Plasmablastic lymphoma: An update
Abstract
Plasmablastic lymphoma (PBL) is a highly aggressive B cell non-Hodgkin lymphoma frequently associated with immunosuppression, particularly human immunodeficiency virus (HIV) infection. Although PBL is rare globally, South Africa has a high burden of HIV infection leading to a higher incidence of PBL in the region. Laboratory features in PBL may overlap with plasmablastic myeloma and other large B cell lymphomas with plasmablastic or immunoblastic morphology leading to diagnostic dilemmas. There are, however, pertinent distinguishing laboratory features in PBL such as a plasma cell immunophenotype with MYC overexpression, expression of Epstein–Barr virus-encoded small RNAs and lack of anaplastic lymphoma kinase (ALK) expression. This review aims to provide a summary of current knowledge in PBL, focusing on the epidemiology, pathophysiology, laboratory diagnosis and clinical management.
1 INTRODUCTION
Plasmablastic lymphoma (PBL) is a rare and aggressive B cell lymphoma with poor clinical outcomes.1, 2 The disease is typically extranodal and was first described in 1997 by Delecluse et al. in the oral cavity of HIV infected individuals.3 It is characterized histologically by a diffuse proliferation of plasmablasts or immunoblasts with a markedly high proliferation index. The neoplastic cells have a plasma cell immunophenotype with expression of plasma cell markers and absent or dim B cell markers. Unlike plasma cell neoplasms, PBL is frequently associated with MYC overexpression, due to either MYC translocations, MYC amplifications,4 or constitutive activation of STAT3.5, 6 In addition, most PBL cases show Epstein–Barr virus (EBV) co-infection, which is a further differentiating feature from plasmablastic myeloma.1
PBL typically occurs in immunosuppressed adults, particularly those with HIV infection or receiving immunosuppressive therapy.1, 2 South Africa (SA) has a particularly high burden of HIV infection with an estimated 7 800 000 adults and children living with HIV in 2020,7 which has led to a higher incidence of PBL in the region.8 In recent years, the scientific understanding of this rare lymphoma has been deepened by international and local SA research groups who have investigated the genomic landscape of PBL, including those with and without associated HIV infection.4, 6, 9-11 Furthermore, the association of PBL with activating MYC rearrangements and EBV has been further elucidated.12 These findings, which will be summarized in this review, have provided an improved understanding of the pathogenesis of this intriguing lymphoma and have revealed new potential targets for personalized medicine.
2 EPIDEMIOLOGY
PBL usually affects immunocompromised individuals with only scanty reports of PBL in individuals with an apparently intact immune system.1, 2 In SA, the majority of PBL cases occur in the context of HIV infection and HIV-associated PBL comprises a significant proportion of newly diagnosed lymphomas. PBL comprised 8.3%–13.6% of 4122 lymphomas diagnosed in Johannesburg between 2004 and 2009.8, 13 Of 759 new lymphomas diagnosed at our tertiary referral centre in Cape Town, between 2005 and 2010, there were 34 (4.5%) new cases of PBL, 29 (85%) in HIV positive (+ve) individuals, and 6 (20.7%) with bone marrow involvement.14 Similarly between 2012 and 2014, PBL was reported to comprise 4.9% of 163 aggressive lymphoma cases diagnosed at our centre, 6.5% of 122 non-Hodgkin lymphoma (NHL) cases, and 15% of 47 HIV-associated lymphomas.15 Of 228 PBL cases in a United States (US) study, the majority, 69%, were HIV-associated.16 Widespread ART use has not reduced the incidence of HIV-associated PBL despite immune-recovery and virological suppression.8
A male predominance is consistently reported for PBL with males comprising up to 75% of cases, and the reason for this is unknown.1, 8, 17 PBL can occur at any age, but is exceedingly rare in children, mainly occurring in the setting of HIV.1, 2, 18, 19 One case of childhood HIV-associated PBL was reported in a review of 75 childhood B-cell NHL diagnosed and treated between 2005 and 2014 at Red Cross Children's Hospital in Cape Town, the second largest paediatric hospital in the Southern Hemisphere.20
3 CLINICAL PRESENTATION
PBL typically presents as a mass in one or more extra-nodal sites, usually the oral cavity and/or gastro-intestinal tract.1, 2, 8, 21 A higher frequency of nodal and skin involvement is reported in immunosuppressed patients post-transplantation, as compared to other PBL subgroups.1, 2, 17 Nodal disease without extra-nodal involvement is rare, though it has been reported.8 Extranodal sites reported in >1% of PBL cases include the genitourinary tract, central nervous system, bone, liver, nasal cavities, lung, and orbits. Rarely, PBL may evolve from indolent lymphomas such as chronic lymphocytic leukaemia or follicular lymphoma.1
Advanced stage disease (Ann Arbor stages III and IV) at presentation is found in >65% of HIV + ve patients, 50% of post-transplant patients, and 25% of apparently immunocompetent patients.1, 2, 4 The reported frequency of bone marrow involvement for HIV +ve versus HIV negative (−ve) PBL cases varies. Castillo et al. reported bone marrow involvement in up to 40% of HIV-associated and 25% of HIV −ve in a large cohort of 590 PBL cases.1 In a smaller SA cohort, Vaughan et al. reported bone marrow involvement in 27% of HIV-associated PBL cases diagnosed in 2017.8
Meer et al. assessed the EBER status and other clinico-pathological characteristics associated with oral versus extra-oral PBL. Extra-oral PBL was found to be identical to its oral counterparts in terms of gender, age distribution, HIV status, morphological appearance, immunophenotypic profile, and Epstein–Barr virus-encoded small RNAs (EBER) status.22
4 LABORATORY FINDINGS
Cytological findings in PBL have only been described in small case series or case reports, which reflects the rarity of this lymphoma.1 Scanty case reports of peripheral blood involvement by PBL have been recorded in the literature. The most common cytological findings include hypercellular specimens, plasmablastic and immunoblastic morphological features, mitotic figures, single cell necrosis, background necrosis and fragments of tumour cell cytoplasm. Tumour cells occur as single cells and/or loosely formed groups without clustering, which is typical of high-grade B cell lymphomas.23
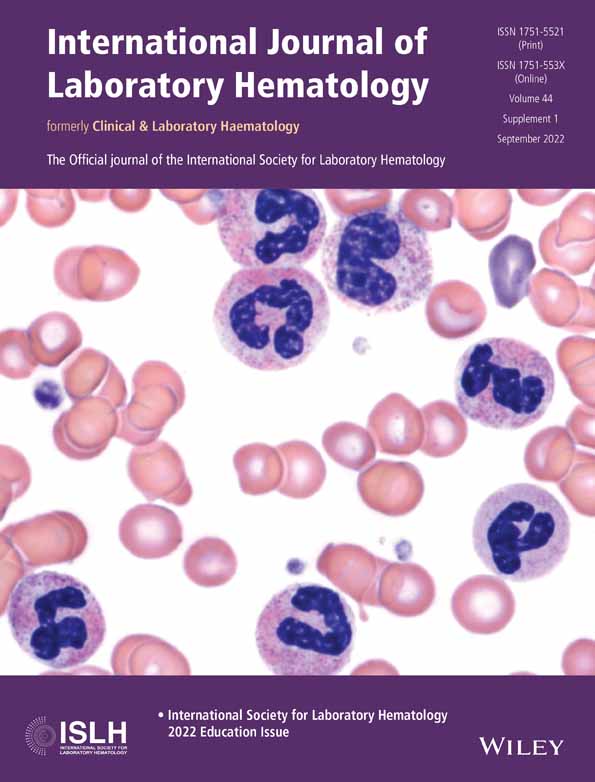
On histological sections, PBL presents as a diffuse proliferation of predominantly plasmablasts or immunoblasts, occasionally with multinucleated and anaplastic forms. Mature lymphoid cells with plasmacytic differentiation are also often present.2, 24-26 The tumour site and patient HIV status influence the tumour histology. For example, cases with monomorphic plasmablastic morphology are common in HIV-associated PBL of the oral mucosa.2 PBL involving other extranodal and nodal sites often shows plasmacytic differentiation, with more mature morphology such as smaller tumour cell size and clumped nuclear chromatin.2, 27, 28 Frequent mitotic figures and tingible body macrophages resulting in a ‘starry-sky’ appearance are also typical histological features of PBL.1, 2, 25 The Ki-67 proliferation index is invariably ≥70%21, 29 and usually >90%.1
Detailed immunophenotypic analysis by flow cytometry can be conducted if there is an appropriate bone marrow aspirate or body cavity fluid sample for analysis. Alternatively, a less detailed immunophenotypic profile may be provided by immunohistochemical (IHC) stains on histological sections. In summary, the immunophenotype of PBL resembles that of terminally differentiated plasma cells1, 2, 30 with similar aberrancies found to those described in plasma cell neoplasms.29 PBL usually expresses CD38, CD138, IRF4/MUM1, PRDMI/BLIPM1, XBP1 and cytoplasmic immunoglobulin light chain restriction (either kappa or lambda), with or without dim CD45 and CD79a. B cell markers such as CD19, CD20 and PAX5 are not expressed or rarely show dim positivity in a small subpopulation.1, 2, 30 Many immunophenotypic aberrances have been described in both PBL and plasma cell myeloma which include the expression of CD56 (seen in up to half of PBL cases),26, 29-32 CD117, uniform positivity for CD28,32, 33 and/or loss of normal plasma cell markers such as CD27 and/or CD81.32 Aberrantly dim and/or partial expression of CD38 or CD138 may also be seen.32 Other aberrancies described in both PBL and plasmablastic myeloma include the expression of CD10,1, 2, 17, 26, 29, 30 epithelial membrane antigen (EMA), and T cell markers in particular CD4.26, 29, 30 BCL2 and BCL6 are usually negative.2 In EBV-associated PBL, which accounts for 70% of cases,1 CD30 and programmed cell death-ligand 1 (PD-L1) expression are well described1, 26, 30, 34, 35 and have implications for potential treatment such as the anti-CD30 therapy brentuximab vedotin,1, 26 or checkpoint blockade in the case of PD-L1 expression by tumour cells.34 In situ hybridisation for EBER should be performed routinely to assess the EBV status with positive expression supporting a PBL diagnosis.2 Typical morphological features of PBL are shown in Figure 1. Table 1 highlights the common immunophenotypic and in-situ hybridization findings in PBL.
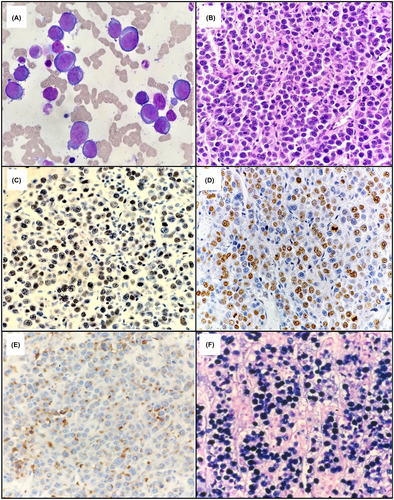
| Marker | Number of patients testeda | Proportion positive for marker (%) |
|---|---|---|
| CD4 (aberrant) | 17 | 5 (29.4) |
| CD20 | 244 | 26 (10.6) |
| CD30 | 38 | 12 (31.5) |
| CD38 | 43 | 41 (95.3) |
| CD45 | 142 | 82 (57.7) |
| CD56 (aberrant) | 88 | 34 (38.6) |
| CD79a | 148 | 77 (52.0) |
| CD138 | 91 | 83 (91.2) |
| BCL2 | 68 | 20 (29.4) |
| EBER | 250 | 176 (70.4) |
| HHV8 LANA | 34 | 0 (0) |
| Ki-67 >80% | 73 | 52 (71.2) |
| MUM1/IRF4 | 84 | 83 (98.8) |
| MYC | 21 | 9 (42.8) |
- a Includes all plasmablastic lymphoma cases described in three publications between 2005 and 2021.
4.1 Other laboratory findings
A monoclonal serum immunoglobulin and bony lytic lesions with or without hypercalcaemia may be detected in rare cases of PBL.2, 28 Cases of HIV-associated PBL have been reported with monoclonal serum immunoglobulins measuring <20 g/L.28
4.2 Genetic studies
A complex karyotype, often with translocations involving the MYC locus at 8q24, may be detected via conventional karyotyping in PBL cases with suitable available samples for analysis.2, 25 MYC aberrations, either due to translocations or amplifications, can be detected using specific fluorescence in-situ hybridisation (FISH) analysis probes.4 These MYC genetic aberrations lead to MYC protein overexpression, which is commonly detected using IHC on histological sections, with a threshold positivity of ≥40% of tumour cells.21, 37, 38
5 PATHOGENESIS
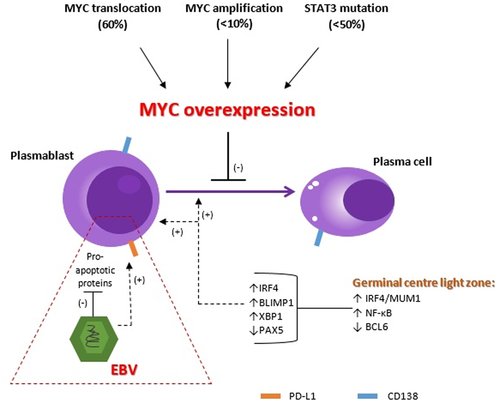
The pathogenesis of PBL, focussing on the most frequent genetic abnormalities, is summarized in Figure 2.
5.1 Role of EBV
Up to 70% of PBL cases are associated with EBV infection,1 and there is a higher frequency of EBV in HIV +ve versus HIV −ve PBL cases.1, 8, 16 EBV is a double-stranded DNA virus that preferentially infects naïve B cells, T cells, natural killer cells, and epithelial cells. Up to 90% of the world's population shows EBV seropositivity.39, 40 After primary infection by EBV, the virus becomes latent in memory B cells and persists by evading detection by the host's immune system.39-41 Viral latency and persistence are mediated via the expression of certain viral gene products such as the Epstein–Barr nuclear antigen (EBNA-1) and EBER.40 EBV has a B cell immortalizing effect via the inhibition of pro-apoptotic proteins and supports cellular survival via various intra-cellular signalling pathways such as NF-κB and NOTCH signalling pathways.39-41 Furthermore, EBV infection may result in the acquisition of oncogenic mutations leading to B cell transformation and tumorigenesis. The prevalence of EBV-driven B cell lymphomas is increased in HIV infected individuals, patients receiving iatrogenic immunosuppressive therapy, and the elderly.39-41
Despite antiretroviral therapy (ART) and viral suppression in those with HIV, individuals with HIV remain at increased risk of EBV-associated malignancies.8, 35, 39 This is likely due to a number of factors including immune evasion, chronic inflammation with an altered cytokine profile, and immune senescence, which persists in people living with HIV (PLWH) despite ART and virological suppression.39 In EBV-positive PBL cases, immune senescence and evasion are in part related to the expression of PD-L1 by PBL cells and tumour associated macrophages (TAM) which prevents anti-tumour cellular responses.6, 34, 42 The absence or decreased expression of major histiocompatibility complex (MHC) class II proteins by EBV infected PBL cells and the secretion of interleukin 10, transforming growth factor beta (TGF-β) and other suppressive mediators by TAM and T regulatory cells further promotes immune evasion.6, 42, 43
In PBL, EBV infection is demonstrated histologically by EBER in-situ hybridisation (ISH) positivity in the tumour cells.1, 2, 37 PBL is associated with a latency type I EBV program,5 although latency type II and III have also been described in post-transplant and HIV-associated cases.1, 12, 26 A recent study performed at our centre in Cape Town, showed EBV latency type 0 program in up to 70% of 49 PBL cases, the majority of which were HIV +ve. Most of this study cohort showed a restricted EBV latency program (0/ I) with 11% showing type II latency.12 Latency type 0/I is associated with a decrease in EBV gene expression as compared to latency types II and III.12, 40, 41 EBV latent membrane protein 1 (LMP1) is not expressed in EBV latency type 0/I and should therefore not be used diagnostically to stain for EBV in PBL tumour cells.1
5.2 MYC
MYC is a proto-oncogene situated on chromosome 8q24 which is involved in cellular metabolism, growth, proliferation, and apoptosis.1, 44 MYC expression is normally inhibited during plasma cell differentiation by transcription factors such as IRF4, BLIMP1 and XPB1.1 Up to 70% of PBL cases show MYC protein overexpression due to MYC translocations or amplifications (Figure 2).4, 5 A higher proportion (up to 87%) of MYC rearrangements in PBL have been reported in a separate study.9 MYC usually translocates to immunoglobulin genes, particularly the immunoglobin heavy chain (IgH) gene on chromosome 14q32.5 A high frequency of MYC aberrations has been reported in a series of 63 HIV-associated PBL cases with MYC amplifications reported in 43% and concurrent MYC translocations and amplifications in 49%.4 Triple hit lymphoma with rearrangements of MYC, BCL2 and BCL6, has not been described in PBL, however a de novo PBL with BCL2 and MYC rearrangements (double hit) has been reported in a case with malignant pleural effusion and leukaemic presentation.45
5.3 Other mutations and aberrant pathways
Other cellular proliferation and survival pathways are frequently aberrant in PBL, particularly the JAK–STAT, ERK–MAPK and NOTCH signalling pathways.5, 6, 9-11 Mutations involving B cell receptor (BCR) signalling, MYD88 (toll-like receptor pathway), genes involved in histone modification, TP53 and MYC have also been described in a subset of PBL cases.5, 6, 10, 11 Frontzek et al. investigated the genomic landscape in HIV + ve and HIV −ve PBL subgroups and found that STAT3 and MYC mutations occur more frequently in the setting of HIV infection.11 STAT3 SH2 domain mutations resulting in constitutive activation of STAT3 (phospho-STAT3) appear to be an alternative or cooperating genetic event to MYC translocations (or amplifications) by independently causing MYC protein overexpression (Figure 2).5, 6
Another study from SA reported the genomic landscape in a large cohort of 110 cases of HIV-associated PBL. Sixty-two percent of mutations detected in this cohort involved the JAK–STAT pathway with STAT3 SH2 domain (42%), JAK1 JH1 kinase domain (14%), and SOCS1 (10%) the most common. In contrast to the STAT3 SH2 domain and JAK1 JH1 gain-of-function mutations, SOCS1 mutations were widely distributed in the gene and resulted in the inactivation and loss-of-function of this tumour suppressor gene. Mutations involving the ERK–MAPK pathway (NRAS, KRAS, BRAF and MAP2K1) and NOTCH signalling pathway were frequent and occurred in 28 and 24% of cases, respectively.10 [Correction added on 18 October 2022, after first online publication: The preceding statement was corrected in this version.]
BLIMP1, a product of the tumour suppressor gene PRDM1, is a transcription factor and the main repressor of MYC expression in post-germinal centre B cells. PRMD1 missense mutations involving domains required for the regulation of MYC gene expression have been described in up to 50% of PBL with aberrant MYC expression and likely represent a ‘second hit’ event.5, 6, 21 MYC overexpression (due to MYC aberrations or STAT3 mutations) and PRDM1 mutations have been noted to occur more frequently in EBV-positive PBL,6 and are likely related to EBV-induced genomic instability caused by activation and proliferation of infected cells.39, 40 Furthermore, cellular apoptosis normally induced by MYC overexpression is inhibited by the EBV latency transcripts EBNA-3A and EBNA-3C.1, 41
Lastly, recurrent somatic copy number alterations (CNA) have also been described in PBL. Recently, two studies identified amplifications involving chromosome 1q and chromosome 7p and 7q and occurred in up to 43%, 32% and 33% of PBL cases, respectively.10, 11 Of particular interest is the amplification of chromosome 1q21.3 which includes the anti-apoptotic protein MCL1,10, 11 the molecular target of novel therapeutic MCL1 inhibitors.11, 46 A number of MCL1 inhibitors have recently entered phase 1 clinical trials for myeloid and lymphoid malignancies including plasma cell myeloma and non-Hodgkin lymphoma.46 CNAs influencing genes involved in histone modification have also been described.10
CD44, a transmembrane glycoprotein required for normal B cell function (including migration, homing and cellular interactions) and survival, has been shown to be highly expressed in most PBL cases, with or without amplifications of CD44. Increased CD44 expression has also been described in other B cell lymphomas and likely confers a growth and survival advantage to the tumour cells.5, 10
6 LYMPHOMAS WITH PLASMABLASTIC DIFFERENTIATION
The World Health Organization (WHO) Classification of tumours of haematopoietic and lymphoid tissues (revised 4th edition, 2017) includes distinct lymphomas with plasmablastic features. A summary of their immunophenotypes and discerning laboratory findings is presented in Table 2.
| Malignancy | Immunophenotype | Discerning laboratory findings |
|---|---|---|
| Plasmablastic lymphoma | Plasma cell markers including CD38, CD138, IRF4/MUM1, PRDM1/BLIMP1, and XBP1 | Lack of pan-B cell markers, HHV8 −ve. EBER +vea |
| Plasmablastic myeloma | Plasma cell markers expressed: CD38, CD138, IRF4/MUM1, PRDM1/BLIMP1, and XBP1. +/− cyclin D1b | Monoclonal serum or urine immunoglobulin, serum free light chain ratio ≥100, renal dysfunction, lytic bone lesionsc |
| Extra-osseous plasmacytoma with plasmablastic morphology | Plasma cell markers expressed: CD38, CD138, IRF4/MUM1, PRDM1/BLIMP1, XBP1 | MYC rearrangements −ve; EBER –ve |
| ALK-positive large B cell lymphoma | Plasma cell markers expressed: CD38, CD138, IRF4/MUM1, PRDM1/BLIMP1, XBP1, BOB1 and OCT2. ALK positivity (granular cytoplasmic pattern) using IHC | Lack of pan-B cell markers and CD30. Frequently, t(2;17) (p23;q23). Rarely, t(2;5) (p23;q35) |
| Extra-cavitary primary effusion lymphoma | Expression of pan B cell markers (CD19, CD20, CD79a and PAX5)d | HHV8 LANA1 nuclear +ve. EBER +ve in 65% of cases |
| HHV8–positive diffuse large B cell lymphoma, NOS | Express IRF4/MUM1 and cytoplasmic IgM lambda +/−CD20. CD138 –ve | HHV8 LANA1 nuclear +ve. EBER –ve |
| Immunoblastic DLBCL and EBV-positive DLBCL, NOS | Expression of pan B cell markers | Lack of plasma cell markers. BCL6 +/−ve in germinal centre derived DLBCL. EBER +ve in EBV-positive DLBCL, NOS. MYC aberrations in 8%–14% of DLBCL, NOS |
- Abbreviations: ALK, anaplastic large cell lymphoma kinase; CTLC, cutaneous T cell lymphoma; DLBCL, diffuse large B cell lymphoma; EBER, Epstein–Barr virus-encoded small RNAs; HHV8, human herpes virus 8; IHC, immuno-histochemistry; LANA1, latency associated nuclear antigen; NOS, not otherwise specified; NPM1, nucleophosmin.
- a Considering the EBV latency program in PBL, EBV LMP1 IHC staining should not be used in PBL.
- b Cyclin D1 detected in cases with CCND1 gene rearrangements.48
- c Monoclonal serum immunoglobulin and lytic bone lesions have been described in rare case of HIV-associated PBL.2, 28
- d Primary effusion lymphoma (PEL) is limited to effusion fluid (a defining feature distinct from PBL) and classically does not express B cell markers.26
In particular, distinguishing PBL from plasmablastic myeloma can be challenging due to similar morphological and immunophenotypic features.26, 29-31, 49 Plasmablastic myeloma is a cytomorphological subtype of plasma cell myeloma (PCM) which accounts for 8.2% of PCM cases.50 Consideration of the clinical, radiological and other laboratory findings is required to make a distinction between PBL and plasmablastic myeloma.30, 31 EBER ISH positivity occurs in 75% of PBL cases, and is exceedingly rare in plasmablastic myeloma, where it is associated with plasmablastic morphology.1, 2, 29, 37, 51 HIV infection and younger age do not invariably support a diagnosis of PBL, especially in high HIV burden countries. PBL may occur in the elderly1, 2 and plasmablastic myeloma may occur in younger patients in the setting of HIV.52
Patients with plasmablastic myeloma usually have a significant serum monoclonal immunoglobulin, which is not present in PBL.1, 30 In addition, patients with PBL often have anaemia and/or renal impairment due to underlying chronic disease or drugs, which may lead to confusion with plasmablastic myeloma. According to WHO diagnostic criteria, the diagnosis of plasma cell myeloma requires clonal bone marrow plasma cells of at least 10%, or biopsy proven plasmacytoma, as well as at least one myeloma defining event. These myeloma defining events include end organ damage attributable to the plasma cell proliferation such as hypercalcaemia, renal impairment, anaemia and lytic bone lesions.36, 48 However, lytic bone lesions have also been described in rare case of HIV-associated PBL,2, 28 and both PBL and plasmablastic myeloma may present with bone marrow involvement, MYC overexpression,26, 53 and a high Ki-67 proliferation index (>80%).26, 29 However, MYC rearrangements occur as late events in plasmablastic myeloma and in 50% of cases do not involve immunoglobulin loci, in contrast to PBL.49, 53, 54 Mori et al. have proposed a diagnostic algorithm where the absence of EBER expression plus a Ki-67 <80% supports a diagnosis of plasmablastic myeloma. The diagnosis of plasmablastic myeloma is further supported by a serum free light chain ratio of ≥100.36
Extramedullary plasmacytoma with plasmablastic or anaplastic morphology may be particularly challenging to distinguish from PBL. Apart from similar morphology, immunophenotype and localisation to PBL, extramedullary plasmacytoma is defined by the absence of bone marrow involvement and end organ damage, which are found in PCM.26, 36, 48 Importantly, PCM may show extramedullary disease with plasmablastic morphology at initial diagnosis or relapse.55 In addition, 20% of plasmacytoma cases show a small clonal serum immunoglobulin peak on serum or urine protein electrophoresis. Rare plasmacytoma cases show EBER ISH positivity; however, MYC rearrangements do not occur.48 Where distinction is not possible, the WHO suggests that a descriptive diagnosis is made such as ‘plasmablastic neoplasm, consistent with PBL or anaplastic plasmacytoma’.2
Other aggressive lymphomas with plasmablastic or immunoblastic morphology should also be considered in the differential diagnosis of PBL.30
7 HIV STATUS AND THE IMPACT OF ANTIRETROVIRAL THERAPY
PLWH have an increased risk of developing PBL1, 2 and present at a younger age.16, 56 Prognosis in PBL, regardless of immune status, is invariably dismal with a median overall survival (OS) of <12 months.1, 17 Furthermore, PBL in the setting of HIV is associated with more advanced stage disease compared to apparently immunocompetent patients.2, 16, 57 Despite this, survival rates in PBL do not appear to be adversely affected by HIV infection, however further larger studies are needed.16, 17
Patients with HIV-associated PBL have been reported to respond better to chemotherapy compared to HIV negative PBL cohorts. This is likely due to starting ART in ART naïve HIV +ve patients newly diagnosed with PBL, which results in improved immune surveillance associated with immune recovery.16 In addition, PBL in HIV −ve patients is more likely to occur in the elderly, a population with poorer performance status and organ reserve, and able to tolerate less intense chemotherapy regimens.16 Improved clinical outcomes have been noted in EBV-positive HIV-associated PBL compared to EBV-negative HIV-associated PBL cases, which possibly reflects the effect of ART, subsequent virological control and reduced lymphomagenesis in EBER +ve HIV patients.8
Similar OS has been reported in ART experienced and ART naïve HIV-associated PBL cases,1, 8 Further studies including larger cohorts of PBL patients are needed to better assess the impact of CD4 count recovery and ART on OS, and to compare the ART experienced and naïve groups.57 For HIV-associated PBL treated with chemotherapy, Castillo et al. reported an overall response rate to chemotherapy using CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) or more intense chemotherapy regimens of approximately 77%, however overall survival was poor at 14 months with no evidence of longer survival with more intense regimens. The poor prognosis in PBL has been attributed to a high relapse rate, chemotherapy refractory disease and superimposed infections.58 Similar findings have been reported by other study groups.56, 57
8 OTHER PROGNOSTIC FACTORS
PBL with MYC aberrations appears to be associated with a particularly poor OS.1, 2 Furthermore, advanced stage disease57, 59 and a poor performance status (ECOG ≥2) are consistently associated with worse outcomes which supports the use of the age-adjusted International Prognostic Index (aaIPI), or enhanced IPI scores (such as the revised IPI or National Comprehensive Cancer Network [NCCN]-IPI), as prognostic tools in PBL.1, 56, 60 Scanty and conflicting data exist for other prognostic variables such as age, EBER status, bone marrow involvement, LDH levels, and Ki-67 expression.1
9 TREATMENT
There is no accepted evidence-based systemic therapy for PBL due to the rarity of this disease. CHOP remains a commonly used treatment regimen particularly in resource-constrained settings.57 The National Comprehensive Cancer Network (NCCN) guideline on AIDS-related B cell lymphomas (version 5.2021), however, recommends more intense regimens in PBL1, 56, 61, 62 and suggests dose-adjusted (DA)-EPOCH (etoposide, vincristine and doxorubicin with bolus doses of cyclophosphamide and prednisone) as a preferred alternative.62 More intensive treatment regimens have not demonstrated a clear advantage over CHOP in two retrospective studies.57, 59 A systematic review found no significant difference in objective response rates when comparing CHOP and DA-EPOCH, however, the use of more aggressive chemotherapy resulted in improved overall survival.63 The addition of bortezomib to EPOCH has yielded excellent results in some case series.64, 65
The International Prognostic Index (IPI) has been shown to be a useful prognostic tool,56, 60 and advanced stage is associated with a higher risk of relapse.57, 59 The use of chemotherapy and the achievement of a complete remission (CR) improve immediate outcomes in PBL.56, 61 A survival benefit is seen with the addition of involved site radiotherapy in patients with limited stage disease and those with disease in the head and neck region.66, 67 Radiotherapy is an easily accessible and more affordable modality in low and middle income countries where costly biologic therapies and stem cell transplant are not always available. Table 3 lists the current treatment regimens used in PBL. Three international groups have active clinical trials assessing the role of EPOCH in combination with other therapies for PBL registered on ClinicalTrials.gov (Table 3).
| Chemotherapy regimen | Current active clinical trials and ClinicalTrials.gov identifier | |
|---|---|---|
| CHOP | Cyclophosphamide, doxorubicin, vincristine and prednisone | |
| EPOCH | Etoposide, vincristine and doxorubicin with bolus of cyclophosphamide and prednisone | NCT04139304: multi-centre, open-label, feasibility study for dose-adjusted EPOCH plus daratumumab in newly diagnosed PBL. Status: Recruiting, early phase I. NCT01092182: dose-adjusted EPOCH plus rituximab in adults with untreated Burkitt lymphoma and MYC +ve DLBL and PBL. Status: active, phase II. NCT02481310: dose-adjusted EPOCH plus Rituximab (DA-EPOCH-R) plus ixazomib in MYC +ve lymphoid malignancies including PBL. Status: active, phase I/II |
| CODOX-M/IVAC | Cyclophosphamide, vincristine, doxorubicin, methotrexate alternating with ifosfamide, etoposide, and cytarabine | |
| Hyper-CVAD-MA | Hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with methotrexate and cytarabine | |
Despite achieving complete remission, most patients with PBL relapse with a median progression free survival of 6 months.61 Autologous stem cell transplant in rare eligible patients at first CR or the use of novel or immunomodulatory drugs remain an option in resource rich settings,61 however, prospective clinical trial data is lacking due to the scarcity of PBL.
Other novel drugs and treatment approaches for PBL currently undergoing phase II clinical trials include belantamab mafodotin for relapsed or refractory PBL (NCT04676360), and daratumumab plus dexamethasone plus bortezomib in relapsed or refractory PBL (NCT04915248; phase II).
10 CONCLUSION
PBL is a rare and aggressive lymphoma with a dismal prognosis despite intensive chemotherapy regimens and virological suppression in the setting of HIV. Other aggressive lymphomas with plasmablastic morphology may lead to diagnostic dilemmas, however, a systematic laboratory diagnostic approach, including EBER status, typically clarifies the precise diagnosis of PBL. In countries such as SA with a high prevalence of HIV, a high index of suspicion for PBL and other aggressive lymphomas is needed despite wide-scale ART use and virological suppression.
ACKNOWLEDGEMENTS
The medical and nursing staff at Groote Schuur Hospital caring for these patients.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Jenique Bailly and Jessica J. Opie conceptualized the article and content. Jenique Bailly and Jessica J. Opie wrote, edited and reviewed the initial drafts. Nicholas Jenkins photographed the morphology. All authors reviewed, edited and approved the final manuscript.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable as no new data generated.