Review of D-dimer testing: Good, Bad, and Ugly
Abstract
D-dimer assays are commonly used in clinical practice to exclude a diagnosis of deep vein thrombosis or pulmonary embolism. More recently, they have been also been used to guide patients with unprovoked venous thromboembolism (VTE) when faced with the decision to continue or stop anticoagulation after initial treatment is complete. D-dimer assays vary widely with respect to the antibody used, method of capture, instrumentation required, and calibration standard. These differences have an important influence on the operating characteristics of the assays. Consequently, the evidence available in the literature for one assay cannot simply be extrapolated to another. In this review, we will outline the general properties of D-dimer assays, discuss the concept of raising the D-dimer threshold used in diagnosis of VTE according to pretest probability and age, and provide clinical perspective on the role of D-dimer testing in the diagnosis and prognosis of VTE.
Learning Objectives
- At the conclusion of this presentation, participants should be able to do the following:
- Summarize the general properties of D-dimer assays
- Explain the concept of varying D-dimer thresholds according to pretest probability and age
- Describe the role of D-dimer testing in the diagnosis and prognosis of venous thromboembolism
1 Introduction
1.1 What is D-dimer?
During hemostasis, the formation of fibrin clots by the coagulation system in response to vascular injury is balanced by the breakdown of clot by the fibrinolytic system. D-dimers are one of several fragments that are produced when plasmin, an enzyme activated through the fibrinolytic pathway, cleaves fibrin to break down clots. It consists of two covalently bound fibrin D domains that were cross-linked by factor XIII when the clot was formed. This fragment forms unique epitopes that can be targeted by monoclonal antibodies in D-dimer assays to confirm that the coagulation cascade is generating thrombin.
The role of D-dimer assays in clinical medicine has evolved over time. When D-dimer assays were first introduced in the 1970s, they were primarily used to check for evidence of disseminated intravascular coagulation, a condition that promotes rapid turnover of clot formation and breakdown within the microvasculature. These first-generation assays detected both fibrinogen and fibrinogen degradation products and were therefore more of a blunt tool than a fine-tuned diagnostic instrument.
With the generations that followed, there was dramatic improvement in the performance characteristics of D-dimer assays and consequently a shift of their clinical utility into the field of acute thrombosis medicine. Now, clinicians routinely use them as part of a diagnostic algorithm to exclude the diagnosis of thrombosis within the lower limbs (deep vein thrombosis; DVT) or the lungs (pulmonary embolism; PE). More recently, D-dimer assays have also been used to predict which patients are more likely to experience recurrent thrombosis when anticoagulants are stopped.
In the discussion below, we will provide a brief overview of the general properties of D-dimer assays; compare and contrast assay types; discuss the importance of the threshold chosen to define a positive and negative result; and provide clinical context for the role of D-dimer testing in the diagnosis and prognosis of venous thromboembolism (VTE).
1.2 Determinants of D-dimer concentration
Low levels of D-dimers are detectable in healthy individuals, as small amounts of fibrinogen are converted to fibrin physiologically. D-dimer levels in healthy individuals increase with age; in a population-based cohort, the upper 95th percentile for D-dimer was 2.5 times greater in people over the age of 70 compared to people less than 50 years old.1
D-dimer levels are increased in almost all cases of acute VTE. However, any process that increases fibrin production or breakdown also increases D-dimer levels. Examples of these conditions include pregnancy, inflammation, cancer, and surgery. Therefore, an elevated D-dimer is not a specific test for VTE.
Among patients with VTE, D-dimer levels vary with clot burden, timing of measurement, and initiation of treatment. Among patients with confirmed PE, higher D-dimer levels are observed among patients with larger emboli, such as those involving more than 50% of the lung volume, compared to patients with smaller thromboses.2 Similarly, among patients with DVT, higher D-dimer levels are observed among patients who have thrombosis extending above the level of the knee compared to patients who have thrombosis confined to their calf (proximal vs distal DVT).3 D-dimer levels fall with increasing duration of VTE symptoms. Patients with confirmed DVT who have had symptoms for more than 7 days have lower D-dimer concentrations compared to patients with DVT and a shorter duration of symptoms.3 D-dimer levels also fall rapidly after initiation of treatment (eg heparin, low-molecular-weight heparin, vitamin K antagonists). Within 24 hours of heparin treatment, D-levels fall by approximately 25%.4 Therefore, D-dimer testing is most accurate in patients with larger clot burden who have symptoms for less than a week and who have D-dimer testing performed before initiation of therapy.
1.3 Principles of D-dimer measurement
The process of measuring D-dimer can be conceptually divided into two steps: (i) D-dimer fragments must be captured by monoclonal antibodies, and (ii) the D-dimer plus monoclonal antibodies must be detected and quantified. Captured antibodies are immobilized on a larger structure, such as a microplate well or membrane, or linked to a latex bead or red cell. Detection antibodies can be labeled and produce a colorimetric or fluorescent reaction that is quantified after binding captured D-dimer or can be linked to a large particle such as a bead or red blood cell to induce agglutination. In sandwich-based assays, the capture and detection antibodies may have specificity for different epitopes on the D-dimer molecule. It follows that a D-dimer molecule must have the two different epitopes present to be detected by such assays.5 In agglutination based assays, the same monoclonal antibody serves as both capture and detection antibody. Only D-dimer fragments with multiple identical epitopes are captured with these assays.5
1.4 Assay types
The range of commercially available D-dimer assays is daunting. A recent publication reported 30 different assays using 20 different monoclonal antibodies.6 The assays differ in the D-dimer epitope targeted by the antibody, method of capture and detection (as discussed above), instrumentation required, and calibration standard. A brief overview of the major categories of D-dimer assays is given below.7, 8 (Table 1) For a more comprehensive listing of assay types, see the review by Riley et al.8
| ELISA | ELFA | Latex-enhanced immunoturbidimetric | Whole-blood point of care | |
|---|---|---|---|---|
| Description | Quantitative | Quantitative | Quantitative | Qualitative |
| Turnaround time | 2-4 h | 35 min | 15 min | 2-5 min |
| Sensitivitya (95% CI) | 94% (86-97) | 96% (89-98) | 93% (89-95) | 83% (67-93) |
| Specificitya (95% CI) | 53% (38-68) | 46% (31-61) | 53% (46-61) | 71% (57-82) |
| Advantages | High sensitivity |
High sensitivity Fully automated |
Comparable sensitivity to ELISA Fully automated |
Can be performed at bedside Higher specificity |
| Disadvantages |
Labor-intensive Moderate specificity |
Moderate specificity | Moderate specificity |
Observer dependent Lower sensitivity |
- ELISA, enzyme-linked immunosorbent assay; ELFA, enzyme-linked immunofluorescence assay.
- a Summary estimates for diagnosis of DVT reported in systematic review by Di Nisio et al.11
1.4.1 Enzyme-linked immunosorbent assays (ELISA)
Microplate ELISA
Plasma is added to microtiter wells that are coated with D-dimer antibody. After incubation, a second labeled antibody also specific for D-dimer is added to the well that binds to immobilized D-dimer molecules and then produces a colorimetric reaction. Due to high sensitivity of these assays to low levels of D-dimer, they are considered the reference standard assay for D-dimer. However, they are time consuming and require specialized personnel and therefore are impractical for routine D-dimer determination.
1.4.2 Enzyme-linked immunofluorescence assays (ELFA)
Designed along the same principles as the microplate ELISA, ELFAs are fully automated and binding of the detection antibody produces a fluorescent product. These assays have a rapid turnaround time and can be performed on single samples.
1.4.3 Latex assays
These assays use latex particles coated with D-dimer antibodies. They are available in qualitative, semiquantitative, and quantitative formats. Second-generation latex-enhanced immunoturbidimetric assays are fully automated, quantitative assays that measure transmission of light with a photometric analyzer. If D-dimer antigen is present in the plasma, the antibody-coated latex beads agglutinate around molecules of D-dimer producing a large aggregate. The degree of light absorption by these larger particles can then be measured. These assays have similar operating characteristics to ELFAs.
1.4.4 Whole-blood assays
A drop of whole blood is added to a slide followed by a reagent containing a hybrid, bispecific antibody that binds to D-dimer and a red blood cell membrane antigen. If D-dimer antigen is present, erythrocyte agglutination occurs. The agglutination is detected visually by the operator. Other rapid point-of-care assays apply a similar principle, using slides with a chromatography membrane containing immobilized monoclonal antibodies conjugated to colloidal gold particles or fluorescent-labeled monoclonal antibodies that produce reactions that are read by portable fluorometers.
From a laboratory perspective, D-dimer testing is further complicated by lack of a standardized unit of measure. D-dimer levels are reported as a unit of mass per volume. However, there are two different units used to describe D-dimer mass: (i) purified D-dimer units (DDUs) or (ii) fibrinogen equivalent units (FEUs). FEUs express the mass of D-dimer as the equivalent mass of fibrinogen that would be needed to produce the D-dimer in the sample. One FEU has approximately two times the mass of one DDU; therefore, 1 μg/L of D-dimer as measured in DDU is about equal to 2 μg/L in FEU.5, 9 There is no standardization with respect to the mass unit used to report D-dimer levels, with some laboratories reporting D-dimer concentration using DDU, while others report FEU. Many laboratories also do not clearly identify which mass unit they use. Furthermore, the magnitude of the unit of measure varies widely across laboratories using the same assays (eg ng/mL, μg/mL, μg/L). An additional problem is the lack of a universal calibrator that can be used to standardize D-dimer concentration across assay types.6, 10
From a clinician's perspective, variation in assay types means variation in operating characteristics. Systematic reviews and meta-analyses of studies that have evaluated D-dimer assays for diagnosis of DVT and PE have reported sensitivities ranging from 69% to 97% and specificity from 43% to 99%.11 Consequently, the results of clinical studies performed using one assay cannot simply be extrapolated to another.
Key point: All D-dimer assays are not made equal. Each assay must be independently and prospectively validated within the target population of interest.
2 D-dimer testing for diagnosis of DVT and PE
A D-dimer assay should not be used as stand-alone test to diagnose VTE. As outlined above, elevated D-dimer levels are nonspecific; consequently, a positive D-dimer result in a patient suspected to have VTE should be followed by an imaging test to confirm the diagnosis (eg ultrasound of leg, computed tomography pulmonary angiogram of lungs).
A negative D-dimer result can exclude the diagnosis of VTE without further testing, but only if the sensitivity of the test is high (>98%).12 However, high sensitivity comes at the cost of specificity. A D-dimer with high sensitivity (eg ELISA) will miss few patients who have VTE, but due to low specificity, it will also produce a large number of false-positive results. False-positive D-dimer results lead to unnecessary ultrasounds and CT scans. Ultrasounds cost money and time, but are relatively innocuous. On the other hand, CT scans require exposure to radiation and the risk of contrast dye-induced nephropathy.
To improve the utility of D-dimer testing in patients with suspected VTE, D-dimer measurement is usually combined with other tests as part of a diagnostic algorithm. (Figure 1) A key component of validated diagnostic algorithms is the application of a clinical decision rule before performing any diagnostic tests, including D-dimer measurement. Clinical decision rules are comprised of independent predictors for disease (ie signs, symptoms, risk factors) that have been derived from clinical studies and incorporated into a score. The score divides patients into different groups according to their likelihood of having DVT/PE (eg low/moderate/high, or likely/unlikely). Several clinical decision rules for diagnosis of DVT and PE have been developed, but the most validated include the Wells’ score and the Geneva score.13-18
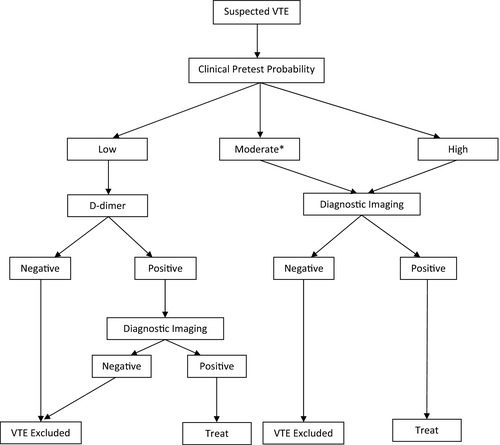
In a typical VTE diagnostic algorithm, patients who are scored as “low” or “unlikely” to have VTE have a D-dimer level drawn. (Figure 1) If the D-dimer result is negative, VTE is considered excluded and no further testing is performed. This is the primary role of a D-dimer assay—to safely exclude the diagnosis of VTE without requiring any further investigations. On the other hand, if the D-dimer result is positive, imaging tests are ordered. If the patient is scored as “moderate/high” or “likely” to have VTE, D-dimer testing is omitted and imaging tests are performed. A D-dimer result is unhelpful when the pretest probability of VTE is “likely” for three reasons: (i) the result is unlikely to be negative even if VTE is not present (ie many of the factors that increase the risk of VTE will also increase D-dimer levels, eg cancer, recent surgery); (ii) a positive result still requires confirmatory imaging; and (iii) there is uncertainty that D-dimer testing can safely exclude VTE in this subgroup. Algorithmic approaches similar to this one have been well validated in clinical studies.19, 20
Key point: D-dimer assays play an important role in diagnostic algorithms for VTE. They should always be used within the context of pretest likelihood for VTE.
2.1 D-dimer thresholds
The D-dimer level which defines the boundary between a “positive” and “negative” result is referred to as the threshold or cutoff level. The threshold is determined by the manufacturer, and it is important to note that it is not equivalent to the upper limit of the reference interval for the assay. Traditionally, the threshold used to define a positive test was intentionally set low to maximize the sensitivity of D-dimer testing and limit the likelihood of missing patients with DVT or PE. However, as discussed previously, maximizing sensitivity comes at the cost of lowering specificity. If specificity is too low, the end result is reduced clinical utility of the assay because few patients will have a negative result (ie the majority will have a positive test and need to proceed to diagnostic imaging).
Investigators have attempted to improve the clinical utility of D-dimer testing by raising the threshold that defines a positive test, primarily among patients with “low” or “unlikely” clinical pretest probability (C-PTP), as determined by a clinical prediction rule. The principal behind this approach is that the negative predictive value (NPV) of a diagnostic test (ie the probability that a patient with a negative result truly does not have the disease) is dependent on the prevalence of disease within the population being tested. If the prevalence is low, the negative predictive value of a test will increase because a negative result is more likely to be a true negative. Reciprocally, if prevalence is low, the positive predictive value (PPV) of a test will decrease because a positive result is more likely to be a false positive. In other words, if the prevalence of DVT in a population is low, elevated D-dimer levels are more likely to be due to conditions other than DVT; therefore, PPV is low and NPV is high.
2.1.1 Varying D-dimer threshold according to C-PTP
Varying the threshold according to a patient's baseline C-PTP for VTE has been shown to improve the clinical utility of D-dimer testing. As previously discussed, the Wells’ score is a validated clinical decision rule that separates patients into pretest probability categories—”low” (5% prevalence of VTE), “moderate” (25%), or “high” (60%)—based on the presence or absence of certain risk factors, physical signs, and symptoms.13 VTE prevalence is low in patients with a low Wells’ score; therefore, a high D-dimer threshold (less sensitive) can be used to exclude VTE within this subgroup. Conversely, VTE prevalence is high in patients with a high Wells’ score, which lowers the NPV of D-dimer testing and limits the safety and usefulness of D-dimer testing in this subgroup. Furthermore, hospitalized patients commonly have other reasons to have elevated D-dimer levels (eg malignancy, infection, surgery); therefore, a negative D-dimer result in this subgroup is both unlikely and unhelpful. Consequently, varying the D-dimer threshold according to C-PTP and patient admission status may be more efficient than using a one-threshold-fits-all approach.
To test this strategy, Linkins et al. randomized patients with suspected first acute DVT either to a uniform strategy where the same D-dimer threshold was used for all patients (<500 μg/L; STA®-Liatest® D-Di; Stago, Asnières sur Seine Cedex, France), or a selective strategy where the D-dimer threshold varied according to C-PTP.21 In the selective strategy, a higher D-dimer threshold (<1000 μg/L) was used for patients with low C-PTP; the manufacturer's recommended threshold (<500 μg/L) was used for patients with moderate C-PTP; and D-dimer testing was not performed in outpatients with a high C-PTP or inpatients (irrespective of C-PTP). The incidence VTE at 3 months was the same in both arms (0.5%; difference 0.0%, 95% CI: −0.8% to 0.8%) which confirmed the hypothesis that a higher D-dimer threshold can safely be used in patients with low C-PTP. Furthermore, the selective strategy resulted in 22% fewer patients requiring D-dimer testing and 8% fewer requiring ultrasonography compared with uniform testing. The primary limitation of this strategy is concern about the ability to extrapolate the results to other D-dimer assays.
2.1.2 Varying D-dimer threshold according to age
Another strategy that improves the clinical utility of D-dimer testing is to vary the threshold according to patient age. It is known that D-dimer levels naturally increase with age; therefore, older patients are less likely to have a negative result even if they do not have VTE.1 Righini et al. proposed that the D-dimer threshold for patients above 50 years of age could be safely increased by multiplying their age in years by 10 (eg D-dimer threshold for a 60-year-old patient would be 600 μg/L instead of the manufacturer's threshold of 500 μg/L).22
In their prospective trial, consecutive patients presenting to emergency departments with suspected PE had C-PTP determined by the Wells' score (likely or unlikely) or the revised Geneva score (likely or unlikely). Patients who were categorized as “PE likely” proceeded directly to diagnostic imaging. Patients who were categorized as “PE unlikely” underwent D-dimer testing. Six different quantitative D-dimer assays were used (VIDAS D-Dimer Exclusion [bioMérieux, Marcy l'Etoile, France]; Tina-quant [Roche, Basel, Switzerland]; Cobas h 232 [Roche, Basel, Switzerland]; STA-Liatest D-Dimer [Stago, Asnières sur Seine Cedex, France]; D-Dimer HS 500 [IL Diagnostics, Orangebury, NY]; and Innovance D-Dimer [Siemens AG, Munich, Germany]). For patients <50 years of age, the threshold for a positive test was 500 μg/L. For patients ≥50 years of age, the threshold for a positive test was calculated by multiplying the patient's age in years by 10. Patients with a positive D-dimer underwent diagnostic imaging, patients with a negative D-dimer had no further testing and were not anticoagulated, and all patients were followed for 3 months.
The investigators reported that the 3-month rate of VTE in patients who had PE excluded based on a D-dimer level higher than 500 μg/L, but lower than their age-adjusted threshold, was 0.3% (95% CI: 0.1%-1.7%). Furthermore, in patients over age 75 with pretest probability of “PE unlikely,” using the age-adjusted threshold increased the proportion of patients who had PE excluded without the need for imaging by 23%.
Others have questioned the use of the age-adjusted D-dimer. In an analysis of individual patient data from two clinical studies, Takach Lapner et al. compared the proportion of patients who had VTE excluded using (i) age-adjusted D-dimer interpretation, (ii) a uniformly higher D-dimer threshold in all patients, and (iii) a higher threshold only in younger patients (ie an opposite age-adjusted strategy, but the same average D-dimer threshold as age-adjusted interpretation).23 The results from this analysis showed that the gain in specificity with the age-adjusted strategy merely reflected use of a higher average D-dimer threshold overall (ie 620 μg/L) and not just the selective use of a higher threshold in the elderly. Furthermore, variation in D-dimer results with different commercial assays can make interpretation of the age-adjusted threshold difficult. For example, in one retrospective study, an 86-year-old patient with PE had D-dimer levels that ranged from 590 to 1170 ng/mL across five commercial assays.24
Key point: The clinical utility of D-dimer testing is limited by a one-threshold-fits-all approach. Varying the threshold according to C-PTP or age improves utility, but the safe threshold values differ from one assay to another. Laboratories and clinicians must be familiar with the literature on the D-dimer assay used at their institution to interpret results.25
3 D-dimer testing for Prognosis
In recent years, the role of D-dimer testing has expanded to include prediction of risk of recurrent VTE. VTE in patients who have no obvious provoking risk factors (eg recent surgery, immobilization of a limb, cancer) is referred to as “unprovoked” or “idiopathic.” After 3 months of anticoagulant therapy, patients with unprovoked VTE are informed that the risk of recurrence while off of anticoagulant therapy is 10% in the first year and 30% over 5 years.26 Anticoagulant therapy carries a risk of bleeding that increases with age; therefore, it is not a benign treatment. Some patients will be uncomfortable with this level of risk of recurrent VTE and choose to remain on anticoagulation indefinitely, whereas others will choose to stop it. However, a substantial proportion of patients are undecided about what to do. D-dimer testing can be helpful in these cases to aid decision-making.
From our discussion about diagnostic testing for VTE, we know that D-dimer levels increase with acute thrombosis. For most patients, the level drops over time; however, for some patients, the level remains elevated which is thought to indicate a persistent thrombotic state. Meta-analyses of clinical trials have shown that the risk of recurrent VTE in patients with a positive D-dimer after 3 months of anticoagulant therapy is higher (8.9% per year; 95% CI: 5.8% to 11.9%) than in patients with a negative D-dimer (3.5% per year; 95% CI: 2.7% to 4.3%).27 Overall, the evidence suggests that a positive D-dimer after completion of 3 months of anticoagulant therapy in patients with a first unprovoked VTE predicts a risk of recurrence that is approximately double the risk in patients with a negative D-dimer.
While D-dimer testing to predict recurrence is used at many centers, individual practice varies. As with diagnosis of VTE, there are concerns about extrapolating the results of studies using one D-dimer assay to another assay. There is also controversy over the timing of when the prognostic D-dimer level should be drawn after stopping anticoagulant therapy (3 weeks? 2 months?) and the threshold that should be used to indicate a positive result (manufacturer's threshold? a different threshold?). One meta-analysis using patient-level study data suggested that while timing and threshold level may not influence the predictive ability of D-dimer testing, gender does appear to be a factor.28 For reasons that are not entirely clear, a negative D-dimer in patients who have completed 3 months of treatment does not predict a lower risk of recurrence in men with the same accuracy as it does in women.
As with diagnosis of VTE, D-dimer testing should not be used as a stand-alone test for predicting risk of recurrence. Clinical prediction rules which include the result of D-dimer testing as one factor in the score have been developed.29-31 Furthermore, it is important to consider the risk of bleeding on anticoagulant therapy as well as the patient's personal preferences when making this important decision.
Key point: D-dimer testing after 3 months of anticoagulant therapy for a first unprovoked VTE can guide decisions about duration of therapy, but should be used as part of a clinical decision model.
4 Conclusions
D-dimer testing plays an important role in the exclusion of VTE and, to a lesser extent, in the prediction of recurrence in patients with a first acute unprovoked VTE. However, wide variation in the types and operating characteristics of D-dimer assays means study results with one assay cannot be extrapolated to another. Much work remains to be done with respect to standardization of the performance and reporting of D-dimer assays as well as translation of results of D-dimer studies into clinical practice.
Conflicts of Interest
Linkins received D-dimer assays in-kind from BioMérieux and Stago for a peer-funded research study. Takach Lapner reports no conflict of interests.




