Laboratory testing for antiphospholipid syndrome
Summary
This is a practical report on laboratory tests for the diagnosis of antiphospholipid syndrome (APS). After a general definition of APS, this study deals with appropriateness and timing in requesting the determination of antiphospholipid (aPL) antibodies. Lupus anticoagulant (LAC), anticardiolipin (aCL), and anti β2-glycoprotein I (aβGPI) are the mandatory tests to be performed, while other tests are not yet validated for clinical use. Interpretation of results is an important discussed issue that implies a close liaison between clinical pathologists and clinicians. Finally, a personal definition of APS according to aPL antibody profile closes the manuscript.
Venous or arterial thrombosis and pregnancy loss are the recognized clinical features of antiphospholipid syndrome (APS). As these clinical manifestations are common, the diagnosis of APS is essentially based on the detection circulating antiphospholipid (aPL) antibodies 1. In sensitive coagulation tests, these immunoglobulins (aPL) cover the procoagulant phospholipid (PL) surface that results in a prolongation of the coagulation time. This phenomenon is called lupus anticoagulant (LA), a misnomer, as its presence is associated with thromboembolic events. aPL antibodies can also be detected using immunological techniques. Initially, an enzyme-linked immunosorbent assay (ELISA) to detect anticardiolipin (aCL ELISA) antibodies was the only performed immunological test. Subsequently, β2-glycoprotein I (βGPI) was identified as the major autoantigen in APS and a specific ELISA (aβ2GPI ELISA) was introduced in the laboratory diagnosis of APS 1.
In Whom and When Checking for the Presence of Antiphospholipid Antibodies
Search for aPL antibodies should be reserved to young subjects (less than 50 years of age) with idiopathic venous thromboembolism (VTE) or VTE in uncommon sites. Moreover, tests are required in young patients with cryptogenic stroke in general and when this event is associated with other concomitant conditions such as dementia, livedo reticularis, epilepsy, and valvular heart disease in the absence of overt atherosclerosis. Suspicion is higher if associated autoimmune diseases are present or in case of unexplained recurrence of venous or arterial thromboembolism. Tests should be also performed in women with pregnancy loss (especially in case of fetal death) and in case of unexpected aPTT prolongation in otherwise healthy subjects. Timing for testing is important. In patients with VTE, the ideal time for testing is before the initiation of any drug as LA determination may be impaired after the initiation of anticoagulation.
Actually, aPL antibody screening in VTE is needed when deciding to prolong or discontinue anticoagulant treatment. On the other hand, in patients with arterial thromboembolism (ATE), it is important to check for aPL antibodies initially, to decide the use of antiplatelet or anticoagulant drugs or both. In women with pregnancy loss, aPL test can be performed any time to establish an appropriate prophylaxis during and outside pregnancy.
Which Test should be Performed?
According to guidelines 1, all the three tests (LA, IgG and IgM aCL, IgG and IgM aβ2GPI) are needed to assess the aPL profile and classify the patients according to the presence of one or more positive test. To avoid detection of transient antibodies, tests must be positive on two or more occasions, at least 12 weeks apart.
Coagulation tests (lupus anticoagulant)
Using platelet-poor plasma (double centrifugation), the presence of LA is checked by performing two different tests that represent different assay principles 2. The test of choice is the diluted Russell Venom Time (dRVVT), and the second test is an aPTT-based test. Three steps are recommended to evaluate the presence of LA: (i) screening, (ii) mixing, and (iii) confirmatory studies. The screening test is the coagulation time obtained in patient plasma. The result of screening test must be reported as the ratio between the coagulation time of screening and normal pooled plasma. Similarly, the mixing test must be reported as the ratio of coagulation time of 1 : 1 mixing of screening and normal pooled plasma to that of normal plasma, and confirmatory test using an excess of PL or hexagonal PL as the ratio of the coagulation time of screening to normal pooled plasma. If the ratio of mixing test is higher than the reference ratio, the confirmatory test defines the positivity of LA. Skipping the mixing step may determine false-positive 3 and false-negative results 4, 5. Different factors affect the sensitivity of reagents to LAC. Among these, the type (linear anionic PL molecules or hexagonal phase PL that quench LA activity) and concentration of phospholipids and type of activators (kaolin, ellagic acid, silica,celite) are important components. An ideal reagent should contain low PL concentration and a type of activator that is sensible to LAC and less to heparin or factors deficiency. Tripodi et al. 6 investigated the responsiveness of commercial APTT reagents for screening of LAC-positive plasma samples and showed that the sensitivity of silica-based reagents to LAC was higher than that of ellagic acid-based reagents. On the other hand, another study concluded that the sensitivity of APTT reagents for LAC is dependent on phospholipid concentration and not the activator 7. It is very important to exclude the presence of anticoagulants such as heparin and non-antivitamin k oral anticoagulants (NOACs) as screening and mixing test are prolonged. The presence of heparin and dabigatran can be readily excluded by testing patient plasma with thrombin time (TT). In case the patient is treated with anti-Xa oral anticoagulants, the determination of LA becomes more complicated as two tests are probably needed, one of which is PL-independent as the ecarin clotting time (ECT). However, in most cases, there is no need to determine the presence of LA when the patients are on anticoagulant drugs but is important when deciding its suspension.
Immunological tests (aCL/aβ2GPI ELISA)
IgG and/or IgM in serum or plasma are defined positive at initial testing when present in medium or high titer (i.e., >40 GPL or MPL, or > the 99th percentile). Both aCL and anti-β2GPI ELISAs should be performed according to standardized procedures. A large variability in results from these assays has been reported 8, and despite many attempts to produce consensus guidelines, some issues remain unanswered 9, 10. A guidance from the Scientific Subcommittee of International Society of Thrombosis and Haemostasis for the detection of aCL and anti-β2GPI antibodies in solid-phase assays was recently published in the Journal of Thrombosis and Haemostasis 11. To address the large variability among assays, the committee issued a ten-point recommendation that extends from patient selection to the interpretation of results. An important issue is the reference material.
Although different from human oligoclonal aPL antibodies, monoclonal antibodies directed against β2GPI should be used as a primary standard. The use of an in-house reference-positive plasma to which a value of 100 units is arbitrarily assigned may be of value to overcome this problem; however, tested sample results may vary from one laboratory to another. In the case of a strong positive in-house reference plasma, low or very low levels will consequently be reported for tested plasma; conversely, using a weak positive plasma, high levels will be reported for tested plasma. Thus, the choice of consistent in-house positive reference plasmas of intermediate strength for each run is crucial. A further control for any run of ELISA is the introduction of at least two in-house known negative and positive controls. In this way, positive and negative values can be determined and their evaluation gives feedback on the quality of every day session. Concordance of isotype positivity in aCL and aβ2GPI ELISA is essential. The use of chemiluminescent methods and automatic instruments to detect both aCL and aβ2GPI antibodies is very accurate and promising.
Other immunological tests
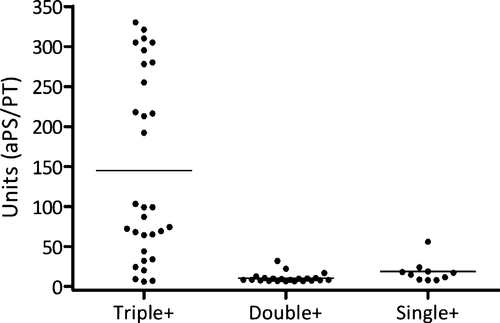
Recently, other tests have been proposed for the diagnosis of APS. Among them, the determination of subclasses of aβ2GPI antibodies, those directed against Domain 1 and Domain 4/5 of the molecule, sounds interesting. Our group found a significant association between positive IgG aβ2GP1-Dm1 and thromboembolic events 12, while no association to IgG aβ2GP1-Dm4/5 was found 13. In association with the classic tests, the detection of antibodies directed toward specific domains of β2GP1 may be useful in clinical practice in identifying individuals at high risk of developing thromboembolic events. Promising but undetermined results come also from the determination of antibodies directed to phosphatidylseine/prothrombin (aPS/PT) complex. We found the presence of aPS/PT antibodies in a subgroup of high-risk individuals with triple positivity and in none of those with double or single positivity (Figure 1).
Although some reports correlate the presence of IgA aCL and IgA a β2GP1 with thrombosis-related events, there are still insufficient data to attach importance to these antibodies 14.
How to Read the Results?
Reading and interpretation of aPL antibodies profiles may be challenging, and collaboration between clinical pathologists and clinicians is particularly desirable in this field. Consistency of data is the first point at issue. aCL and aβ2GP1 ELISAs should be both positive or negative for the same isotype (IgG or IgM): If not, the determination must be repeated. A full positive profile (triple positivity) reflects the presence of large amounts of anti-β2GPI antibodies directed to Domain 1 of β2GPI 12. Its identification is easy whichever method is employed 15 as both coagulation tests (dRVVT and aPTT) are strongly positive and titers in ELISAs are of medium–high titers. Triple positivity (LA+, aCL+, aβ2GP1+, same isotype) is the most reliable profile that is always confirmed after 12 weeks 16 and is associated with thromboembolic events and pregnancy loss 17, 18. On the other hand, single positivity is confirmed after 12 weeks in less than 50% of cases 12 and is not associated with thromboembolic events 19-23. In light of this background, it is clear that clinical studies should be always performed in cohort of patients with homogeneous pattern of aPL antibodies.
How Definite is the Diagnosis of APS?
Triple-positive patients constitute a high-risk group (definite APS) with an observed high recurrence rate (one-third of patients in 6 years follow up). Young age (less than 50 years), unprovoked VTE or VTE in unusual site or in microcirculation, cryptogenic stroke, late pregnancy morbidity (including fetal death, eclampsia/severe pre-eclampsia, or placental insufficiency), IgG isotype, high titer of aCL and anti-β2GP1, and strong potency of LAC test, all reinforce the diagnosis of definite APS 17, 24, 25. Some of these patients can develop the most feared form of APS, that is, catastrophic APS that is fatal in some 50% of cases. Lower risk patients are those positive in aCL and anti-β2GPI ELISAs but negative for LAC. Relevant anti-β2GPI antibodies are probably involved but at a titer not sufficient to induce LAC activity in plasma. In fact, dRVVT becomes positive at an aCL concentration that exceeds 50GPL units 8. Lower amounts of antibodies are probably not associated with thromboembolic events but may be relevant in pregnancy morbidity, where lower titers of antibodies are frequently encountered 19, 21. It must be stressed here that positivity in a single aPL test raises serious questions about their association with thrombosis. Venous or arterial thromboembolism are very common conditions that accounted for two of three deaths in 2010 26 and are in the majority of cases unrelated to the presence of these antibodies.




