Impact of different preanalytical conditions on results of lupus anticoagulant tests
Abstract
Introduction
The currently recommended preanalytical conditions for lupus anticoagulant (LA) analysis require analyzing samples in fresh or freshly frozen platelet-poor plasma. The aim of this study was to evaluate whether alternative and less cumbersome preanalytical procedures for LA testing give significantly different results compared to recommended conditions.
Materials and Methods
Citrated blood samples were drawn from 29 study participants, 15 with negative and 14 with positive LA results. The samples were processed according to the ISTH guideline for LA testing and compared to several alternative preanalytical conditions. Measurements were performed using the dilute Russell's viper venom time (DRVVT) and silica clotting time (SCT), both screen and confirm, on a STA-R Evolution analyzer. Stability criteria were based upon biological variation.
Results
All DRVVT tests (normalized screen, confirm, and screen/confirm ratio) met the stability criteria for all the preanalytical conditions. The SCT tests (normalized screen, confirm, and screen/confirm ratio) met the stability criteria only when treated according to the ISTH guideline, except for SCT normalized screen/confirm ratio which also met the stability criteria for double-centrifuged aliquoted plasma stored in room temperature for 24 hours and then analyzed “fresh” or after being frozen. One warfarin-treated patient was reclassified from positive to negative for DRVVT after the preanalytical modifications, while 2 of 29 participants became falsely positive for 2 of 8 conditions for SCT.
Conclusions
The DRVVT assays met the criteria for stability for all preanalytical conditions tested, while the SCT assays should be interpreted with caution if the preanalytical guidelines from ISTH are not followed.
1 INTRODUCTION
The results of lupus anticoagulant (LA), together with anticardiolipin and anti–beta2-glycoprotein I antibodies, will guide the anticoagulant treatment after an acute episode of thromboembolism and influence the use of prophylaxis during pregnancy.1-3 According to the guidelines for LA testing,4-6 a LA panel should consist of at least dilute Russell's viper venom time (DRVVT) and an activated partial thromboplastin time (eg, with silica as activator; silica clotting time [SCT]).
Preanalytical factors that shorten or prolong clotting times may alter LA results significantly.7-9 The latest guideline recommends freezing the plasma if LA cannot be analyzed within 4 hours after sampling.4 Freezing plasma may cause rupture of platelet membranes, resulting in excess phospholipids in the samples, again leading to decreased sensitivity for antiphospholipid antibodies and the potential of falsely negative results.7, 10, 11 Consequently, guidelines for LA testing recommend centrifugation of plasma until the platelet count is less than 10 × 109/L,4-6, 9 and laboratories usually perform double centrifugation to achieve this. There are some recent published studies on the stability of LA results for different sample handling conditions.11-14 One study showed that LA testing in fresh plasma (stored in room temperature) was stable up to 6-8 hours, but did not test for longer duration.13
Labor-intensive and cumbersome procedures are prone to errors, and if possible, the preanalytical recommendations for LA testing should be simplified.15 If simplification is not possible, laboratories still need information on the preanalytical conditions that cause the largest errors and the magnitude of the expected errors. Such knowledge is useful in guiding clinical decision making as less optimal preanalytical conditions may occur. The aim of this study was to investigate how different preanalytical conditions change the LA results and their interpretation.
2 MATERIALS AND METHODS
2.1 Subjects
The study was approved by the Regional Ethical Committee (REC number 2010/2037-4). Patients >18 years, living in the proximity of the hospital laboratory, who had positive or negative results of LA testing, were identified by searching the laboratory information system. Patients were invited to participate by regular mail and reminded by phone call from the Department of Rheumatology. A total of 29 study participants (aged 22-81 years, 21 women) gave informed written consent and were included. Warfarin was used by 9 participants (international normalized ratio [INR] range 1.5-2.6), and one used low molecular weight heparin.
2.2 Methods
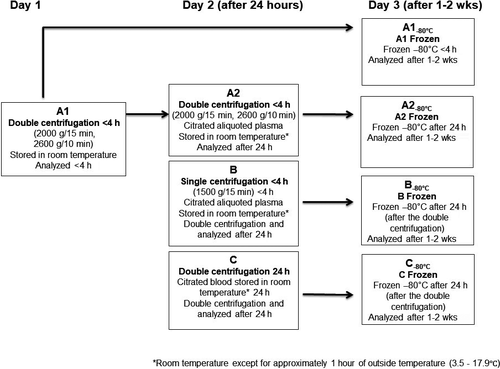
Blood sampling was performed at the Department of Medical Biochemistry and Pharmacology (Haukeland University Hospital) on three separate days from February through May 2014. The samples were collected in 3.2% sodium citrate tubes (Vacuette®, Greiner Bio-One GmbH), and the samples from each study subject were processed at eight different preanalytical conditions (Figure 1). The first tube from each patient was handled according to the ISTH guideline5 with double centrifugation (2000 g in 15 minutes, then 2600 g in 10 minutes) to achieve platelet-poor plasma (<10 × 109/L) and analyzed within 4 hours (stored in room temperature) after the blood draw (Figure 1, condition A1). In condition A2, aliquoted plasma from A1 was stored for 24 hours before analyzed (to mimic double-centrifuged aliquoted “fresh” plasma being received from another hospital). In condition B, citrated blood was centrifuged at 1500 g (single centrifugation) within 4 hours, then double-centrifuged after 24 hours and then analyzed (to mimic fresh single-centrifuged aliquoted plasma received from smaller centers not familiar with double centrifugation). In condition C, citrated blood was double-centrifuged after 24 hours and then analyzed (to mimic “fresh” citrated blood received from smaller centers). The aliquoted samples (secondary tubes) in A2 and B and the citrated blood (primary tubes) in C were transported outdoors in a vertical position in a bag for 30 minutes on two separate occasions (to mimic two short transportation legs). The samples were not agitated in-between transport. In the remaining storage time, the samples were stored at room temperature (18-22°C). Double-centrifuged aliquoted plasma from conditions A1, A2, B, and C was frozen at minus 80°C in 1-mL microtubes with screw cap (A1−80°C, A2−80°C, B−80°C, and C−80°C), mimicking the situation when the laboratory does not analyze the samples immediately. Conditions A1 and A1−80°C are in accordance with the guidelines for LA testing.4-6, 9 The frozen samples were rapidly thawed for 5 minutes in a 37°C water bath and mixed thoroughly before being analyzed in batches after 1-2 weeks in the freezer. The platelet counts after single centrifugation at 1500 g and after double centrifugation were <22 × 109/L and <8 × 109/L, respectively.
2.3 Reagents, instruments, and reporting of results
All samples were analyzed by the DRVVT screen and confirm (STAGO) and the APTT test SCT screen and confirm (Instrumentation Laboratories) on the STA-R Evolution instrument (STAGO). The DRVVT and SCT tests consist of an initial screen test that utilizes low concentration of phospholipids (DRVVTscreen and SCTscreen) and a confirm test with high concentration of phospholipids (DRVVTconfirm and SCTconfirm). Phospholipid dependence, that is, that the prolonged clotting time for the screen test is accompanied by a significantly shortened clotting time for the confirm test, is indicative of a positive LA result. This will be evident by a normalized screen/confirm ratio higher than the cutoff (99th percentile derived from healthy persons as recommended by the ISTH guideline5). In patients treated with warfarin, results should be interpreted with caution, as both screen and confirm tests may be prolonged, and the normalized screen/confirm ratio may be higher than in nonanticoagulated patients (“false-positive” results). Mixing tests were not performed in this study. The DRVVT and SCT reagents used in the present study contain a heparin inhibitor to avoid interference of unfractionated heparin and low molecular weight heparin up to certain levels (ie, 0.5-0.8 IU/mL and 1 IU/mL, respectively).
As recommended, normal pooled plasma (NPP) was analyzed in every run. A single batch of NPP, prepared in-house from 40 healthy donors according to the protocol A1−80°C, was used throughout the study. The uncertainty in the NPP result was reduced by analyzing it four times in every run. Each patient sample result was divided by the mean NPP result (normalization). Results were given as normalized DRVVTscreen ratio (DRVVTSR) (ie, DRVVTscreen patient [seconds]/DRVVTscreen NPP [seconds]) and normalized DRVVTconfirm ratio (DRVVTCR) (ie, DRVVTconfirm patient (seconds)/DRVVTconfirm NPP (seconds). Normalized SCT results were reported similarly, as SCTSR and SCTCR. The outcome of the LA test was finally determined based on the normalized screen/confirm ratio (DRVVTnormalized ratio [DRVVTNR]) (ie, DRVVTSR/DRVVTCR) and SCTNR (ie, SCTSR/SCTCR). In accordance with the ISTH guideline,5 the 99th percentiles (DRVVTNR ≥ 1.28 and/or SCTNR ≥ 1.31) were used as the cutoff for LA positivity in the present study. These cutoffs were based upon analyzing samples from 126 healthy volunteers <50 years old.
The same lot of reagents (DRVVT screen [lot nr 110292], DRVVT confirm [lot nr 110522], and SCT screen and confirm [lot nr 520073]) were used. The within-run analytical variations (CVA), based on NPP measured in duplicates, were 1.8%, 1.6%, and 1.7% for DRVVTSR, DRVVTCR, and DRVVTNR, respectively, and 2.4%, 1.8%, and 2.3% for SCTSR, SCTCR, and SCTNR, respectively. Total analytical CVs (within- and between-run variation) calculated from internal quality control materials and NPP were less than 5% (Table S1).
INR (Owren) was measured with the STA SPA+ reagent (STAGO) on the STA-R Evolution instrument, and the platelet count was measured by Cell-Dyn Sapphire (Abbott Diagnostics Division).
2.4 Statistics
2.4.1 Criteria for sample stability according to allowable bias and total error (TE)
For each preanalytical condition, the results were calculated as a percentage of the corresponding results from condition A1. The samples were defined as stable if (a) the limits of the 90% confidence interval (CI) of the mean were within 100% ± allowable bias, and (b) 95% of the individual results were within 100% ± allowable TE (ie, ~2 individual results could be outside the limits for allowable TE). This is a general statistical method,16, 17 which is applicable also for clotting assays. Allowable bias was defined as maximum allowable bias, that is, 0.375 × total biological variation = 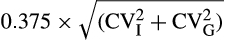 , where CVI is within-subject biological variation, and CVG is between-subject biological variation. Allowable TE was defined as 1.65 × maximum allowable imprecision + maximum allowable bias, where maximum allowable imprecision is 0.75 × CVI.18 The CVI and CVG used for calculation of stability requirements in the present study were derived from the only study found on biological variation for DRVVTSR and DRVVTNR.19 Based on biological variation data for DRVVTSR (CVI 7% and CVG 10%),19 allowable bias and allowable TE were calculated to be 5% and 13%, respectively. We did not find any studies describing biological variation for SCT, but allowable bias may be derived from the width of the reference interval, as shown in Table S2. The calculations based on the reference interval for SCTNR supported the use of the widest limits (5% and 13%) as quality specification (Table S2), and consequently, these were chosen for evaluation of both tests.
, where CVI is within-subject biological variation, and CVG is between-subject biological variation. Allowable TE was defined as 1.65 × maximum allowable imprecision + maximum allowable bias, where maximum allowable imprecision is 0.75 × CVI.18 The CVI and CVG used for calculation of stability requirements in the present study were derived from the only study found on biological variation for DRVVTSR and DRVVTNR.19 Based on biological variation data for DRVVTSR (CVI 7% and CVG 10%),19 allowable bias and allowable TE were calculated to be 5% and 13%, respectively. We did not find any studies describing biological variation for SCT, but allowable bias may be derived from the width of the reference interval, as shown in Table S2. The calculations based on the reference interval for SCTNR supported the use of the widest limits (5% and 13%) as quality specification (Table S2), and consequently, these were chosen for evaluation of both tests.
2.4.2 Reclassification of results based on sample stability
The clinical significance of alternative preanalytical conditions for LA testing was evaluated based upon whether the classification of the results from condition A1 changed category from negative to positive or vice versa. To avoid reclassification based purely on analytical variation, results deviating less than ±1.96 times the within-run analytical variation for the assay were not evaluated as reclassifications.
Descriptive statistics (median, 10 and 90 percentiles) were calculated by use of spss version 23.0 (SPSS Inc).
3 RESULTS
The initial analysis (guideline-recommended preanalytical condition A1) of the 20 nonwarfarin participants resulted in 12 negative (both DRVVTNR and SCTNR) and eight positive LA results (six positive for both DRVVTNR and SCTNR and one positive for either DRVVTNR or SCTNR). The initial analysis of the nine warfarin-treated participants showed three negative and six positive results (one was positive for both DRVVTNR and SCTNR, the others only for DRVVTNR) (Figure S1). The medians, and 10 and 90 percentiles for DRVVT and SCT for the eight different preanalytical conditions are shown in Table 1.
| Preanalytical conditions (see Figure 1 for details) | ||||||||
|---|---|---|---|---|---|---|---|---|
| A1 | A1−80°C | A2 | A2−80°C | B | B−80°C | C | C−80°C | |
|
Cent1: Double Storage: 4 h Temp: Rt |
Cent1: Double Temp: Rta/−80°C |
Cent1: Double Storage: 24 h Temp: Rt-1 |
Cent1: Double Temp: Rt-1a/−80°C |
Cent1: Single Storage: 24 h Temp: Rt-1 Cent2: Double |
Cent1: Single Temp: Rt-1a/−80°C Cent2: Double |
Cent1: NA Storage: 24 h Temp: Rt-1 Cent2: Double |
Cent1: NA Temp: Rt-1a/−80°C Cent2: Double |
|
| Assay |
Median (10 and 90 percentiles) |
|||||||
| DRVVTNR | 1.24 (1.06-2.10) | 1.22 (1.07-1.98) | 1.20 (1.05-2.06) | 1.20 (1.07-1.90) | 1.19 (1.06-2.0) | 1.22 (1.06-2.0) | 1.21 (1.05-1.99) | 1.24 (1.06-1.94) |
| DRVVTSR | 1.28 (1.09-2.60) | 1.31 (1.13-2.59) | 1.31 (1.08-2.67) | 1.34 (1.11-2.76) | 1.32 (1.09-2.54) | 1.37 (1.10-2.68) | 1.28 (1.05-2.47) | 1.39 (1.05-2.55) |
| DRVVTCR | 1.07 (0.99-1.49) | 1.08 (1.00-1.47) | 1.08 (0.99-1.48) | 1.09 (1.00-1.51) | 1.08 (1.01-1.47) | 1.09 (1.01-1.54) | 1.04 (0.98-1.49) | 1.07 (0.99-1.51) |
| SCTNR | 1.07 (0.90-2.32) | 1.04 (0.89-2.15) | 1.09 (0.96-2.13) | 1.07 (0.92-2.10) | 1.11 (0.94-2.12) | 1.08 (0.91-2.16) | 1.10 (0.99-2.02) | 1.05 (0.93-2.15) |
| SCTSR | 1.29 (0.99-2.96) | 1.25 (0.96-2.84) | 1.34 (1.02-2.93) | 1.31 (1.02-2.92) | 1.33 (1.05-2.71) | 1.29 (1.03-2.84) | 1.34 (0.96-2.67) | 1.33 (0.96-2.58) |
| SCTCR | 1.09 (0.94-1.46) | 1.10 (0.95-1.52) | 1.14 (0.98-1.56) | 1.14 (1.01-1.62) | 1.14 (0.99-1.58) | 1.13 (0.99-1.59) | 1.11 (0.97-1.57) | 1.16 (0.94-1.62) |
Note
- Cent1, immediate centrifugation condition; Cent2, centrifugation condition after 24-h storage (before analyzing or freezing); storage: time from blood sampling until analyzing. Temp, temperature; Rt, room temperature; Rt-1, room temperature, except for approximately 1 h of outside temperature (3.5-17.9℃); h, hour; wk, week; NR, normalized screen/confirm ratio; SR, normalized screen ratio; CR, normalized confirm ratio. NA, not applicable.
- a Time and temperature from blood sampling until freezing.
- b Time in freezer before thawing and analyzing.
3.1 Sample stability according to allowable bias and TE
The DRVVT assays (DRVVTNR, DRVVTSR, and DRVVTCR) met both the criteria (stated in Methods section) for allowable bias and allowable TE for all preanalytical conditions tested (Figure 2A-C).
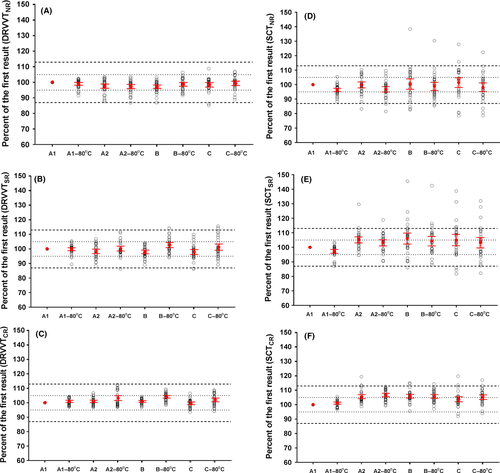
The SCTNR assay met the allowable bias criterion for all conditions tested, while the allowable TE criterion was met only for conditions A1−80°C, A2, and A2−80°C (Figure 2D). The SCTSR and SCTCR assays only met the criteria for allowable bias and TE for A1−80°C (Figure 2E,F).
3.2 Influence of warfarin treatment
Prolongation of both DRVVTSR and DRVVTCR results was seen for eight of the nine warfarin-treated participants in condition A1, while four of them had prolonged SCTSR and SCTCR results. The results remained prolonged regardless of preanalytical modifications, except in one DRVVT sample (Table 2). Excluding samples from warfarin-treated study participants did not alter the main conclusions (data not shown).
| Test/ID | Preanalytical conditions* | |||||||
|---|---|---|---|---|---|---|---|---|
| A1 | A1−80°C | A2 | A2−80°C | B | B−80°C | C | C−80° | |
|
Ratio 95% CI |
Ratio 95% CI |
Ratio 95% CI |
Ratio 95% CI |
Ratio 95% CI |
Ratio 95% CI |
Ratio 95% CI |
Ratio 95% CI |
|
| DRVVTNR | ||||||||
| Subject 28 (warfarin) |
1.35 1.31-1.39 |
1.31 1.27-1.35 |
1.21** 1.17-1.25 |
1.25** 1.21-1.29 |
1.27 1.23-1.31 |
1.44 1.40-1.48 |
1.37 1.33-1.41 |
1.44 1.40-1.48 |
| SCTNR | ||||||||
| Subject 13 (nonwarfarin) |
1.01 0.96-1.06 |
0.96 0.91-1.01 |
0.98 0.93-1.03 |
0.92 0.87-0.97 |
1.40 ** 1.34-1.46 |
1.32 ** 1.26-1.38 |
1.10 1.05-1.15 |
1.11 1.06-1.16 |
| Subject 20 (nonwarfarin) |
1.14 1.09-1.19 |
1.08 1.03-1.13 |
1.05 1.0-1.10 |
1.06 1.01-1.11 |
1.06 1.01-1.11 |
1.07 1.02-1.12 |
1.46 ** 1.41-1.53 |
1.40 ** 1.34-1.46 |
| Change in classification (significant difference) | NA |
0/29 0% |
1/29 3.5% |
1/29 3.5% |
1/29 3.5% |
2/29 6.9% |
2/29 6.9% |
2/29 6.9% |
Note
- 95% CI: DRVVTNR ± 1.96 × CVa × DRVVTNR, CVa = 0.0156 (1.56%) or 95% CI: SCTNR ± 1.96 × CVa × SCTNR, CVa = 0.0233 (2.33%).
- Lupus anticoagulant positive results in bold (>99th percentile).
- Abbreviations: DRVVT, dilute Russell's viper venom time; NR, normalized screen/confirm ratio; SCT, silica clotting time.
- * Preanalytical conditions are explained in more detail in Figure 1.
- ** Significant different from the result in preanalytical condition A1.
3.3 Reclassification of study participants based on sample stability
None of the participants in the study were reclassified when the guideline-recommended condition (A1−80°C) was used, neither for DRVVT nor for SCT. One warfarin-treated participant was reclassified from DRVVT positive to negative in conditions A2, A2−80°C, and B (Table 2). Two nonwarfarin participants were reclassified from SCT negative to positive, one in conditions B and B−80°C and the other in conditions C and C−80°C (Table 2).
4 DISCUSSION
The most important finding in this study was that the DRVVT assays were stable for all the preanalytical conditions which were tested, based upon the statistical criteria, while the SCT assays were less robust to the different preanalytical conditions. The SCTNR assay did not change in conditions A1−80°C, A2, and A2−80°C, but results should still be interpreted with caution as SCTSR and SCTCR assays, used for the calculation of the SCTNR, were stable only in condition A1−80°C. Reclassifications occurred in few patients and were related to warfarin treatment in one patient or preanalytical handling being quite different than those recommended (ie, conditions B and C) in two patients (Table 2).
4.1 Strength and limitations
The major strength of this investigation is that both positive and negative samples for LA were included. Several different preanalytical conditions commonly faced by laboratories were investigated, and the study also included an evaluation of the potential clinical consequences for the study participants. According to our knowledge, such a comprehensive range of preanalytical conditions has not been tested before. Another strength of the present study is that acceptance criteria for stability were defined both for bias and for TE. Several studies evaluating changes in hemostasis parameters only use acceptance criteria for bias. These studies state that the mean change should be within clinical significant limits (definitions differ from ±5% to ±20%),12, 20-24 some including 99% CI of the mean,20, 24 or define the desirable bias based on biological25, 26 or analytical27 variation. Our study demonstrates the important fact that storage conditions may lead to increased analytical variability, and consequently, several results may exceed the allowable TE even though the bias is reasonably low (Figure 2D; B, B−80°C, C, C−80°C). If a bias of 10% had been used as the sole criterion in the present study,20, 24 the assays would have met this criterion for all conditions except SCTSR in conditions B and C.
A limitation of our study is that the number of study participants included is relatively low. This is compensated for by including the 90% CI for the bias in the quality specification, ensuring (ie, with 90% confidence) that the bias criterion is met. However, it cannot be excluded that more reclassifications would occur if more patients were included or if the samples were more agitated by longer transportation. In addition, the 99th percentile was used as the cutoff as recommended by the ISTH guideline. Only one study on biological variation for LA was found, using a different assay and instrument (HemosIL DRVVT/ACL Top; Instrumentation Laboratory) than the present study, implicating some uncertainty of the biological variation data used to calculate the allowable limits for bias and TE.19 However, the suggested limits were confirmed by additional calculations of biological variability based on the local reference values for the test (Table S2). Published biological variation data for APTT were not used in the present study because of a large variation of the CVI in the literature and uncertainty whether biological variation for a “simple” APTT measured in seconds will represent that for a SCT normalized screen/confirm ratio.
Ideally, LA testing should not be performed in patients using anticoagulants as anticoagulants usually cause prolonged clotting times, which may increase the risk of erroneous interpretation.28-30 It could be argued that samples from warfarin-treated patients should be excluded, especially as neither mixing test results nor cutoff especially for these patients were evaluated. In addition, the group of warfarin-treated patients are few; thus, it cannot be drawn firm conclusions regarding reclassifications. However, information of the influence of different preanalytical conditions on such samples is useful. In addition, the results were evaluated both with and without warfarin-influenced samples, and conclusions seemed robust. Only one coagulation instrument with one SCT reagent and one DRVVT reagent was tested in this study; it is however plausible that other APTT reagents and DRVVT reagents could be affected to a similar extent by these preanalytical conditions.
4.2 Stability of LAs according to the chosen allowable bias and allowable TE
DRVVT assays were stable for all tested preanalytical conditions, while SCT assays were affected by several of the conditions, especially conditions B and C. SCTSR and SCTCR were stable only for A1−80°C. This information should be notified by laboratories, which only perform confirmatory tests when screen results are prolonged. The reason for DRVVT being more “robust” than SCT may be that the DRVVT tests are sensitive only to the coagulation factors in the common coagulation pathway (fibrinogen, FII, FV, and FX), while the SCT tests are also sensitive to changes in the intrinsic pathway (factors VIII, IX, XI, and XII). Consequently, the rapid decrease in both FV and FVIII during storage in room temperature of citrated blood20 and aliquoted plasma24 may affect SCT more than DRVVT. The reason why some SCT results (SCTSR results from C and C−80°C) decreased is unclear, especially as the platelet count was less than 10 × 109/L in all samples before freezing. A decrease after freezing, in spite of a low platelet count, was shown in two other-recent studies, but these studies used only single centrifugation.11, 12 The large variation, especially for SCTSR results from conditions B and C, demonstrates that the effect of different preanalytical condition is not predictable. Therefore, our findings support the recommendations from the CLSI guideline advocating follow-up LA tests when a strong clinical suspicion of APS remains despite a negative LA result.4
4.3 Clinical consequences (reclassification) of different preanalytical conditions
In contrast to the studies by Froom11 and Gosselin,12 where several patients were reclassified, only one warfarin-treated participant was reclassified from positive to negative for three of the different preanalytical conditions. In addition, two negative non–warfarin-treated participants were reclassified to positives, without any good explanation, as these results did not follow the same pattern as the other participants and could be analytical outliers. A follow-up sample in at least 12 weeks is recommended in all patients with a first positive result to confirm the result to avoid diagnosing antiphospholipid syndrome in patients where this is not persistent. However, our study indicates that additional follow-up samples, preferentially taken at a hospital laboratory, should also be performed in study participants with results close to the cutoff (both negative and positive), especially if a strong clinical suspicion of antiphospholipid syndrome is present.
5 CONCLUSION
The study demonstrates that the DRVVT assays (normalized screen, confirm, and screen/confirm ratios) in nonanticoagulated patients are robust regarding suboptimal preanalytical conditions compared to the SCT tests. As SCTSR and SCTCR assays are less stable, the results of SCT should be interpreted with caution if the preanalytical procedures recommended in the ISTH guidelines are not followed.
ACKNOWLEDGEMENTS
Thanks to Clara Gram Gjesdal and the Department of Rheumatology at Haukeland University Hospital for recruiting study participants and the Biomedical Laboratory Scientists at the Department of Medical Biochemistry and Pharmacology at Haukeland University Hospital for excellent technical assistance in blood sampling, preanalytical sample handling, and sample analyzing, and additional appreciations to the Western Norway Regional Health Authority for supporting Ann Helen Kristoffersen with a postdoctoral fellowship.
CONFLICT OF INTEREST
The authors have no competing interests.
AUTHOR CONTRIBUTION
AHK, IJH, SV, and KMA designed the study. IJH recruited participants. SV performed the laboratory measurements. AHK, IJH, SV, AÅ, and KMA analyzed and interpreted the data. AHK drafted the manuscript. All of the authors contributed to review and to finalize the manuscript.




