Current and emerging approaches for evaluating platelet disorders
Summary
Platelets play a central role in physiological hemostasis and also in pathological thrombosis. It is well established that congenital or acquired abnormalities of platelet function are associated with a heightened risk of bleeding of variable severity and excessive hemorrhage after surgery or trauma. Several kinds of different platelet function tests have been developed over the years to identify or diagnose platelet function disorders. The use of these tests for the assessment of thrombotic risk or for monitoring the effects of drugs inhibiting platelet function is not well established. Light transmission aggregometry (LTA) is the gold standard for the study of patients with defects of platelet function. Its results are affected by several pre-analytical and analytical variables. The Subcommittee on Platelet Physiology of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis published official guidelines for the standardization of the variables affecting LTA, which should be followed to harmonize the procedures across different laboratories worldwide. The lumi-aggregometer, a modification of LTA that measures platelet secretion in parallel with aggregation, is preferable to LTA for diagnosing inherited defects of platelet function, because it is more sensitive to the most common disorders, which are characterized by abnormalities of platelet secretion. LTA (or lumi-aggregometry) is useful as a first screening test of patients with the clinical suspicion of defects of platelet function, because it helps to provide an interim diagnostic hypothesis, which can then be confirmed or discounted using appropriate and specific tests.
Introduction
Platelets play a central role in physiological hemostasis and also in pathological thrombosis. When a blood vessel is injured, platelets adhere to the exposed subendothelium, are activated, produce thromboxane A2, and secrete their granules’ contents, including the platelet agonists adenosine diphosphate (ADP) and serotonin, which, like thromboxane A2, interact with specific platelet receptors to form and stabilize platelet aggregates. In addition, platelets provide the necessary surface of procoagulant phospholipids for assembly of coagulation complexes.
It is well established that congenital or acquired abnormalities of platelet function are associated with a heightened risk of bleeding of variable severity and excessive hemorrhage after surgery or trauma 1. Several kinds of different platelet function tests have been developed over the years to identify or diagnose platelet function disorders and will be reviewed here.
The use of platelet function tests has also been advocated to improve the efficacy and safety of treatment with antiplatelet agents, which are used to reduce the risk of recurrences in patients with previous acute episodes of ischemic or thrombotic events of the coronary or cerebral vascular district. The antiplatelet agents that are commonly used for these indications are acetylsalicylic acid (ASA, or aspirin) and antagonists of the platelet P2Y12 receptor for adenosine diphosphate (ADP). In the last decade, reports of variability of the individual response to these agents have been published. However, more accurate studies revealed that most patients on treatment with aspirin display good inhibition of platelet thromboxane A2 production 2. In contrast, all observational studies that have been published so far confirmed that about 30–40% of patients treated with the thienopyridine pro-drug clopidogrel exhibit high on treatment platelet reactivity (HTPR) and, as a consequence, are not adequately protected from cardiovascular events 2. Therefore, poor pharmacological response to clopidogrel represents a clinical relevant problem that needs a solution. It has been proposed to identify patients with HPTR on clopidogrel with platelet function tests and treat them using alternative, more effective therapeutic approaches. Unfortunately, many issues about this strategy still need to be solved 2. Most importantly, the large intervention randomized clinical trials that have been published so far failed to demonstrate that this strategy is effective 2. As a consequence, according to the criteria of Evidence Based Medicine, monitoring clopidogrel treatment by platelet function tests should not be implemented in the clinical setting yet. Therefore, the issue of monitoring the effects of drugs inhibiting platelet function will not be addressed in detail.
Classification of Congenital Disorders of Platelet Function
There are several different classifications of platelet function disorders PFD), which are based on different criteria. In 2003, I proposed a classification that is based on abnormalities of platelet components that share common characteristics 1 (Table 1): (i) platelet receptors for adhesive proteins, (ii) platelet receptors for soluble agonists, (iii) platelet granules, (iv) signal transduction pathways, and (v) procoagulant phospholipids. A 6th category of miscellaneous disorders would include platelet function that is less well characterized.
| 1. Abnormalities of the platelet receptors for adhesive proteins |
| GPIb-IX-V complex: Bernard–Soulier syndrome, platelet-type von Willebrand disease |
| GPIIb-IIIa (αIIbβ3): Glanzmann thrombasthenia |
| GPIa-IIa (α2β1) |
| GPVI |
| 2. Abnormalities of the platelet receptors for soluble agonists |
| P2Y12 receptor |
| Thromboxane A2 receptor |
| α2-Adrenergic receptor |
| PAR-1 defect |
| 3. Abnormalities of the platelet granules |
| δ-Granules: nonsyndromic δ-storage pool deficiency, Hermansky–Pudlak syndrome, Chediak–Higashi syndrome, MRP4 deficiency, thrombocytopenia with absent radii syndrome, Wiskott–Aldrich syndrome |
| α-Granules: gray platelet syndrome, Quebec platelet disorder, 11q terminal deletion disorder, white platelet syndrome, Medich platelet disorder, X-linked macrothrombocytopenia with thalassemia, arthrogryposis renal dysfunction, and cholestasis (ARC) syndrome |
| α- and δ-Granules: α,δ-storage pool deficiency |
| 4. Defects of signal transduction |
| Abnormalities of the arachidonic acid/thromboxane A2 pathway: defects in phospholipase A2, cyclooxygenase, thromboxane synthetase |
| Abnormalities of GTP binding proteins: Gαq deficiency, Gαi1 defect, hyper-responsiveness of platelet Gsα |
| Defects in phospholipase C activation: partial selective PLC-β2 isozyme deficiency |
| Abnormalities in transcription factors |
| Abnormality in GPVI/FcRc signaling leukocyte adhesion deficiency III (LAD-III) |
| CalDAG/GEFI defect |
| 5. Abnormalities of membrane phospholipids |
| Scott syndrome |
| Stormorken syndrome |
| 6. Miscellaneous abnormalities of platelet function |
| Primary secretion defects |
| Other (osteogenesis imperfecta, Ehlers–Danlos syndrome, Marfan syndrome, hexokinase deficiency, glucose-6-phosphate deficiency) |
Clinical Evaluation of Patients with Suspected Platelet Function Disorders
PFD should be suspected based on the personal history for abnormal bleeding. Typically, patients with PFD experience mucocutaneous bleeding (e.g., epistaxis, gum bleeding, menorrhagia, and easy bruising) and excessive, early onset hemorrhage after surgery or trauma 3. Bleeding into the central nervous system rarely occurs. The severity of the bleeding episodes varies with the severity and nature of the defect, but also among patients with the same disorder. Bernard–Soulier syndrome, Glanzmann thrombasthenia, Quebec platelet disorder (QPD), and Scott syndrome are associated with the most severe bleeding manifestations. Spontaneous bleeding in patients with the remaining, milder disorders is rare, but postsurgical bleeding may occasionally be severe. Other, partially unknown factors contribute to the bleeding risk, because the severity of the bleeding history may vary widely among patients with the same defect. No single symptom can be considered distinctive or pathognomonic of a specific platelet disorder. Although QPD and Scott syndrome may be associated with mucocutaneous bleeding, they are usually characterized by delayed onset bleeding after hemostatic challenges and ‘coagulation-type’ bleedings (e.g., joint bleeds) 1.
The ISTH bleeding assessment tool (ISTH-BAT) is useful to identify patients with lifelong history of abnormal bleeding, but is unable to predict the presence of abnormalities of platelet function that can be diagnosed using the currently available laboratory tests 4.
Due to space limitations, this review will focus on platelet function tests only, without addressing the issue of the diagnostic workup of platelet function disorders.
Global Tests of Primary Hemostasis
Bleeding time
The bleeding time is an invasive technique that measures the time needed to arrest bleeding from a standardized incision (usually 10 mm long and 1 mm deep) in the forearm of the patient. It is a poorly reproducible, even when carried out by experienced personnel, and is influenced by several variables in addition to platelet function, which include platelet count, plasma factors, red blood cells count and volume, the vessel wall and the skin tropism. For this reason, it displays low sensitivity to mild/moderate abnormalities of inherited and acquired (including drug-induced) defects of platelet function 3: As a consequence, its diagnostic utility is very poor. In addition, the bleeding time does not allow to distinguish between patients with abnormalities of platelet-dependent hemostasis (primary hemostasis) due to abnormalities of platelets or abnormalities of von Willebrand's factor, which is a frequent cause of congenital or acquired defects of primary hemostasis.
PFA-100® and innovance PFA-200®
The PFA-100® system measures the time needed for a sample of anticoagulated blood to occlude a 150-μm aperture in a membrane (mimicking the injured part of the vessel wall), after its aspiration through a capillary, which mimics the resistance of a small artery: the end-point is therefore termed ‘closure time’. Two cartridges are available for testing: In one, the membrane with the 150 μm aperture is coated with collagen plus ADP (C-ADP), while in the other cartridge, the membrane is coated by collagen plus epinephrine (C-EPI). The closure of the aperture in the membrane is brought about by the formation of a platelet plug, as a consequence of the high shear applied to the system and the interactions of the blood sample with the thrombogenic surface of the membrane 5. The system is user-friendly, automated, and quick and requires a small volume of blood sample. It is very sensitive to type-3, type 2B, and type 2A von Willebrand disease (but less sensitive to type 1) and to severe abnormalities of platelet function, for example Glanzmann thrombasthenia and Bernard–Soulier syndrome 6. In contrast, its sensitivity and to mild/moderate inherited and drug-induced defects of platelet function is low 6, 7. Closure times (CT) of the C-EPI cartridge may be prolonged in some, but not all patients with platelet secretion defects or on treatment with antiplatelet agents, such as aspirin or clopidogrel 8. The low sensitivity in these disorders may be explained by its sensitivity to many variables (platelet count, red blood cells, platelet reactivity to collagen and, above all, plasma VWF), the effects of which on platelet aggregate formation can outweigh the effects of mild platelet dysfunction 8.
Due to its poor sensitivity to mild abnormalities of platelet function, which are by far the most prevalent PFD, PFA-100 is not recommended for the screening of patients with suspected PFD. However, it has been suggested that the combined evaluation of the collagen/ADP and collagen–epinephrine cartridges of PFA-100 may be useful to distinguish between mild PFD and VWD, because patients with VWD usually display prolonged closure times with both cartridges, while patients with mild/moderate forms of PFD usually display prolonged closure time of the collagen/epinephrine cartridge only 7. Although this approach is promising, it needs to be validated before being implemented in the clinical setting.
The modified INNOVANCE PFA P2Y test cartridge has been shown to be sensitive to severe and mild congenital abnormalities of the platelet P2Y12 receptor for ADP 9 and has been proposed also for monitoring patients on treatment with the P2Y12 inhibitory drug clopidogrel.
Thromboelastography
Thromboelastography measures the viscoelastic changes that occur during clot formation in whole blood. It is influenced by platelet function, coagulation factors, natural inhibitors of coagulation, and fibrinolysis. It is considered superior to standard coagulation assays for the management of patients with bleeding events, especially when associated with acquired disorders of coagulation 10. Its use has also been suggested for diagnosing platelet function disorders or monitoring antiplatelet therapy 11; however, its use in the clinical setting needs to be validated.
Specific Tests of Platelet Function
Light transmission aggregometry
Light transmission aggregometry (LTA) measures the light transmission through a sample of platelets in suspension (platelet-rich plasma [PRP], washed platelets, or gel-filtered platelets) that increases when platelets are aggregated by an agonist 12. LTA is a time-consuming and technically challenging technique that is affected by many pre-analytical and analytical variables, which must be carefully controlled for by expert personnel. For this reason, LTA should be performed only in highly specialized laboratories.
Upon stimulation with a platelet agonist of the platelet suspension, which is placed in the aggregometer at 37 °C and continuously stirred at 1000 rpm, platelets change their shape from discoid to spiny spheres, an event that is associated with a transient and short-lived increase in optical density, which is rapidly followed by a brisk increase in light transmission, indicative of ongoing platelet aggregation. The increase in light transmission may either reach a plateau, without deflecting toward baseline (irreversible aggregation), or else return toward the baseline (reversible aggregation). Occasionally, when platelet aggregation is stimulated by weak agonists at critical concentrations, the aggregation curve has a biphasic appearance: an initial wave of aggregation (primary wave) is followed by a secondary wave of aggregation, which is usually irreversible. At higher concentrations of the agonist, the primary and secondary waves of aggregation usually fuse in one single, irreversible wave. At lower concentrations of the agonist, or in the presence of some abnormalities of platelet function, usually associated with defects of platelet secretion, the primary wave may deaggregate and the secondary aggregation does not occur.
The lumi-aggregometer is a modified version of light transmission aggregometry, which allows to measure the secretion of platelet delta-granules in parallel with platelet aggregation 12. The ATP that is released by platelets is determined using firefly extracts of luciferin and luciferase, which react with ATP, followed by oxidative decarboxylation of luciferin, resulting in light emission. Considering that mild platelet secretion defects may not be paralleled by abnormalities of platelet aggregation 13, the combined evaluation of platelet aggregation and secretion by lumi-aggregometry may be superior to LTA alone 3, 14.
Several agonists are used to stimulate platelet suspensions in a light transmission aggregometer. The most commonly used agonists include ADP, epinephrine, collagen, arachidonic acid, and the thromboxane A2 analogue U46619 3, 15. Another commonly used reagent is ristocetin, which should not be considered an agonist, as it induces platelet agglutination, a passive process during which platelets form clumps without being activated. Ristocetin is useful to diagnose abnormalities of the interaction between the platelet glycoprotein Ib (GPIb) and von Willebrand's factor (VWF) and thus critical for evaluation of Bernard–Soulier syndrome and some forms of von Willebrand disease (VWD), including the ‘platelet-type’ VWD. Many other agonists are available for studies of platelet function in the clinical setting and, more commonly, for research purposes (e.g., thrombin, the thrombin receptor (PAR-1)-activating peptide (TRAP), convulxin, the calcium ionophore A23187, γ-thrombin, PAF-acheter, vasopressin, and others).
Factors affecting the results of LTA
Blood samples from which PRP is prepared should be handled very carefully, following standardized procedures, to minimize the risk of in vitro platelet activation and subsequent platelet desensitization. The several pre-analytical and analytical variables that may affect the results of LTA 15 include blood sampling, the type of anticoagulant used to prevent clotting of the blood sample, the temperature (which should be maintained constant at 37 °C), the speed at which platelet suspensions are stirred (as already mentioned, the commonly used speed is 1000 rpm), the time elapsed since blood sampling and preparation of platelet suspension and others. In contrast to what it was originally believed, LTA is not sensitive to changes in the platelet count within the physiological range (150–40 000/μl) in platelet suspensions 16, 17.
The Subcommittee on Platelet Physiology of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis published official guidelines for the standardization of the variables affecting LTA, which should be followed to harmonize the procedures across different laboratories worldwide 15. Due to lack of investigations that directly compared different methodologies to perform LTA studies, there were insufficient data to develop evidence-based guidelines: As a consequence, these guidelines are to be considered recommendations by experts in the field.
Impedance aggregometry
Impedance aggregometry, which was first described in 1980 18, allows the measurement of platelet aggregation in whole blood, because it is sensitive to the increase in electrical impedance that is caused by the formation of a platelet aggregate on platinum electrodes upon the addition of a platelet agonist to a diluted whole-blood sample, in which the electrodes are immersed. A relatively recent instrument, the Multiplate, has been used for monitoring the extent of platelet aggregation inhibition in patients on treatment with dual-antiplatelet therapy with aspirin and clopidogrel 19. The technique is less technical demanding and time-consuming than LTA; however, most drawbacks that have been listed for LTA also apply to this technique. Moreover, impedance aggregometry is sensitive to many variables, including the platelet count, which significantly affects the results even for slight variations within the normal range, and the hematocrit 17. Impedance aggregometry has never been validated for the diagnosis of platelet function disorders. In addition to its sensitivity to the platelet count in platelet suspensions, it has drawbacks that theoretically hamper its validity as a diagnostic test for PFD (Figure 1): (i) It provides no information on the ‘resting’ state of platelets at baseline, which can be evaluated by inspecting the PRP (smoky appearance) and the presence of oscillations of the baseline tracing of LTA; (ii) it does not give information on the change in shape of platelets upon their stimulations with agonists; (iii) it does not give information on the reversibility of platelet aggregates, which is an important hallmark of some defects of platelet function (i.e., defects of platelet secretion, defects of the platelet P2Y12 receptor for ADP). For these reasons, the use of Multiplate for diagnosing defects of platelet function should be discouraged, until its accuracy in distinguishing among different types of platelet function disorders will be clearly established in ad hoc studies.
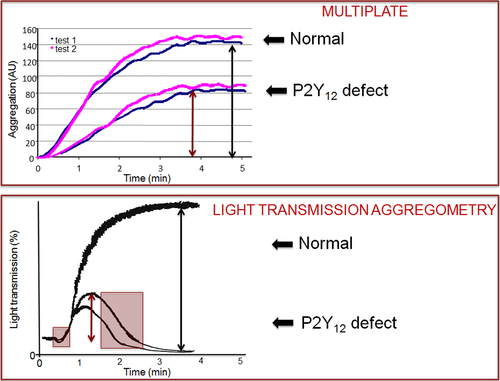
Whole-blood platelet aggregation measured by platelet counting
The method is based on the principle that single platelet counts in whole blood decrease as a consequence of the formation of platelet aggregates, after exposure of the sample to platelet agonists in vitro 20. It is more sensitive than LTA, because it can detect very small aggregates, formed by 2–3 aggregated platelets. A commercial device (Plateletworks™, Helena Laboratories, Beaumont, TX) is available 21.
Flow cytometry
The flow cytometer is a sophisticated and expensive instrument, which is now available in most institutions. It allows the measurement of platelet reactivity in vitro and the detection of activated platelets, platelet–leukocyte aggregates, and platelet-derived microparticles in the circulation 22. Flow cytometry has been used to monitor the degree of inhibition of platelet function be antiplatelet agents, such as aspirin 23 and clopidogrel. The inhibition by ADP of phosphorylation of vasodilator-stimulated phosphoprotein (VASP) 24, which is mediated by P2Y12 through the inhibition of adenylyl cyclase, has been proposed as a highly specific method to measure the antiplatelet effects of clopidogrel, and other drugs inhibiting the platelet P2Y12 receptor for ADP 25-27. It is also useful as confirmatory test of moderate/severe inherited defects of P2Y12, although it proved insensitive-to-mild abnormalities of the receptor, such as inherited heterozygous deficiency 25. There was a good correlation between ADP-induced platelet aggregation and VASP phosphorylation assay in detecting P2Y12 inhibition 11, 28, 29 and circulating levels of the active metabolite of clopidogrel 28.
Flow cytometry allows the study of platelet secretion (CD62 and CD63) and platelet procoagulant activity. Platelet aggregation can be measured either using a recently developed dual color assay 30, or by evaluating a surrogate end-point, namely the exposure of activated GPIIb/IIIa receptor, upon stimulation of small volumes of whole blood in the presence of an antibody, such as PAC-1, that recognizes only the activated form of GPIIb/IIIa. The technique is also extremely useful to detect defects of membrane glycoproteins, such as GPIIb/IIIA, GPIb, GPVI, on resting platelets, to confirm the diagnosis of specific platelet disorders that can be suspected based on well-defined abnormalities of platelet aggregation.
Traditional flow cytometry and dual-color flow cytometric method to study platelet aggregation may be particularly useful for studying pediatric patients, from whom only small amounts of blood samples can be drawn, or patients with moderate/severe thrombocytopenia who cannot be studied using LTA 31, 32.
Clot retraction
Clot retraction is a very simple test, which is based on the inspection of the amount of serum that is squeezed out of a retracting clot, formed after incubation of non-anticoagulated blood sample at 37 °C. In addition to giving important information on platelet function, based on the GPIIb/IIIA fibrin-dependent retraction of blood clot, it allows to save a sample of serum in which thromboxane B2 can be measured to rule out surreptitious intake of nonsteroidal anti-inflammatory drugs and/or to diagnose inherited abnormalities of the arachidonate pathway of platelet activation 3.
Serum thromboxane B2
Serum thromboxane B2 (TxB2) reflects the total capacity of platelets to synthesize thromboxane A2, of which it is a stable metabolite, and is therefore the most specific test to evaluate congenital or acquired abnormalities of the arachidonic acid pathway in platelets 33. Both congenital abnormalities of cyclooxygenase-1 and phospholipase A2 enzymes result in severely reduced levels of serum thromboxane B2 3, 34. The ability of platelets to synthesize TxB2 after in vitro stimulation with an agonist, such as collagen, can also be considered a useful test for studying abnormalities of the arachidonic acid pathway 33.
Urinary levels of the TxB2 metabolite, 11-dehydrothromboxane B2
Urinary metabolites of TxB2 represent a time-integrated index of TxA2 biosynthesis in vivo 35. This urinary TxA2 metabolite reflects systemic TxA2 formation, which largely, albeit not exclusively occurs in the platelets: as a matter of fact, up to about 30% of the urinary metabolite derives from extra-platelet sources. It is important to note that the contribution of extra-platelet sources of the metabolite may substantially increase in pathological conditions, such as in inflammatory diseases. Therefore, measurement of urinary TxB2 metabolites should not be considered a specific test to be implemented for diagnosing abnormalities of the platelet arachidonic acid pathway or the pharmacological effects of aspirin administration on the ability of platelets to synthesize thromboxane A2.
The Ultegra Rapid Platelet Function Assay (RPFA)-VerifyNow
This point-of-care test measures the agglutination of fibrinogen-coated beads by platelets stimulated by an agonist in citrated whole blood. It was initially developed to measure the antiplatelet effects of GPIIb-IIIa antagonists. Later, it was slightly modified to become more sensitive to aspirin (RPFA-VerifyNow ASA), for monitoring aspirin treatment 36, and RPFA-VerifyNow P2Y12 37, which is more specific than ADP-induced platelet aggregation with LTA for monitoring clopidogrel treatment 38. It has not been validated for the evaluation of congenital PFD.
Tests of platelet secretion
Platelet secretion may be measured by means of many laboratory tests. The most commonly used one is lumi-aggregometry, which measures platelet secretion and ATP secretion in parallel (see before). Other methods include the following: (i) measurement of the platelet release of preloaded radioactive serotonin, which has the disadvantages of the use of a radioactive reagent; another important drawback is that it cannot be used in patients with defects of platelet delta-granules, because radioactive serotonin cannot be loaded into the granules and, as a consequence, is metabolized in the platelet cytoplasm; (ii) measurement of released serotonin by the o-phtalal-dehyde method, ELISA, HPLC–fluorometric detection, or liquid chromatography–mass spectrometry; (iii) measurement of secretion of alpha-granules proteins, such as beta-thromboglobulin, PF4 by ELISA methods; (iv) measurement of expression of P-selectin on the platelet membrane 39.
Conclusions
Global tests of primary hemostasis are not be useful in the diagnostic workup of patients with suspected PFD. Although it was first introduced almost 50 years ago, LTA is still the most commonly used method to study platelet function. LTA is a time-consuming and technically challenging technique that is affected by many pre-analytical and analytical variables, and these must be carefully controlled for by expert personnel. For this reason, LTA should be performed only in highly specialized laboratories. LTA is useful in the initial screening of patients with defects of platelet function. However, its sensitivity to the most common inherited platelet function disorders, which are associated with abnormalities of platelet secretion, is suboptimal. Lumi-aggregometry, which measures platelet aggregation and secretion simultaneously, might be preferable to LTA for the diagnostic workup of these patients. Additional platelet function tests should be implemented to test the diagnostic hypotheses that can be raised, based essentially on the results of platelet aggregation-based assays.




