Migraine and erythrocyte biology: a review
Summary
Migraine is a common disabling headache disorder that is conventionally classified according to the presence or absence of aura. The pathogenesis of this disorder entails a complex interplay of neurovascular factors, that trigger reduction of cerebral blood flow followed by reactive vasodilatation. Despite major emphasis has been placed on the investigation of putative biomarkers that could predict response to specific treatments and prophylaxis, less focus has been directed at the association between migraine and erythrocytosis. Erythrocytosis is typically accompanied by hyperviscosity, that is now considered a crucial determinant in the pathogenesis of migraine. The results of some epidemiological investigations are in substantial agreement to confirm the existence of a significant relationship between increased haemoglobin levels and migraine, whereas some case reports have also reported an effective improvement of symptoms after reduction of erythrocyte count by therapeutic venesection. Interesting evidence has recently emerged from the assessment of red blood cell distribution width (RDW), a simple and inexpensive measure of anysocytosis that has been also associated with a variety of ischaemic and thrombotic disorders other than migraine. The aim of this review was to provide an overview of the current clinical and epidemiological evidence linking migraine and erythrocyte biology.
Introduction
According to the International Headache Society (HIS) 1, headache is conventionally defined as pain located above the orbitomeatal line. The HIS has also recently proposed a hierarchical classification of this condition [i.e., the International Classification of Headache Disorders 2nd (ICHD-II)], which substantially entails primary (including migraine), secondary headaches and other forms of headaches (Table 1) 1. As specifically regards migraine, this common disabling headache disorder can be basically classified according to the presence or to the lack of ‘aura’, that is conventionally defined as a transient focal neurological phenomenon that occurs before or during the headache.
| Primary headaches |
| Migraine (with or without aura) |
| Tension-type headache |
| Cluster headache and other trigeminal autonomic cephalalgias |
| Other primary headaches |
| Secondary headaches |
| Headache attributed to head and/or neck trauma |
| Headache attributed to cranial or cervical vascular disorder |
| Headache attributed to nonvascular intracranial disorder |
| Headache attributed to a substance or its withdrawal |
| Headache attributed to infection |
| Headache attributed to disorder of homoeostasis |
| Headache or facial pain attributed to disorder of cranium, neck, eyes, ears, nose, sinuses, teeth, mouth or other facial or cranial structures |
| Headache attributed to psychiatric disorder |
| Other headaches |
| Cranial neuralgias and central causes of facial pain |
| Other headache, cranial neuralgia, central or primary facial pain |
The migraine with aura, which is also referred to as ‘classic’, complicated, ophthalmic, hemiparaesthetic, hemiplegic or aphasic migraine, typically occurs with recurrent pain attacks that last for minutes, and which are anticipated (by nearly 60 min) by one or more fully reversible aura symptoms (i.e., visual, sensory, speech and/or language, motor, brainstem and retinal) that last between 5 and 60 min 1. Interestingly, at least one aura symptom is fully monolateral and spreads gradually in approximately 5 min, suddenly followed by the onset of two or more other symptoms. The visual involvement (e.g., a zigzag figure near the point of fixation or scotoma) is by far the most common type of symptom, developing in more than 90% of subjects. Sensory involvement is also frequent, and is characterized by dizziness or onset of pins and/or needles that slowly spread from the site of origin to involve the face or the entire body. In some subjects, the aura can be preceded by ‘premonitory’ symptoms, which begin several hours before aura and encompass fatigue, problems of concentration, nausea, hypersensitivity to lights and/or sounds.
As regards the pathogenesis, cerebral blood flow imaging reveals an initially reduced blood flow in the cortical affected area before or at the same time with the onset of aura. The decreased blood flow typically starts from the back and spreads to the front, but usually remains over the ischaemic threshold. After a variable period of time (i.e., from 1 to 12 h), a gradual transition to reactive vasodilation and hyperaemia occurs in the same area, and this is thought to be the leading mechanism responsible for head pain 1, 2. It is now well established that the cortical spreading depression (CSD) of Leão is an accompanying phenomenon, that manifests as a depression of electroencephalographic activity, moving across the cortex at a rate of 3–6 mm/min 2. It is noteworthy that aura was considered for a long peculiar vascular process (Wolff 1948). The further discovery of CSD has, however, complemented this notion, and it is now widely accepted that abnormal neuronal activity occurs in combination with alterations of cerebral blood flow 2. Even more interestingly, vasomotor changes in cortex advance at a faster speed than neuronal abnormalities, thus suggesting that the pathogenesis of aura more deeply involve the vasculature than the neuronal component 2. It is also noteworthy that the visual cortex of migraine subjects has a much lower inhibitory activity, and this may ultimately translate into a lower threshold for head pain. When trigeminal fibres are sensitized, a sterile meningeal inflammation develops along with thalamus activation. The signal is then transmitted to the cortex where it causes the subjective perception of pain 1.
The migraine without aura, which is conventionally known as common migraine or hemicrania simplex, is a recurrent headache disorder which manifests in attacks that typically last between 4 and 72 h 1. The conventional symptoms include monolateral localization, pulsating pattern, intensity comprised between moderate and severe, typical worsening by routine physical activity and frequent association with nausea, photophobia and phonophobia. In children and adolescents (i.e., those aged 18 years or younger), the migraine attacks may often be bilateral and tend to be shorter (i.e., 2 to 72 h). Occasionally, the pain may also have a facial location (i.e., ‘facial migraine’). Interestingly, the attacks may occur in association with menstrual cycle in women (i.e., pure menstrual migraine or menstrually related migraine). At variance with migraine with aura, regional cerebral blood flow imaging fail to reveal suggestive signs of CSD during the attacks, even if cortical changes secondary to pain activation and minor blood flow changes may still develop in the brainstem. Despite this disorder has been considered for long as of primarily vascular origin (identification of bilateral spreading oligaemia may support the presence of silent aura), recent evidence suggests that glial waves along with additional cortical phenomena may be involved in the pathogenesis, with an active participation of some key messengers such as nitric oxide (NO), 5-hydroxytryptamine (5-HT) and calcitonin gene-related peptide (CGRP). Therefore, an active involvement of neuronal components has been supposed, wherein increased sensitization of pain pathways is now regarded as a complementary mechanism 1. This basically involves a dysfunction of raphe nucleus, locus coeruleus and periaqueductal grey matter, which lead to anomalous activation of the trigeminal system and, ultimately, to a lower threshold for head pain.
Although no conclusive data about the epidemiology of migraine are available because even the most reliable statistics may suffer from a substantial bias due to underestimation, it has been suggested that not <40% of people has experimented at least one headache some time in life. Headache is also one of the most frequent cause of consultation in both general practice and neurological clinics, thus emerging as a public healthcare issue. Considering that headache is a (often) disabling condition causing remarkable sufferance and quality of life impairment, it also poses a dramatic socioeconomic burden 3. Merikangas has recently performed a comprehensive review of the scientific literature about the prevalence of migraine subtypes and tension-type headache defined in accordance with the ICHD-II criteria, including 19 studies in adults and totalling 272 731 people from 17 countries 4. The resulting aggregate weighted estimate of 12-month prevalence of migraine was 18.5% (11.5% definite and 7% probable migraine). The weighted aggregate rates of migraine with aura, chronic migraine and tension-type headache were 4.4%, 0.5% and 13%, respectively. In a recent review of 64 cross-sectional studies in 32 different countries and including 227 249 children and adolescents, Wöber-Bingöl 5 also estimated a mean prevalence of 54% (95% CI, 43–66%) for headache and 9.1% (95% CI, 7.1–11.1%) for migraine. It has also been reported that women in the age between 30 and 49 years are at particularly increased risk of migraine, and are more likely to use emergency care services for this condition 6.
Migraine and Red Blood Cells
It has been for long appreciated that migraine may be a frequent symptom of polycythemia with or without iron overload 7, so that the hypothesis of a neurovascular pathogenesis which may also involve erythrocyte biology has been put forward 8. Therefore, with the aim to review the current clinical and epidemiological evidence linking migraine and red blood cell (RBC) biology, we searched Medline, Scopus and Web of Science using the keywords ‘migraine’ and ‘red blood cells’ or ‘h(a)emoglobin’ or ‘h(a)ematocrit’ or ‘red cell distribution width’ (RDW), with no language or date restriction. The reference of retrieved articles was also searched for identifying additional articles about this topic. Only those articles using standardized criteria for diagnosing migraine (i.e., those of the IHS) and reporting data about the epidemiological association between RBC biology and migraine were finally included.
Clinical evidence
The electronic search according to the specified criteria yielded 182 items. After careful reading of title, abstract and full text (when available), this review finally included five clinical studies exploring the epidemiological association between RBC biology and migraine, along with three reports of clinical efficacy (on frequency and severity of migraine) after lowering haemoglobin by therapeutic venesection.
The first study that has investigated the potential association between RBC biology and migraine was carried out by Arregui et al., in 1994 9. The investigation, which involved 379 adult men with permanent residence at altitude (i.e., 4300 m), reported a prevalence of migraine of nearly 32% (mostly with aura) and a prevalence of tension-type headache of approximately 15%. Notably, men with migraine or with ≥2 migraine attacks per month had the highest haemoglobin levels as compared with subjects without migraine attacks.
In a following study, Aamodt et al. 10 performed a population-based cross-sectional study (the HUNT Study) including 2385 women aged from 20 to 55 years, who responded to a headache survey and had blood samples available for assessment of haemoglobin and ferritin. In the fully adjusted multivariate analysis, a positive correlation was observed between decreased values of haemoglobin (<115 g/L) and reduced prevalence of migraine [Odds Ratio (OR), 0.4; 95% CI, 0.2–0.9; P = 0.01]. No correlation was instead observed between reduced values of ferritin (<10 μg/L) and prevalence of migraine (OR, 1.1; 95% CI, 0.8–1.5; P = 0.55).
Hermans et al. 11 correlated the prevalence of migraine (with or without aura) with haemoglobin and haematocrit levels in 40 subjects with congenital heart disease, and found that haemoglobin levels significantly predicted the presence of migraine in stepwise logistic regression analysis (r = 0.39 and P = 0.007 in the entire cohort of patients; r = 0.50 and P = 0.036 in patients suffering from migraine with aura).
Forcelini et al. 12 performed a cross-sectional study including 17 subjects with migraine without aura, 17 with chronic migraine, 17 with medication-overuse headache and 17 subjects without headache. In all categories of patients with migraine or headache the values of haemoglobin were significantly higher (i.e., 142 ± 10 g/L in migraine without aura, 142 ± 11 g/L in chronic migraine and 146 ± 13 g/L in medication-overuse headache) than in the control population (137 ± 8 g/L; P < 0.05 for all comparisons). In patients with migraine without aura, no significant difference was found in haemoglobin value between the ictal and interictal phases (142 ± 10 vs. 142 ± 9 g/L, P = ns).
More recently, Celikbilek et al. 13 performed a cross-sectional study including 100 migraine patients (52 with aura) and 100 age- and gender-matched control subjects. Although the median haemoglobin values were found to be similar between migraneus and control men [150 g/L; Interquartile Range (IQR), 149–153 g/L vs. 156 g/L; IQR, 139–161 g/L; P = 0.63], and slightly decreased in migraneus females than in control women (130 g/L; IQR, 124–136 g/L; P < 0.001), the median value of RDW was found to be significantly increased in patients with migraine than in controls (13.7%; IQR, 13.1–14.6% vs. 13.1%; IQR, 12.7–13.4%; P < 0.001). This association remained significant in females (13.8%; IQR, 13.1–14.8% vs. 13.1%, IQR, 12.6–13.4%; P < 0.001), but not in males (13.3%; IQR, 12.9–13.7% vs. 13.3%; IQR, 13.0–13. 5%; P = 0.97). In the entire study population, the value of RDW was also found to be significantly associated with migraine in fully adjusted multivariate analysis (OR, 3.6; 95% CI, 2.1–6.3; P < 0.001). The RDW value was also found to be positively associated with attack duration in the entire cohort of patients with migraine (P = 0.037). It is noteworthy, however, that the overall number of men included in this study (12 controls and 10 with migraine) was very limited as compared with the female population (88 controls and 90 with migraine), so that data on the male gender should be interpreted with caution.
Indirect evidence of a potential relationship between increased haemoglobin values and migraine also emerges from studies of therapeutic venesection. Stovner et al. 14 described the case of a 60-year-old woman with hereditary haemochromatosis and chronic migraine. Therapeutic venesection was started, with removal of 500 mL of venous blood weekly for 2 months. By 10 weeks after the first venesection, the frequency of migraine attacks dramatically dropped and the patient suspended all prophylactic and acute medications a few weeks later. Gaul et al. 15 also described the case of a 41-year-old man with hereditary hemochromatosis, who was admitted to a neurology department because of migraine of increasing frequency. The erythrocyte count on admission was 5.0 × 1012/L (reference limits 4.6–6.2 × 1012/L), and decreased by 11% (4.5 × 1012/L) after therapeutic venesection with removal of 500 mL venous blood once weekly for 3 weeks. This treatment was also associated with a reduction of frequency (from daily to once a week), intensity (visual rating scale decreased from 8 to 5) and duration (from approximately 12 h to 3 h) of migraine attacks. Another interesting case has recently been reported by Stanzani Maserati 8, who studied a 30-year-old man that had suffered from migraine with aura from the age of 16. The haematological profile revealed substantial abnormalities, including a very high haematocrit value of 0.57 (reference range 0.36–0.45), a haemoglobin value of 198 g/L (reference range 125–150 g/L) and a RBC count of 6.7 × 1012/L (reference range 4.0–5.0 × 1012/L). A final diagnosis of polycythemia vera was made, supported by identification of JAK2 V617F mutation and exclusion of secondary causes of erythrocytosis. The patient was then subjected to periodic phlebotomies, which were effective to prevent further attacks of migraine.
Biological evidence
The epidemiological association between increased erythrocytosis (and/or increased haemoglobin values) and migraine is also supported by reliable biological evidence. The leading pathogenetic mechanism is indeed attributable to an increased whole blood viscosity consequent to erythrocytosis, regardless of the underlying cause of polycythemia (either primary or secondary). In an original report published more than 35 years ago, Thomas et al. 16 showed that cerebral blood flow was significantly lower in patients with haematocrit values comprised between 0.47 and 0.53 than in those with haematocrit values in a lower range (i.e., 0.36–0.46). It was also proven that cerebral blood flow increased by approximately 50% after reduction of haematocrit by venesection, an improvement that was principally attributed to decreased blood viscosity. In a following investigation, Grotta et al. 17 also reported that cerebral blood flow was inversely correlated with haematocrit in 53 subjects (r = −0.29; P < 0.05). Tang et al. 18 studied 116 polycythemic neonates by craniocerebral ultrasonic examination, and found a significant reduction in perfusion of cerebral blood flow along with impaired cerebral oxygenation, which was then followed by cerebral ischaemic injury and long-term neurological complications. According to pooled evidence, a decrease of 25–30% of cortical blood flow can hence be recorded in patients with migraine, especially in those with aura 19. The decreased cerebral blood flow persists for up to 150 min into the headache during the aura-like symptoms, thus triggering cerebral hypoxia, reactive vasodilatation and neurogenic inflammation 20 (Figure 1).
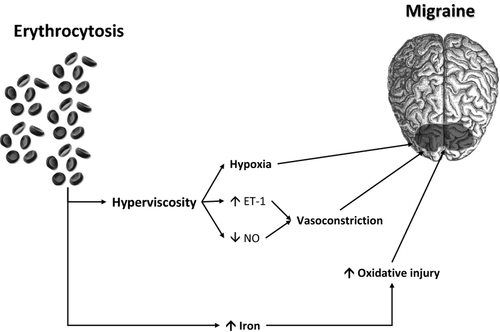
Hyperviscosity due to erythrocytosis may also exert effects other than hypoxia on brain metabolism, with shear stress-dependent activation of blood compounds and endothelium 11. In particular, a decreased concentration of vasodilatant metabolites of NO and an increased concentration of the potent vasoconstrictor peptide endothelin (ET-1) have been observed in patients with migraine 21. Interestingly, the ictal levels of ET-1 were found to be markedly increased at the beginning of the migraine attack, but returned to baseline in the following 4–6 h of an attack of migraine 22. As regard NO metabolism, several lines of evidence implicate RBC as modulators of NO metabolism, by impairing formation of this potent vasodilatant agent and inhibiting its cellular signalling. Although physical compartmentalization of haemoglobin within the erythrocyte limits its ability to scavenge NO, the intrinsic rate of NO scavenging by RBCs is still significant. This phenomenon is particularly magnified by intravascular haemolysis, through direct haemoglobin scavenging or impairment of arginine metabolism 23.
A complementary pathway that may link increased haemoglobin values with migraine involves iron metabolism in the brain. Kruit et al. 24 studied the concentration of this metal in seven deep brain nuclei in 138 patients with migraine and in 75 controls, showing that increased iron concentration in deep nuclei involved in migraine pathophysiology were significantly associated with repeated migraine attacks. Kruit et al. 25 also studied 295 migraineurs and 140 controls, and found an increased iron concentration in putamen, globus pallidus and red nucleus in patients with migraine compared with controls. Considering that the repeated episodes of hypoxia and hyperoxia that occur in patients with migraine may trigger the generation of free radical and subsequent cellular damage 26, it seems reasonable to expect that the greater availability of haemoglobin-transported iron in the cerebral circulation would predispose patients with erythrocytosis to an increased production of free radicals, lipid-peroxidation, demyelination, denudation of axons and neurodegeneration in specific areas, ultimately triggering the classic attacks of migraine 27.
Conclusions
Migraine, with or without aura, is a leading source of disability and a major cause of healthcare services occupation. The remarkable advantages in our understanding of the pathophysiology of this disorder has allowed to develop some therapies specifically tailored to target those pathways that are most frequently involved in migraine attacks. Despite major emphasis has been placed on investigation of putative biomarkers that could predict response to specific treatments and prophylaxis 28, less focus has been directed at the association between migraine and erythrocytosis and/or increased haemoglobin values. Erythrocytosis is typically accompanied by hyperviscosity, that is now considered a crucial determinant of hypoxia in the pathogenesis of this condition, as also confirmed by studies of effective improvement of symptoms by administration of high-flow 100% oxygen in cluster headache as well as in patients with migraine 29, 30. The results of the epidemiological investigations that have explored the association between haemoglobin and migraine are in substantial agreement to confirm the existence of a significant association between erythrocytosis and migraine, whereas some case reports have also described an effective improvement of symptoms after therapeutic venesection. It is also noteworthy that we failed to identify any reliable report in the current scientific literature where anaemia has been significantly associated with classic or common migraine, thus reinforcing the epidemiological and biological relationship with erythrocytosis. Interesting evidence has also emerged from assessment of RDW in patients with migraine, a simple and inexpensive measure of anysocytosis that has recently been associated with a variety of cardiovascular disorders 31. At variance with other putative migraine biomarkers such as the neuropeptide CGRP or other inflammatory cytokines, haemoglobin, haematocrit and RDW can be routine measured with easier, faster and less expensive techniques, thus representing very attractive biomarkers of migraine. It is also noteworthy that a deeper understanding of the intriguing relationship existing between erythrocyte biology and migraine would help identify new therapeutic perspective to decrease the burden of this painful and disabling disorder.