). PTCP is often caused by the anticoagulant EDTA, but can also occur independently of EDTA or be caused by preanalytical errors such as poor mixing of sample and anticoagulant.1 The prevalence of PTCP varies in different populations and is reported to occur in 0.07–0.10% of outpatients and up to 2% in hospitalized patients. The incidence is probably even higher in patients with thrombocytopenia of unknown cause.1 Several guidelines recommend how laboratories should investigate for PTCP to avoid reporting falsely low results.2-4 In our experience laboratories use different platelet count cut-offs to decide which samples to investigate. Investigation for PTCP is time-consuming and results in delayed reporting of results.
Modern hematology instruments can flag samples for suspected presence of platelet aggregation. Such flags are incorporated into some of the guidelines, but there is limited documentation on their diagnostic accuracy.2-4 Sysmex XN instruments utilize impedance (PLT-I) and/or fluorescence flowcytometry (PLT-F) for platelet counting. The flagging for suspicion of platelet aggregation originates from the WNR/WDF channel (for counting leucocytes) and/or PLT-F channel. According to Sysmex the diagnostic accuracy of the flags from the two channels is similar.5
Platelet aggregation is mostly referred to as a qualitative phenomenon, being either present or absent. However, aggregation clearly exists on a quantitative scale. None of the mentioned guidelines describe how to quantify aggregation, and not all guidelines have a precise definition of aggregation. Accordingly, previous studies of PTCP from hematology instruments have used various or unspecified definitions of aggregation, raising the concern that the studies may not be comparable.6-10
The aim of this study was to investigate the diagnostic accuracy of the flag PLT-Clumps from the WNR/WDF and the PLT-F channels from Sysmex XN. Furthermore, we aimed to study how different cut-offs for investigation for PTCP and different definitions of platelet aggregation affected the diagnostic accuracy.
This prospective observational study is reported according to the standards for the reporting of diagnostic accuracy studies (STARD).11
2 MATERIALS AND METHODS
2.1 Inclusion of samples
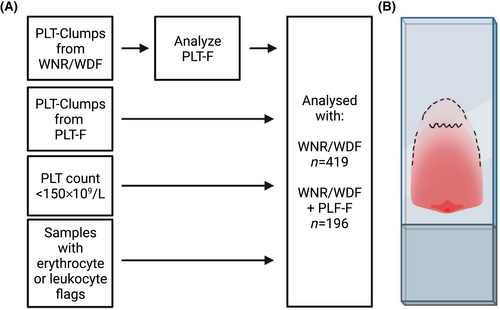
Blood was collected by venipuncture into EDTA tubes (BD Vacutainer K2 [EDTA] 7.2 mg Plus Blood Collection Tubes, Franklin Lakes, New Jersey, USA) from outpatients and hospitalized patients from October 2020 to January 2021 and analyzed on Sysmex XN-10/XN-20 (Sysmex Corporation, Kobe, Japan). Samples with platelet count <150 × 109/L and samples with the flag PLT-Clumps from either WNR/WDF or PLT-F were included (Figure 1A). Additionally, we included samples that were investigated with blood smears due to leukocyte or erythrocyte flags. This was done to include samples without PLT-clumps flag and samples with PLT-count >150 × 109/L as it was not possible to make and evaluate blood smears for every sample submitted for platelet counting in the period. The samples were analyzed on Sysmex XN-10/XN-20. All samples with PLT-I count <100 × 109/L and samples with the flag PLT-Clumps from WNR/WDF were automatically reanalyzed with PLT-F. Rules were defined in the middleware Sysmex Extended IPU (Sysmex Europe GMBH, Norderstadt, Germany) to identify samples for inclusion in the study. The laboratory is accredited by the ISO15189 standard for analysis of platelets and perform regular internal and external quality control.
2.2 Investigation for PTCP
According to the guidelines, investigation for PTCP should be performed by microscopic examination of blood smears.2-4 Blood smears were made by the laboratory staff as soon as possible and within 6 h from blood collection, either manually or semiautomated by using HemaPrep (CellaVision/J.P Gilbert Co, Pennsylvania, US). Smears were dried for 15 min and stained with a MCDh staining kit in the automated stainer RAL-Stainer (RAL Diagnostics, Martillac, France).
There are various definitions of PTCP. Principally, there are two necessary dimensions to define: the minimum number of aggregated platelets necessary to define a cluster as an aggregate (size) and how many such aggregates is necessary to cause PTCP (quantity). In the International Society of Laboratory Hematology «consensus guidelines» a positive finding of platelet clumping is defined as « > rare/occasional».2 The Groupe Francophone de'Hematologie Cellulaire (GFHC) defines aggregation as the presence of at least five clustered platelets.3 However, they state that presence of rare clumps may indicate a sampling problem rather than EDTA-induced PTCP. The Norwegian quality improvement of laboratory investigations (Noklus) defines PTCP as the presence of at least 3–5 clustered platelets.4 Neither GFHC nor Noklus specify the quantity of aggregates needed to cause PTCP. Internal procedures at the Laboratory for Medical Biochemistry at Lovisenberg Diaconal Hospital defines a positive finding of platelet aggregates as a minimum of 3 clusters containing a minimum of 3 platelets.
Microscopy was performed by one person using Olympus B-50 40x objective. 30 fields were investigated: five in the top edge, 10 in the tongue, five in the bottom edge and 10 in monolayer (Figure 1B). The number of clustered platelets in each aggregate in the 30 fields was registered and PTCP classified according to the Noklus and GFHC-definitions in Microsoft Excel (Microsoft Corporation, Redmond, USA).
Inspired by two studies of PTCP in veterinary medicine, guidelines, and internal procedures, we designed a system for quantification of platelet aggregation in smear review, shown in Table 1.3, 4, 12, 13
| Size | Average number of platelets per aggregate (based on 30 fields with 40× objective) | ||
|---|---|---|---|
| 0 | <3 aggregates in 30 fields | ||
| 1 | Small | 3–6 platelets | Based on the definitions from Noklus |
| 5–6 platelets | Based on the definitions from GFHC | ||
| 2 | Medium | 7–19 platelets | |
| 3 | Large | ≥ 20 platelets | |
| Quantity | Number of aggregates (based on 30 fields with 40× objective) | |
|---|---|---|
| 0 | <3 aggregates in 30 fields | |
| 1 | Small | 3–10 aggregates in 30 fields |
| 2 | Moderate | 11–20 aggregates in 30 fields |
| 3 | Large | >21 aggregates in 30 fields |
2.3 Statistical analysis
Sysmex XN reports a Q-value in the range 0 to 300 with increments of 10 for the flag PLT-Clumps with higher results reflecting increased probability of aggregation. To analyze the diagnostic accuracy of the flag PLT-Clumps from both WNR/WDF and PLT-F channel, receiver operating characteristic (ROC) analysis was performed in the Microsoft Excel statistical add-in Analyze-it (Analyze-it Software Ltd, Leeds, United Kingdom.) with the continuous Q-value for the flag PLT-Clumps as the diagnostic test and the findings of aggregates in blood smears (yes/no) as the reference standard. The flag PLT-Clumps is reported when the Q-value is ≥100, and therefore this was chosen as the cut-off for estimation of sensitivity and specificity.
We performed ROC analysis with various combinations of PTCP definitions and inclusion criteria reflecting common laboratory approaches to investigate all samples with platelet count <150 × 109/L or < 100 × 109/L. For samples that are flagged with Giant Platelets, a Sysmex algorithm suppresses the Q value and the PLT Clumps flag to avoid false flagging for platelet aggregation. Thus, samples with this flag could not be included in ROC analysis. Sysmex recommends that laboratories should follow their internal procedures for samples with the Giant Platelets flag. Therefore, it is possible that laboratories will perform blood smear review independently of suspicion of platelet aggregation for these samples.
We did not predefine the required sample size, but rather sought to maximize the statistical power by including as many samples as possible in the predefined inclusion period.
PTCP is in most cases EDTA dependent.14 Thus, in samples with EDTA dependent PTCP, thrombocyte measurement in citrate blood is expected to represent the true result while measurement in EDTA blood will be falsely low.1, 3, 15 We hypothesized that for samples with EDTA dependent PTCP there should be a positive association between quantification of platelet aggregation and the difference between thrombocyte counts in citrate and EDTA blood. That is, with few and/or small aggregates the difference between measurement in citrate and EDTA blood would be small, with large and/or many aggregates the difference would be large. If such a relationship exists, it can possibly be used to determine quantitatively what constitutes clinically significant PTCP. To investigate this, platelet count was performed in EDTA blood and uncentrifuged citrate blood (within 3 h of sample collection) when this was available from the same venipuncture. Platelet counts in citrated blood were corrected for the dilution factor 1.1, and only tubes with more than 90% filling were accepted. The difference between the measurements was compared to the number of aggregates in 30 fields.
2.4 Ethical considerations
The study was a part of the laboratory's work for quality improvement and the data was collected from analysis of anonymized samples submitted for platelet counting. Therefore, the study was not subject to application to the Norwegian Regional Committees for Medical and Health Research Ethics. The data protection officer at Lovisenberg Diaconal Hospital approved the project.
3 RESULTS
We included 419 samples in the study (Figure 1A). All samples were analyzed with WNR/WDF, 196 samples were analyzed with both WNR/WDF and PLT-F. For samples with platelet count between 100 × 109/L and 150 × 109/L 45 of the 229 samples were analyzed with PLT-F. The number of samples classified with platelet aggregation with the Noklus and GFHC definitions in our selected study population were 40% and 25%, respectively (Table 2). This difference was caused by samples with small platelet aggregates (3–5 platelets) which were classified as aggregates with the Noklus-definition, but not with the GFHC definition. The small aggregates of 3–5 platelets also affect the average size. However, the quantity in percent was similar with both definitions.
| Number of samples | NOKLUS = minimum 3 platelets at least 3 times | GFHC = minimum 5 platelets at least 3 times | ||
|---|---|---|---|---|
| Total number of smears reviewed | 419 | 419 | ||
| Without platelet clumping | 251 | 60% | 316 | 75% |
| With platelet clumping | 168 | 40% | 103 | 25% |
| Size 1 | 98 | 58% | 5 | 5% |
| Size 2 | 70 | 42% | 96 | 93% |
| Size 3 | 0 | 0% | 2 | 2% |
| Quantity 1 | 108 | 64% | 70 | 68% |
| Quantity 2 | 29 | 17% | 15 | 15% |
| Quantity 3 | 31 | 18% | 18 | 17% |
- Abbreviations: GFHC, Groupe Francophone de'Hematologie Cellulaire; Noklus, Norwegian quality improvement of laboratory investigations.
Forty samples were flagged in the WNR/WDF-channel and 49 samples were flagged in the PLT-F-channel. Thirty samples were flagged in both channels. Twenty-four samples were missing the Q-value due to the flag Giant Platelet which automatically overrule the PLT Clumps flag, and therefore excluded from ROC-analysis. Using the GFHC definition platelet aggregation was found in four of these samples.
As shown in Table 3, there was a large variation in diagnostic accuracy for both WNR/WDF and PLT-F when various combinations of inclusion criteria and PTCP definitions were used. The area under the curve (AUC) was in general higher with the GFHC definition than with the Noklus definition and higher when lower cut-offs for inclusion of samples were used (150 × 109/L and 100 × 109/L). We found higher AUCs for PLT-F than WNR/WDF. It is important to note that samples included for ROC analysis as presented in Table 3 are only partly overlapping for WNR/WDF and PLT-F. Comparative ROC analysis for the 196 samples that were analyzed with both channels were consistent with the results presented in Table 3 with significantly higher AUCs for PLT-F than for WNR/WDF (Supplemental Table 1).
| Number of samples without presence of aggregates in smear | Number of samples with presence of aggregates in smear | AUC 95% CI | Sensitivity at Q-value = 100 95% CI | Specificity at Q-value = 100 95% CI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WNR/WDF | PLT-F | WNR/WDF | PLT-F | WNR/WDF | PLT-F | WNR/WDF | PLT-F | WNR/WDF | PLT-F | |
| Noklus All | 205 | 125 | 152 | 68 | 0.56 0.50–0.61 |
0.80 0.73–0.87 |
0.20 0.14–0.28 |
0.57 0.45–0.69 |
0.96 0.92–0.998 |
0.92 0.86–0.96 |
| Noklus All & quantity 2 + 3 | 304 | 159 | 53 | 34 | 0.62 0.53–0.72 |
0.88 0.81–0.96 |
0.40 0.37–0.57 |
0.82 0.66–0.93 |
0.94 0.90–0.96 |
0.87 0.81–0.92 |
| Noklus PLT ≤150 | 198 | 121 | 102 | 46 | 0.54 0.47–0.61 |
0.76 0.67–0.85 |
0.15 0.09–0.23 |
0.50 0.35–0.65 |
0.97 0.93–0.99 |
0.95 0.90–0.98 |
Noklus PLT ≤150 Quantity 2 + 3 |
279 | 148 | 21 | 19 | 0.59 0.44–0.74 |
0.88 0.78–0.99 |
0.38 0.18–0.62 |
0.79 0.54–0.94 |
0.95 0.92–0.97 |
0.91 0.85–0.95 |
| Noklus PLT ≤100 | 74 | 90 | 30 | 35 | 0.50 0.38–0.61 |
0.73 0.62–0.84 |
0.17 0.06–0.35 |
0.43 0.26–0.61 |
0.96 0.89–0.99 |
0.96 0.89–0.98 |
| Noklus PLT ≤100 Quantity 2 + 3 | 93 | 113 | 11 | 12 | 0.60 0.40–0.80 |
0.86 0.70–1.00 |
0.36 0.11–0.70 |
0.83 0.52–0.98 |
0.96 0.98–0.99 |
0.92 0.85–0.96 |
| GFHC All | 260 | 149 | 97 | 44 | 0.57 0.50–0.64 |
0.84 0.76–0.92 |
0.26 0.18–0.36 |
0.71 0.55–0.83 |
0.94 0.91–0.97 |
0.88 0.82–0.93 |
GFHC All & quantity 2 + 3 |
327 | 175 | 30 | 18 | 0.49 0.37–0.62 |
0.93 0.90–0.97 |
0.27 0.12–0.46 |
0.89 0.65–0.97 |
0.90 0.87–0.93 |
0.81 0.75–0.87 |
| GFHC PLT ≤150 | 244 | 141 | 56 | 26 | 0.56 0.47–0.64 |
0.82 0.71–0.93 |
0.21 0.12–0.34 |
0.65 0.44–0.83 |
0.96 0.93–0.98 |
0.92 0.86–0.96 |
GFHC PLT ≤150 Quantity 2 + 3 |
284 | 153 | 16 | 14 | 0.57 0.38–0.75 |
0.97 0.94–0.99 |
0.44 0.20–0.70 |
0.93 0.66–1.00 |
0.95 0.91–0.97 |
0.90 0.84–0.94 |
| GFHC PLT ≤100 | 88 | 108 | 16 | 17 | 0.49 0.33–0.65 |
0.79 0.64–0.94 |
0.25 0.07–0.52 |
0.65 0.38–0.86 |
0.96 0.89–0.99 |
0.93 0.86–0.97 |
GFHC PLT ≤100 Quantity 2 + 3 |
95 | 116 | 9 | 9 | 0.59 0.35–0.83 |
0.98 0.96–1.00 |
0.44 0.34–0.79 |
1.00 0.66–1.00 |
0.96 0.90–0.99 |
0.91 0.85–0.96 |
- Abbreviations: AUC, area under curve; CI, confidence interval; GFHC: Groupe Francophone de'Hematologie Cellulaire; Noklus, Norwegian quality improvement of laboratory investigations; PLT, Platelet Count ×109/L; PLT-F, Platelet Fluorescence method for platelet counting on Sysmex XN instrument; Q-value, numeric value for the PLT-Clumps flag on Sysmex XN instrument; WNR/WDF, measurement channel for leucocyte count on Sysmex XN instrument.
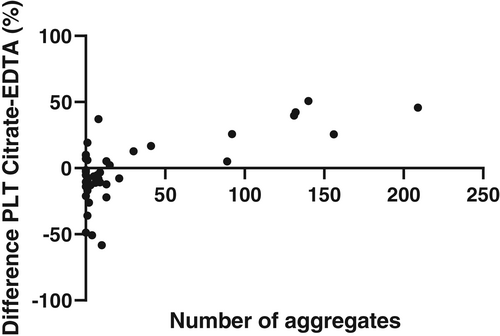
We also investigated whether there was a relationship between quantification of aggregation and the difference between platelet counts in citrate and EDTA blood in samples with platelet aggregation in blood smears. In Figure 2 we show the percentage difference between platelet counts in EDTA and citrate blood as a function of the observed number of aggregates in 30 fields with 40× objective. As expected, there is a wide dispersion of results at low numbers of observed aggregates, while at higher numbers there is clear tendency for higher platelet counts in Citrate-blood. The number of samples with PTCP is quite low. For samples with a low number of aggregates there appears to be higher thrombocyte counts in EDTA blood than Na-citrate blood. This may be due to errors or uncertainties in the dilution-factor in citrate tubes (e.g., underfilling).
4 DISCUSSION
In this study, we found that the diagnostic accuracy of PLT-F from Sysmex XN-20 for identification of PTCP was very good and superior to the WNR/WDF channel. Furthermore, we demonstrated that the diagnostic accuracy is highly dependent on the definition of PTCP, and to a lesser degree on the platelet count cut-off for investigation of samples for PTCP. PTCP is defined differently or not clearly in the guidelines, and we have suggested a way to establish a quantitative or semi-quantitative cut-off for platelet aggregation that leads to PTCP.
Our study is, to our knowledge, the first to compare the performance of the PLT-clumps flag from the PLT-F and WNR/WDF channels from Sysmex XN instruments. The PLT-F channel separates immature platelets from mature platelets and calculates an immature platelet fraction (IPF). An increased IPF together with information from the WNR and or/WDF scattergram allows the system to distinguish between giant platelets and platelet clumps. Interestingly, Hardy et al. has recently described a greater effect of PTCP on platelet counts when using impedance compared to fluorescence.16 Thus, it may seem that PLT-F is both better at detecting PTCP and more robust to the effect of PTCP.
Hawkins et al. studied the Sysmex XE-5000 ability to detect platelet clumps and found a sensitivity of 57% and specificity of 99% for the PLT-Clumps flag.7 This instrument utilizes impedance and optical detection for platelet count, not fluorescence, and the definition of platelet aggregation was unspecific, making it difficult to compare sensitivity. In 2015 Bruegel et.al compared five different hematology analyzers and found overall low sensitivity for platelet clumps flagging.17 Although the study used the PLT-F method on Sysmex XN-2000, the criteria for a positive smear finding was not specified, and the number of samples with PTCP was very small (n = 4).
We found that the diagnostic accuracy was greatly dependent on the definition of aggregation. Guidelines for identification of PTCP use different thresholds or lack definition of the threshold for identification of PTCP. There is probably ample experience in laboratories about what constitutes clinically significant platelet aggregation, but to our knowledge, there are no published studies that examines this. This is problematic for two reasons. First, it may lead to laboratories reporting PTCP in too many or too few samples. This can cause unnecessary confusion and retesting, or the reporting of spuriously low platelet counts. Second, it makes it difficult to examine and compare the performance of platelet aggregation flags from hematology instruments. We have suggested that PTCP can be quantified by the number of aggregates in 30 fields. Alternatively, it could be quantified by combination of the two dimensions aggregate size and the quantity of aggregates. In either case, the quantification of platelet aggregation can then be compared to the difference in platelet count between EDTA and citrated blood for samples with EDTA dependent PTCP. When analysing a small number of samples, we found a positive association between the number of aggregates and the difference between the platelet count in EDTA and citrated blood. While we cannot infer the cut-off for definition of PTCP in this study we believe such an analysis with a higher number of samples with clear proof of EDTA dependent platelet aggregation could be useful in future studies. There are also other potential methods to quantify platelet aggregation. Hardy et al. recently published a study where platelet aggregation was estimated as the ratio between aggregates and platelets in microscopic examination.16
Examination of samples for PTCP is time consuming. Since PTCP will not be found in most samples, it leads to an unnecessary delay in the reporting of results. We have previously found that examination for PTCP with blood smears delayed the result by a median 119 minutes in our laboratory.18 Platelet clumps flags can potentially be used both to identify samples that should be controlled for PTCP, and to exclude PTCP in samples with low platelet count that otherwise would have to be checked for PTCP. In either case, reanalysis with PLT-F is likely to result in a better selection of samples. When the most stringent definition of PTCP is applied (GFHC with quantity 2 and 3) the sensitivity and specificity of the PLT-clumps flag from PLT-F was very high. For laboratories that do not investigate samples with platelet counts higher than 100 × 109/L this may allow investigation of flagged samples with thrombocytopenia, but platelet count higher than 100x109/L without an unacceptable increase in the workload. One could possibly also lower the platelet count threshold for examination for PTCP. At our laboratory we are in discussions to not investigate samples with platelets >50 × 109/L if there is no PLT-Clumps flag from the PLT-F channel. However, the benefit in workload reduction and reduced turn-around time must be weighed against the risk of reporting spuriously low platelet counts. These considerations may vary depending on patient group and the preference in the clinical departments. Furthermore, laboratories should consider investigation of the flagging performance at their institution or look for other confirmatory evidence in published literature before lowering the threshold for investigation for PTCP.
It is important to note that performing PLT-F will add reagent costs that need to be compared to the potential benefit.
Our study has some limitations. First, PLT-F was analyzed for only approximately half of the samples. This project originated as a quality improvement project following the laboratory internal procedures and the recommendations from Sysmex stating that the PLT-clumps flag from WNR/WDF and PLF-F are interchangeable. Since there is a sparsity of previous studies for the diagnostic accuracy of the flag from the two channels and because PTCP is rare we have chosen to present data from all the samples even if this means that included samples for ROC analysis for the two channels are only partly overlapping. This decision is supported by the comparative ROC analysis of the 196 samples analyzed with both channels.
Second, ideally the study should have included all samples submitted for platelet counting in the inclusion period. By selecting groups of samples based on the platelet count and/or presence of PLT clumps flag or other flags such as leukocyte flags, it is possible that we have introduced a bias. However, PTCP is rare, and inclusion of all samples would increase the number of investigated samples a great deal, while probably not increasing the number of samples with PTCP substantially. Since investigation of PTCP is laborious, we chose pragmatic inclusion criteria that resulted in a moderately sized group of samples without platelet aggregation. Although we aimed to include all samples with the slightest suspicion of aggregates, the number of samples with PTCP defined with the most stringent criteria was quite low. This reflects how rare PTCP is in our patient population. Also, our aim was to include mostly samples with thrombocytopenia (platelet count <150 × 109/L) as they have the highest clinical interest. Confirmation of our findings in future studies would be welcome.
Third, blood smear review was performed by a single experienced observer and not by two independent observers, which could potentially have biased the observations of platelet aggregation in blood smears. This was a pragmatic choice based on the resources available in the project that also represents the usual way of investigation for PTCP in clinical laboratories. Furthermore, we argue that characterization of platelet aggregation is a less subjective task than many other morphology review tasks and therefore not particularly prone to inter-observer error.
In conclusion, our study indicates that PLT-F has a very high diagnostic accuracy for identification of PTCP and is superior to WNR/WDF. We suggest that the laboratory community should do further research on the threshold for clinically significant platelet aggregation with the aim of a clearer definition of PTCP in future guidelines.
ACKNOWLEDGEMENTS
We thank the staff at Laboratory of medical biochemistry, Lovisenberg Diaconal Hospital, Oslo, Norway for making and staining the smears.
CONFLICT OF INTEREST
The authors have no competing interests.
Open Research
DATA AVAILABILITY STATEMENT
Anonymized data can be made available upon reasonable request to the authors