State of the art and new developments in molecular diagnostics for hemoglobinopathies in multiethnic societies
Summary
For detecting carriers of thalassemia traits, the basic part of diagnostics consists of measurement of the hematological indices followed by mostly automatic separation and measurement of the Hb fractions, while direct Hb separation either on high pressure liquid chromatography or capillary electrophoresis is sufficient to putatively identify carriers of the common Hb variants like HbS, C, E, D, and O-Arab. A putative positive result is reported together with an advice for parents, partner, or family analysis. For couples, presumed at-risk confirmation at the DNA level is essential. In general, this part of diagnostics is done in specialized centers provided with sufficient experience and the technical tools needed to combine hematological and biochemical interpretation with identification of the mutations at the molecular level. State-of-the-art tools are usually available in centers that also provide prenatal diagnosis and should consist of gap-PCR for the common deletions, direct DNA sequencing for all kind of point-mutations and the capacity to uncover novel or rare mutations or disease mechanisms. New developments are MLPA for large and eventually unknown deletion defects and microarray technology for fine mapping and primer design for breakpoint analysis. Gap-PCR primers designed in the region flanking the deletion breakpoints can subsequently be used to facilitate carrier detection of uncommon deletions in family members or isolated populations in laboratories where no microarray technology or MLPA is available.
Introduction
Hemoglobinopathies (HbPs) constitute the commonest recessive disorder worldwide 1. The severe forms, sickle cell disease (SCD), and β-thalassemia major (TM) are involving a protein essential for life, the hemoglobin tetramer. HbA (α2/β2) is the major protein in adults responsible for oxygen transportation in the body and the major component of the red cells in postnatal life. The disorders are caused by mutations in the globin genes and regulatory elements that may change the structure of the gene products (abnormal hemoglobin) or impair the expression of the genes (thalassemia) 1, 2. Because carriers have been protected for thousands of years against death in infancy due to malaria tropica, at least 7% of the world population is today a (healthy) HbP carrier. Due to historical and recent immigration, hemoglobinopathies have spread from the endemic areas of the old world to all continents and carriers can be found at variable frequencies in many countries that have never been endemic for malaria tropica.
Recessive means that parents who are both healthy carriers have 25% chance of having children who are severely affected with the most common severe forms, that is, sickle cell disease (SCD) and β-thalassemia major (Cooley's anemia) 3-6. These severe forms are caused by defects of the beta-genes and therefore manifest a few months after birth, when the hemoglobin switch from predominantly HbF to HbA expression takes place. Although a correct diagnosis of these severe forms rarely escapes a provisional clinical diagnosis, clinicians still need laboratory confirmation at the molecular level for prognosis, adequate treatment, and genetic counseling of the parents. Carrier detection is especially useful for offering an informed reproductive choice to couples at risk, preferably before conception or retrospectively for the next pregnancy in couples who already had an affected child.
Together with information and counseling, carrier detection is one of the main issues in prevention strategies applied in many countries endemic for hemoglobinopathies as described previously in this journal 7. In 2002, the European Molecular Genetics Quality Network (EMQN) has published best practice guidelines for carrier identification and prenatal diagnosis of hemoglobinopathies. Recently, a revision has been made by experts in the field to reach consensus about recommended strategies and methods to be used in conjunction with other guidelines, for example, those published by the British Society of Haematology 8, the ENERCA recommendations for preconceptional or antenatal screening, prenatal diagnosis and genetic counseling 9, and the UK NHS Sickle Cell and Thalassemia screening program 10 and will soon be available on the EMQN Web site (http://www.emqn.org/emqn/Best+Practice). A strategy applicable in multiethnic societies where a variety of mutations may cause hemoglobinopathies is presented here.
Genetics
In humans, the α- and β-like globin chains are coded by two globin gene clusters on chromosomes 16 and 11, respectively. The embryonic genes (ζ2 and ε only active during early embryonic life, producing Hb Gower-1 (ζ2ε2), Gower-2 (α2ε2) and Hb Portland (ζ2γ2). The duplicated α-genes (4 in diploid genomes) are expressed throughout fetal and adult life producing the α globin needed for the formation of fetal hemoglobin HbF (α2γ2) and the adult HbA (α2β2; 97%) and HbA2 (α2δ2; 2.5%). The latter has no pathological relevance but is of significant interest for the diagnosis of β-thalassemia carriers.
Pathological genotypes involving the 4 α-genes manifest both in pre- and postnatal life while pathological β-globin genes are only significantly expressed in postnatal life. Therefore, as mentioned above, the pathology of sickle cell disease and β-thalassemia major becomes prominent only in the second semester of life, when the normal HbA that should have taken over from fetal hemoglobin is not present or abnormal 1 (Figure 1).
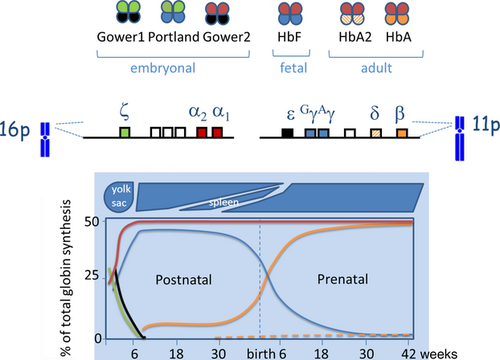
The hemoglobin switch, which is both tissue- and stage specific, has been matter of extensive investigation as this should hold the key to gene therapy 11-13. Manipulating the Hb switch to reactivate, the gamma expression would cure beta-thalassemia major and sickle cell disease by elevation of HbF levels. However, in spite of many years of research, the control of the switch mechanism has not been mastered and a cure by reactivating the silenced gamma-globin genes is not feasible yet.
The molecular basis of the thalassemias and hemoglobin variants is extremely heterogeneous, with over 1150 mutations overall (http://globin.bx.psu.edu/hbvar/). Over 100 different mutations causing α-thalassemia and over 280 causing β-thalassemia have been described worldwide with variable and specific phenotypes. While thalassemia traits are usually associated with mild microcytic anemia, most abnormal hemoglobins, unless unstable or thalassemic, are hematologically silent in the carrier. The majority of the common α-thalassemia determinants are due to deletions that remove part, or all of, the α-globin gene cluster, while the majority of β-thalassemia defects involve point mutations in the beta-gene or in the 5′ or 3′ untranslated region (http://globin.cse.psu.edu/hbvar.html) 14, 15.
Molecular analysis is needed to confirm the hematological and biochemical diagnosis of HbP, to detect complex combinations of α- and β-thalassemias or Hb variants, for the disease prognosis and to offer prevention by early pregnancy molecular diagnostics.
Basic Diagnostic Tools
The tools for basic HbP diagnostics still consist of complete blood count (CBC) and on the separation and quantification of the hemoglobin fractions 7. Dedicated high pressure liquid chromatography (HPLC) and capillary electrophoresis (CE) devices available on the market are highly sensitive in detecting abnormal separations 16. Frequent traits can be confirmed by simple or more complex additional analysis, however, although very sophisticated, HPLC and CE are present, like any analytical method, with inevitable limitations.
A normal individual after the age of 2 will present with about 96–97% HbA, ±3% HbA2, and <1%HbF. Any change in this pattern might hide an hemoglobinopathy and will have to be investigated. The most prevalent and relevant HbP traits in multiethnic societies are those associated with SCD (HbS, C, E, and D) and those causing β- and α-thalassemia.
HbS will be easily detected as an anomalous fraction of about 40% or less in the healthy carrier and of about 80–90% in the presence of fetal hemoglobin (HbF) in untransfused sickle cell patients. New born with sickle cell trait or disease will present with ±80% HbF and about 12% HbA and 8% HbS or with ±20% HbS only, respectively.
Carriers of beta-thalassemia will be diagnosed from the age of 1 by their microcytic hypochromic anemia and elevated HbA2 (>4%, normal range 2.5–3.5%). The child affected with beta-thalassemia major will be severely anemic by the age of 6 months and if not yet transfused, will present with HbF and HbA2 only.
α-Thalassemia is very common in the world population from area's endemic for malaria tropica and can be fairly suspected at the hematological and biochemical level due to microcytic hypochromic parameters not associated with iron depletion and eventually a reduced HbA2 level. Confirmation of alpha-thalassemia is done at the molecular level.
The General Strategy for Molecular Diagnostics for HbPs
The choice of molecular techniques for hemoglobinopathy carrier detection in immigration countries is based on the prevalence of many ethnic minorities originating from areas with a high incidence of hemoglobinopathies as previously described in this journal 7. As it is not uncommon that patients have combinations of alpha- and beta-thalassemia defects, this requires a systematic approach, involving screening for the most common alpha-thalassemia deletions. Patients presenting with microcytic anemia, normal HbA2, and not responding to iron therapy are suspected of being α-thalassemia carrier. If the 7 most common alpha deletions are not found, direct sequencing of the complete alpha and eventually multiplex ligation-dependent probe amplification (MLPA) analysis to detect the less common deletions will follow, depending on the hematological and biochemical evaluation (see Figure 2). For the beta-thalassemia carriers, characterized by an elevated HbA2, direct sequencing is the first choice as more than 90% of beta-thalassemia defects are caused by point mutations in or near the beta-globin gene, followed by MLPA analysis for the less common deletions.
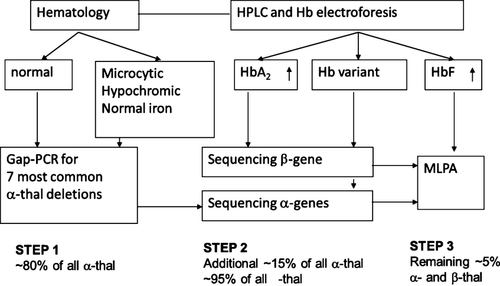
Direct sequencing is considered the golden standard to screen for all mutations including common, rare, and yet unknown mutations. In most ethnic groups, however, the number of prevalent mutations causing thalassemia or Hb variants is specific and well described, and therefore, targeted detection methods such as ARMS or reverse dot-blot can be sufficient for most cases in countries endemic for hemoglobinopathies. However, in countries with different and mixed ethnicities, population-targeted methods may not detect all mutations present and therefore direct sequencing remains the method of choice.
To detect the most common deletions causing alpha- and beta-thalassemia worldwide, a relatively simple, inexpensive multiplex gap-PCR is used 17-21. A limitation to this otherwise excellent system is that primers can be included into the multiplex only when breakpoint sequences are known.
In case no point mutations or common deletions are found, multiplex ligation-dependent probe amplification (MLPA) is the technology of choice. MLPA is based on ligation of probe pairs hybridizing to a region of interest to detect deletions or duplications by quantitative PCR and fragment analysis. Due to the implementation of MLPA for the detection of copy number variation in the alpha- and beta-globin gene clusters, more and more new types of deletions have been detected including the discovery of duplications of the complete alpha-globin gene cluster covering the major conserved regions, explaining unexpectedly severe cases of beta-thalassemia intermedia in beta-thalassemia carriers 22, 23.
Methods to Characterize Alpha-, Beta-Thalassemia Defects and Hb Variants
Gap-PCR for the common α-thalassemia deletions
As mentioned above, gap-PCR is the most established approach in DNA diagnostics to screen for the most common deletion defects causing alpha- or beta-thalassemia. The principle of gap-PCR is based on amplification using two primers complementary to the sense and antisense strand in the DNA region flanking the deletion. If the distance between the two flanking primers is too great to amplify the normal allele, a product is only obtained from the deletion allele. The length of the product is indicative of the deletion type (Table 1). By using different deletion-specific primer sets in a multiplex amplification reaction, more deletions can be detected in a single experiment, which makes it an efficient, inexpensive, and fast screening method. This method requires the use of positive controls for each of the deletions tested, besides a wild-type DNA and a blank.
| Primer mix | Primer name | Primer sequence 5′-3′a | Primer mix volume (μL) from stock of 250 pmol/μLa | Fragment size (bp) | Deletion name classic | Deletion name HGVS NG_000006.1b |
|---|---|---|---|---|---|---|
| A | GAP-3.7 (F) | 5′AAGTCCACCCCTTCCTTCCTCACC 3′ | 24 | 2217(αα) | Normal | |
| GAP-3.7 (R1) | 5′ATGAGAGAAATGTTCTGGCACCTGCACTTG 3′ | 8 | 1963(RW) | -α3.7 or Rightward (RW) | g.34164_37967del3804 | |
| GAP-3.7 (R2) | 5′TCCATCCCCTCCTCCCGCCCCTGCCTTTTC 3′ | 8 | 2440(ααα) | α-triplication | g.34164_37967dup3804 | |
| H2O | +60 = 100 μL | |||||
| B | GAP-4.2 (F) | 5′TCCTGATCTTTGAATGAAGTCCGAGTAGGC 3′ | 20 | 1510(αα) | Normal | |
| GAP-4.2 (R1) | 5′TGGGGGTGGGTGTGAGGAGACAGGAAAGAGAGA 3′ | 10 | 1725(LW) | -α4.2 or Leftward (LW) | ||
| GAP-4.2 (R2) | 5′ATCACTGATAAGTCATTTCCTGGGGGTCTG 3′ | 10 | ||||
| H2O | +60 = 100 μL | |||||
| C | GAP-20.5-F | 5′GGGCAAGCTGGTGGTGTTACACAGCAACTC3′ | 2 | 1187 | -(α)20.5 | g.15164_37864del22701 |
| GAP-20.5-R | 5′CCACGCCCATGCCTGGCACGTTTGCTGAGG3′ | 2 | ||||
| GAP-ALFA / SEA-F | 5′CTCTGTGTTCTCAGTATTGGAGGGAAGGAG3′ | 6 | 1010 (αα) | Normal | ||
| GAP-ALFA-R | 5′TGAAGAGCCTGCAGGACCAGGTCAGTGACCG3′ | 3 | ||||
| GAP-SEA-R | 5′ATATATGGGTCTGGAAGTGTATCCCTCCCA3′ | 3 | 660 | – SEA | g.26264_45564del19301 | |
| GAP-MED-F | 5′CGATGAGAACATAGTGAGCAGAATTGCAGG3′ | 3 | 875 | – Med I | g.24664_41064del16401 | |
| GAP-MED-R | 5′ACGCCGACGTTGCTGCCCAGCTTCTTCCAC3′ | 3 | ||||
| GAP-FIL-F | 5′AAGAGAATAAACCACCCAATTTTTAAATGGGCA3′ | 32 | 550 | – Fil | g.11684_43534del31851 | |
| GAP-FIL-R | 5′GAGATAATAACCTTTATCTGCCACATGTAGCAA3′ | 32 | ||||
| GAP-THAI-F | 5′CACGAGTAAAACATCAAGTACACTCCAGCC3′ | 2 | 411 | – Thai | g.10664_44164del33501 | |
| GAP-THAI-R | 5′TGGATCTGCACCTCTGGGTAGGTTCTGTACC3′ | 2 | ||||
| H2O | +135 = 225 μL |
- HGVS, Human Genome Variation Society.
- a Fifty ng of genomic DNA is added to a mixture containing 1× αPCR buffer (10× αPCR buffer; 0.2 m Tris–HCL pH8.4, 0.5 m KCl, 0.015 m MgCl2, 2 mm of each dNTP), 0.13 μL primer mix A, 5 μL betaine (Sigma B-0300) solution, 1.2 Units Platinum Taq DNA polymerase (Invitrogen, cat.nr.10966-034) and water to an end volume of 25 μL. The other deletions -α4.2, MedI, -(α)20.5, SEA, FIL and THAI, perform best using the commercially available Multiplex PCR kit of Qiagen. The primers for this RW gap-PCR method also detect the α-triplication as a 2440 bp fragment. Thermocycling is performed in a PCR apparatus (any thermocycler with heated lid will do) under the following conditions: 1 cycle, 10 min. at 97 °C (hot start); 30 cycles, 1 min at 97 °C, 1 min. at 65 °C and 2 min. at 72 °C; 5 cycles, 1 min at 97 °C, 1 min. at 65 °C, and 3 min. at 72 °C; and 1 cycle, 10 min at 72 °C. Following completion of the PCR cycling, 5 μl of the PCR reaction is tested by electrophoresis on an agarose gel using a size marker.
- b Reference GenBank sequence.
The method makes use of equipment already available in most diagnostic laboratories and is inexpensive and fast. The most common α-thalassemia mutations are the α+ -thalassemia defects (called α-thalassemia type 2 in the past) which leave a single functional α-globin gene on the chromosome. There are two common types of α+ defects, the first is a deletion of 3.7 kb also known as the right-ward (RW) deletion and the other is a deletion of 4.2 kb, also known as the left-ward (LW) deletion. Besides these two most common ones, there are other deletions called α0-thalassemia defects (called α-thalassemia type 1 in the past) which erase both the α-genes of one chromosome. These are deletions of around 20 kb and larger, which are quite common among populations of the Mediterranean basin (MED I and -(α)20.5 kb deletions) or SE Asia (SEA, FIL, THAI). In the Netherlands, like in other multiethnic countries, more than 80% of all alpha-thalassemia mutations consist of these seven deletions, which makes a prescreening by gap-PCR most cost-effective.
Different protocols are available from literature 17, 18, 24, 25 all with different primers and conditions, but most primer sets can be used together in a multiplex PCR system, some of which are also capable of detecting alpha-triplications. In our experience, the gap-PCR for the -α3.7 deletion is better performing when performed separately and as follows.
The primer names, sequences, and amounts used in preparing primer mix solutions are summarized in Table 1.
Direct sequencing
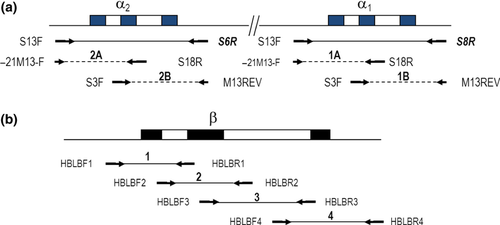
As the globin genes are rather small, direct sequencing, to detect point mutations on the entire β-globin gene and on the α-genes in case no deletion defects are found, is rather easy (Table 2). The selected method is suited to an automated laboratory setting, using 96-well plates for sample preparation and reactions, and the use of universal primer sets for sequencing. This is achieved by the addition of -21M13-F and M13REV sequence ‘tails’ to the amplification primers to generate sequence templates. Direct sequencing of the highly homologous duplicated alpha-globin genes requires a first PCR to amplify specifically α1- and α2-gene, followed by a nested PCR for both α1- and 2 separately. A nested PCR is necessary to obtain the smaller overlapping α1- and α2-specific fragments of approximately 400 bp for direct sequencing. This covers the promoter region from 58 bp upstream from the cap site to the 3′ untranslated region (3′ UTR) 140 bp downstream of the termination codon of each α-globin gene (see Figure 3a).
| Fragment name | Primer name | Primer sequence 5′-3′ | Fragment size (bp) |
|---|---|---|---|
| α-gene | |||
| α2 | S13F | tgtaaaacgacggccagt-cgccagccaatgagcgcc | 996 |
| S6R | caggaaacagctatgacc-tccattgttggcacattccg | ||
| α1 | S13F | tgtaaaacgacggccagt-cgccagccaatgagcgcc | 993 |
| S8R | caggaaacagctatgacc-tgtccacgcccatgctggcac | ||
| 1A and 2A (nested PCR for resp. α1 and α2) | -21M13-F | tgtaaaacgacggccagt | 487 |
| S18R | caggaaacagctatgacc-cgttgggcatgtcgtccac | ||
| 1B and 2B (nested PCR for resp. α1 and α2) | S3F | tgtaaaacgacggccagt-cacggcaagaaggtggccgac | 574 |
| M13REV | caggaaacagctatgacc | ||
| β-gene | |||
| 1 | HBLBF1 | tgtaaaacgacggccagt-tcttagagggagggctgagg | 478 |
| HBLBR1 | caggaaacagctatgacc-ggcagagagagtcagtgccta | ||
| 2 | HBLBF2 | tgtaaaacgacggccagt-gaaactgggcatgtggagac | 471 |
| HBLBR2 | caggaaacagctatgacc-tgatgcaatcattcgtctgtt | ||
| 3 | HBLBF3 | tgtaaaacgacggccagt-gcacgtggatcctgagaact | 678 |
| HBLBR3 | caggaaacagctatgacc-tggtgcaaagaggcatgata | ||
| 4 | HBLBF4 | tgtaaaacgacggccagt-gaatctctttctttcagggcaat | 648 |
| HBLBR4 | caggaaacagctatgacc-aatgcactgacctcccacat | ||
After purification, using QIAquick columns (QIAquick PCR Purification Kit; QIAGEN Benelux BV, Venlo, The Netherlands), the sequencing reaction is set up using 4 μL of the purified PCR product according to the manufacturer's instructions (Big Dye Terminator kit v3.1 cycle sequencing; Applied Biosystems Inc., Foster City, CA, USA) and analyzed using the ABI 3730 or any other automated sequencing device. The sequencing primers are, respectively, -21M13-F and M13REV to obtain sequences of both strands of each overlapping PCR product.
For direct sequencing of the β-globin gene, essentially the same strategy of adding -21M13-F and M13REV sequence ‘tails’ to the primers is applied as for the α-globin gene. The region is divided in four overlapping PCR fragments (Figure 3b) covering a region from −170 from the cap site up to position 175 bp downstream from the termination codon.
Purification and sequencing is performed as previously described for the alpha-globin genes.
Sequencing products are run on any automatic sequencing instrument (such as the ABI310, 3700, 3730), preferably with capillary chemistry separation such as ABI Big Dye Terminator containing ddNTP's labeled with fluorescent dyes in four colors. Data analysis is performed using any appropriate data analysis software, such as the SeqScape software package.
This method is suitable to detect point mutations, small deletions and insertions causing α- and β-thalassemia as well as to confirm common or to characterize rare or new Hb variants.
MLPA
The principle of multiplex ligation-dependent probe amplification is based on detecting deletions or duplications by quantitative amplification of multiple probe pairs hybridized across the alpha- and beta-globin gene clusters and neighboring regions. By using universal tags to each ligated primer set, which differ in length among each other, amplification with a labeled universal PCR primer and separation by an automated fragment analyzer rearrangements are detected by quantifying the fluorescence for each probe. The decrease or increase in signal as compared to a normal control is indicative of, respectively, deletions or duplications across the locus analyzed and represent a valuable alternative or supplementary method to gap-PCR when studying known and unknown rearrangements.
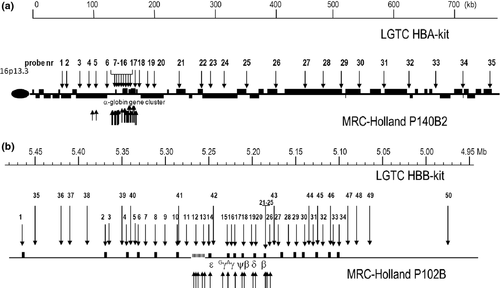
Two types of commercially MLPA kits for each of the α- and β-globin gene clusters are available on the market. The kit from MRC-Holland bv (Amsterdam, The Netherlands, www.mlpa.com) makes use of cloned cosmid probes, which are restricted to the α- and β-gene cluster and to the regulatory elements (respectively, P140B2 and P102B). The kit from LGTC (Leiden Genome Technology Center, Leiden, The Netherlands,www.LGTC.nl) makes use of oligonucleotide probes designed over a larger region allowing the detection of both smaller and more extensive deletions involving the α- and β-globin gene cluster and neighboring regions (respectively HBA- and HBB-MLPA kit; Leiden Genome Technology Center, Leiden, The Netherlands) (Figure 4) 26. The major experimental differences are the probe length, and therefore the optimal separation region on the fragment analyzer, and the use of additional fluorescent dyes. The MRC-Holland probes range in length from approximately 100–480 bp using FAM-labeled primers, while the LGTC probes, which are essentially the probes described by Harteveld et al. 35, have their optimal separation between 40–120 bp using ROX- and FAM-labeled primers. The kits make use of the same components, because MRC-Holland provides the buffers and enzymes for both.
Fragment analysis can be performed according to the manufacturer's instructions on an ABI 3130, 3730 or an equivalent apparatus. Data analysis is performed by using the Genemarker software of Softgenetics.
Tables with probe sequences and probe positions can be found in Harteveld et al. 35 for the LGTC MLPA kits HBA and HBB or the manufacturer's instructions of MRC-Holland for the P102B1 and P140B2 kits.
Latest Developments
Microarray analysis for deletions/duplications
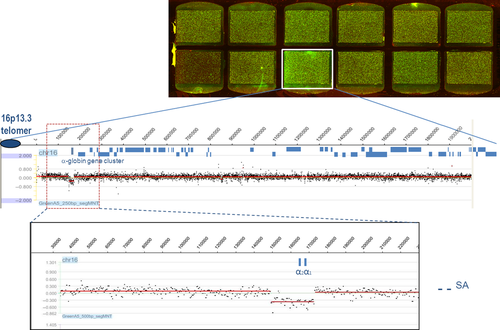
The array comparative genomic hybridization (aCGH) technology is a promising method in characterizing rearrangements and has become an essential routine diagnostic tool in genetics to search for copy number variation. The most significant advantages of aCGH over traditional cytogenetic methods are the high resolution, simplicity, high reproducibility, and precise mapping of imbalances. Fine-tiling oligonucleotide arrays have been used for breakpoint analysis of deletions causing several genetic diseases 17, 18, 27 including thalassemia 28, 29. The principle of aCGH includes the mixing of equimolar concentrations of patient DNA and normal control DNA labeled with different dyes, Cy3 (test samples) or Cy5 (reference samples), respectively, a deletion (or duplication) in the patient DNA becomes visible as a ratio disturbance in a subset of primers covering the region with an altered copy number (deletion or duplicated) (Figure 5). It was shown that fine-tiling aCGH technology was able to detect small and large rearrangements (from ~4 kb up to 2 Mb) in the α- and β-globin gene clusters with high resolution. The information provided by the array supported the design of primers to amplify a relatively short products containing the breakpoint sequence, which can then be easily analyzed by direct sequencing 28. For the thalassemias, arrays were used to fine-map the positions of the breakpoint junctions to design gap-PCR primers for sequencing to determine the exact deletion breakpoints in patients. Furthermore, knowledge of breakpoint sequences might give more insight in the molecular mechanisms giving rise to these rearrangements and may facilitate primer design for gap-PCR to screen for certain common population-specific deletions 19, 30, 31. When designing custom fine-tiling arrays, it is important to include only unique sequence primers as probes to exclude repetitive sequences which are abundant in the genome. This helps to increase specificity and to prevent false positive results. Optimal probe oligonucleotides have a length of 60–80 nt and a mean spacing of ~20 bp apart, indicating a ‘tiling’ or an overlap between probes providing an approximate 3× coverage of the region of interest containing unique sequences 28.
Discussion
The choice of molecular techniques to perform diagnostics for hemoglobinopathies largely depends on the prevalence and mutation spectrum in the population to be examined. In general, the knowledge of the population-specific mutation spectrum supports the selection of the most appropriate methods for molecular analysis. As mentioned above, however, with the advent of global migration, multiethnic societies present with a larger variety of mutations and complex combinations of different mutations. Then, population-specific diagnostic approaches are becoming less reliable, and other diagnostic strategies are required than those applied in geographically isolated areas where only a limited number of mutations are causing thalassemia or sickle cell disease 4, 32-34.
In all cases, and in multiethnic and consanguineous subjects in particular, it is essential to evaluate the hematological parameters in relation to the biochemical and molecular results to exclude the presence of more complex genotypes than the one found in the first place. If these three elements do not perfectly match, other traits can be present making risk assessment more complex.
As most deletions causing alpha-thalassemia involve the seven mutations most commonly found worldwide, many diagnostic laboratories have employed the multiplex PCR using primers described by Liu et al. 17. Based on the extensive mutation spectrum observed in immigration countries like the Netherlands, direct sequencing might be considered the method of choice to screen for the large variety of point mutations causing Hb variants, alpha- and beta-thalassemia.
For less common rearrangements in the alpha- and beta-globin gene clusters, multiplex ligation-dependent probe amplification has become a standard tool in an increasing number of diagnostic laboratories. MLPA is the ultimate solution for unknown deletion defects of either the β- or α-globin genes cluster, as some deletions restricted to certain geographic areas at low incidence are not included in the multiplex gap-PCR 30, 35, 36.
Conclusion
After the basic diagnostic protocols providing the provisional diagnosis of beta-thalassemia and the common Hb variants has been done, gap-PCR for the common alpha and beta deletions and direct sequencing for both nondeletional alpha- and beta-genes mutations is today the procedure of choice for molecular analysis of hemoglobinopathies. If gap-PCR and sequencing do not give the answer, MLPA will provide the characterization of unusual of new deletions with acceptable precision. However, the MLPA method might not be available in all molecular laboratories, and therefore, the characterization of breakpoints for all described deletions by CGH may be helpful to provide accessible gap-PCR methodology. Unfortunately, for most laboratories that are not confronted daily with large deletions, setting up an array CGH for a limited number of samples is not convenient. Then, screening for unusual or unknown deletions by MLPA if available and/or consulting a reference laboratory is the most sensible choice to determine the approximate size of the deletion and the breakpoint more accurately and design a gap-PCR assay to detect the specific deletion. Then, the information provided by the aCGH can be used to design simple and cheaper PCR-based tests to detect the variant alleles in populations or families in areas where specific deletions reach high frequency in the local population. A region-specific multiplex PCR to detect the locally common and less common deletions might increase the diagnostic capacity for many laboratories who can do PCR, but do not have the possibility to implement MLPA as a molecular diagnostic tool 19.




