HCV-HIV co-infection in people who inject drugs: Barriers to treatment and cure of HCV infection in the era of DAAs, a prospective study in Athens, Greece
Abstract
Objectives
HIV/hepatitis C virus (HCV) co-infection among people who inject drugs (PWID) remains a global health problem. The goal of our study was to evaluate, in a real-world setting, success rates of sustained virological response (SVR) using direct-acting antivirals (DAAs) to treat a population of PWID living with HCV/HIV.
Methods
This was a prospective single-center observational study. We collected demographic, socioeconomic, and clinical data pertaining to HIV and HCV infection in PWID with several barriers to care. We identified risk factors for SVR failure.
Results
Among 130 individuals retained to HIV care, we planned HCV treatment in 119/130 (91.5%); 106/119 (89.1%) started treatment with DAAs and 100/106 (94.3%) completed treatment. People not starting treatment were more often in active opioid drug use (odds ratio [OR] 0.25; 95% confidence interval [CI] 0.07–0.97, p = 0.045) and benzodiazepine abuse (OR 0.25; 95% CI 0.07–0.95, p = 0.042). Only 86/100 (86%) were tested for SVR at 12 weeks (SVR12) and 72/86 (83.7%) achieved SVR. PWID in opioid substitution programmes tended to return for SVR12 testing more often (54.7% vs. 30%, p = 0.081). Individuals in active opioid drug use (OR 0.226; 95% CI 0.064–0.793, p = 0.02) or with poor adherence (OR 0.187; 95% CI 0.043–0.814, p = 0.025) were less likely to achieve SVR. At the end of our study period, 113/119 (95%) treatment-eligible patients remained alive. HCV infection was cured in 68/113 (61.1%) people.
Conclusions
Our findings underscore the importance of prioritizing combatting substance use to achieve HCV elimination goals. A systematic approach with effort to overcome barriers to receiving and completing treatment and encourage to enrol in opioid substitution programmes if not possible to completely abstain from use, can help increase chances of HCV cure.
INTRODUCTION
According to the World Health Organization, currently 39 million people are estimated to be living with HIV and 50 million diagnosed with hepatitis C virus (HCV) infection [1, 2]. People co-infected with both viruses present a challenging population for HCV elimination, partly because of their likely exposure to both viruses through intravenous drug use. It is estimated that 15.2% of people who inject drugs (PWID) are infected with HIV, that 52.3% have HCV antibodies and that 38.8% have current HCV infection [3, 4]. Drug use itself, and the socioeconomic factors attached to it, present barriers to healthcare. It is estimated that more than 2 million people worldwide live with HIV and HCV infection. Half of them are PWID [5]. People living with HIV/HCV should be prioritized for HCV treatment as the immunopathological impact of HIV–HCV co-infection is more detrimental than each infection separately. HIV–HCV co-infection has been associated with liver disease progression and with an increased risk of cirrhosis, hepatocellular carcinoma, and overall mortality [6]. Blunted immune responses in the context of HIV–HCV co-infection can be attributed to the reliance of the immune response to HCV infection on CD4 T cells [7]. Bacterial translocation and chronic inflammation related to HIV infection contribute to decompensation of liver disease and accelerate the occurrence of complications, in comparison with mono-infection [8]. Surprisingly, the impact of HIV on the natural history of HCV infection remains prominent after successful HIV virological suppression with effective antiretroviral treatment (ART) [9, 10]. Conversely, HCV infection inhibits immune reconstitution even after effective ART and has been linked to reduced and slower CD4 count increases [11].
The Joint United Nations Programme on HIV/AIDS (UNAIDS) has set the goal of 95-95-95 for HIV elimination: for 95% of people living with HIV to know their HIV status, 95% of people living with HIV to receive ART, and 95% of people on ART to be virally suppressed [12]. In addition since 2018, Greece has initiated a nation-led HCV elimination programme; the restrictions on access to reimbursed direct-acting antiviral (DAA) treatment were lifted and rapid DAA scale-up has occurred [13, 14].
Barriers to care, globally, have been categorized into system-level barriers (lack of providers, lack of patient navigation, lack of coordination between services), provider-level barriers (physician prejudice, referral bias, lack of knowledge) and patient-level barriers (adherence, substance use, socioeconomic problems) [15]. Common barriers to treatment are patients' medical or social priorities and limited access to harm reduction services and mental health treatment [16]. Active injectable drug use poses a major challenge and has been associated with a lower frequency of HCV treatment uptake [17]. People living with HIV–HCV co-infection often face unemployment and food insecurity, where access to food may take precedence over HCV treatment [18]. Moreover, experience from previous complex and long-lasting HCV treatments may deter PWID, and lack of knowledge about the effectiveness of new DAA therapy might be an additional barrier to treatment [19].
The national plan for HCV elimination identifies barriers to treatment and includes an attempt to address them. The authors identify four national policies that need to be implemented, including programmes to raise public awareness, particularly among young people; offering asymptomatic HCV screening to both the general population and those with risk factors for HCV infection; establishing diagnostic and treatment protocols to enable even primary care physicians through education to offer and conclude treatments with DAAs; and establishing programmes for follow-up and chronic care for those requiring it. The authors raise concerns that half of the people diagnosed with HCV infection are lost to follow-up and never receive treatment. Some possible ways to engage people who have not been retained to care include the use of social media and advertising of the benefits of testing and treatment, implementing daily outpatient HCV care departments without the need for prior appointments, educational interventions aimed at hospital and non-hospital healthcare personnel to explain how vulnerable populations like PWID and migrants are best approached, and – finally – visiting addiction facilities or undertaking street work to explain to those in need of care that newer treatments are safer and more effective because previous experience with interferon treatments in the PWID community has been found to act as a deterrent [13].
Just as highly effective ART brought about a paradigm shift on the treatment of HIV, the emergence of DAAs changed the outlook for the treatment of HCV [20]. Overall, success in treatment with DAAs has been over 90% in most populations.
Clinical trials have targeted HCV–HIV co-infected populations. In ASTRAL, 95% (101/106) of participants treated with sofosbuvir/velpatasvir experienced sustained virological response (SVR). In EXPEDITION-2, among 153 HIV–HCV co-infected participants, including 10 with cirrhosis, 98% (150/153) achieved SVR at 12 weeks (SVR12) with glecaprevir/pibrentasvir. In ENDURANCE-1, with 33 HIV–HCV co-infected participants, those receiving glecaprevir/pibrentasvir met a 100% SVR12 rate [21-23]. In the ANCHOR study, which focused on mono-infected PWID starting opioid substitution along with DAAs, 82% (82/100) attained SVR [24].
Beyond clinical trials, SVR percentages have been comparable, but overall cure rates have been low. In a EUROSIDA cohort, although success rates were high, there was great variance between countries with regards to total cure: Belarus had the lowest success at 11.2 and Austria the highest at 87.2% [25]. The SVR rate was 97% in a study from Egypt and 85.9% in a Kenyan cohort [26, 27].
Over the past decade, an ongoing epidemic of HCV and HIV infections has been recorded in Athens and Thessaloniki, Greece. First, during 2011, an increasing number of HIV diagnoses was observed in PWID in Athens, rising from 5.8/100 000 population in 2009 to 8.7 and 10.5 in 2011 and 2012, respectively [28-30]. Epidemiological models have estimated that the incidence (95% confidence interval [CI]) of HCV diagnoses also increased from 640 (495, 842) cases in 2008 to 1260 (1060, 1500) in 2009 [29]. Three programmes were launched: ARISTOTLE HIV (2012–2013) and ARISTOTLE HIV/HCV (2018–2020) in Athens and Alexandros (2019–2021) in Thessaloniki with the aim to “seek, test, and retain” individuals with HIV and/or HCV infections among PWID [31, 32]. The ARISTOTLE HIV programme recorded a decline of 78% in HIV incidence between 2012 and 2013. After the implementation of these programmes, HCV incidence also declined by 64.8% [29]. It is important to highlight that mortality in this population has been very high. In 2530 participants in these programmes, 301 died over 8543 person-years. The standardized mortality rate was 1.5 times higher in people living with HCV and more than double in people living with HIV. PWID with HIV and with HCV had a 1.86- and 1.43-times higher risk of death than those without HIV and HCV, respectively [33].
The goal of our study was to evaluate in a real-world setting, success rates of DAAs in treating a population of PWID living with HCV–HIV. We attempted to investigate potential risk factors for treatment failure and identify barriers to care. Understanding the vulnerabilities of this population in the DAA era will provide key information on current progress and guide efforts toward HCV micro-elimination.
METHODS
Study design
This was a prospective observational study conducted at the Infectious Diseases Unit of the First Department of Internal Medicine at Laiko General Hospital, Athens, Greece. The study period spanned from 1 October 2017 until 17 January 2024, when we locked our database for analysis, while HCV elimination efforts are continuing. Up until the database lock, overall, 417 people living with HIV had been linked and retained in our care. A large proportion of our beneficiaries with HIV–HCV co-infection have been referred to our department through the ARISTOTLE programmes [31, 32]. People diagnosed with co-infection were accompanied by a social worker attached to the programme for an initial visit to our department. Subsequent appointments were reliant upon the beneficiary's adherence.
After obtaining informed consent, we enrolled all patients with HCV antibodies who had been retained in HIV care. People were considered retained in HIV care according to the US Centers for Disease Control and Prevention definition of having two or more visits in our department during the past 12 months [34]. We reviewed medical records and collected data pertaining to HCV infection, HIV infection, laboratory tests, and information pertaining to potential barriers to treatment, that is social security status, migrant status, education, employment, alcohol, opioid and benzodiazepine use, enrollment in opioid substitution or inpatient rehabilitation programmes, imprisonment, concomitant medications, and adherence to treatment information. HCV RNA was measured in all participants at enrollment. To the best of our ability, we informed all participants about DAA treatment and encouraged them to receive it. Participants were referred to other hospitals for elastography measurements, but a significant proportion did not attend their appointments, so a gap exists in these data.
Clinical setting
The process by which patients receive DAA treatment in Greece begins with entering their information into the national HCV registry and applying for treatment approval. A prerequisite for treatment is a Greek social security number and a detectable HCV RNA. Before the advent of pan-genotypic DAAs, the patient's HCV genotype was also required. Physicians can advocate for their patients and apply for DAA treatment approval, the cost of which falls upon the state. Upon treatment approval, beneficiaries need to visit the treating physician to obtain their prescription papers, and then they can obtain medications through state pharmacies.
Treating physicians are responsible for the completion and accuracy of the registration process. People without Greek national security numbers (i.e. migrants) have no means to procure DAA treatment as it cannot be compensated until social services issue them such a number, a process that is expedited for people with HIV and/or HCV. The treatment regimen is chosen by the treating physician based on a selection of possible treatments described in the national HCV treatment plan [13].
The primary outcome measured was successful SVR12 after completion of the first treatment. Secondary outcomes included HCV elimination regardless of number of treatment attempts, improvement in Fibrosis-4 (FIB-4) index for liver fibrosis and aspartate transaminase (AST) to Platelet Ratio Index (APRI) scores after treatment and reinfection rates.
Definitions
SVR was defined as HCV RNA lower than the limit of quantification (LLOQ) at 12 weeks after treatment completion or at any point thereafter.
Due to adherence problems in several cases, we did not have HCV RNA data at exactly the SVR12-week mark. In cases where subsequent measurements at any point thereafter were below the LLOQ, it was safely assumed that this would have been the case at 12 weeks. In cases where subsequent measurements were above the LLOQ, we would accept this as failure in SVR12 even though it would be impossible to determine whether that was due to actual treatment failure or an in-between reinfection.
After treatment, transaminases were measured on every 3–6 months. Participants with elevated liver enzyme results were offered another measurement in 15–30 days; patients with persistent elevations and who were still at risk for new infection were offered HCV-RNA measurement. HCV was considered eliminated/cured if a patient recorded SVR12 and, in case of subsequent measurements of HCV-RNA, undetectable viral loads.
People who did not appear within 3 days of their planned visit to receive their next DAA prescription were regarded as lacking in adherence to HCV treatment. Adherence with regards to daily timely medication receipt was not recorded.
Patients were considered to have advanced fibrosis if they had an FIB-4 score >3.25, an APRI score >1.5, or liver stiffness >11 kpa.
Primary education means having completed 6 years of school, lower secondary means completing 9 years, and upper secondary means completing 12 years.
Statistical analysis
Categorical variables are expressed as absolute and relative frequencies. Continuous variables are measured as means ± standard deviation (SD) (normally distributed) or median and interquartile range (IQR) (non-normally distributed). Normality was tested with the Shapiro–Wilks test and equality of variances by Levene's test. Group comparisons were performed using the Mann–Whitney U test for continuous non-normally distributed variables and Student's t-test for normally distributed variables. Group comparisons for categorical variables were performed using the χ2 test. Comparisons within the same subject before and after treatments were performed using the McNemar and Wilcoxon test. Risk factors are reported using odds ratios (ORs) and 95% CIs). p-Values <0.05 were regarded as statistically significant. Statistical analysis was performed with IBM SPSS Statistics for Windows, version 25.0 (2017, IBM Corp., Armonk, NY, USA).
RESULTS
Baseline characteristics
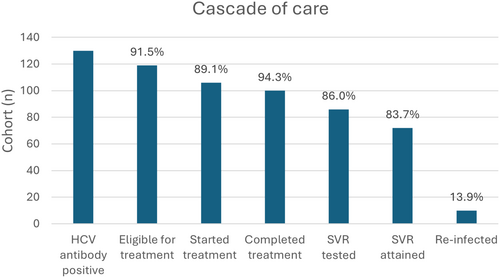
We identified 130/417 (31.2%) individuals with detectable HCV antibodies retained in HIV care in our cohort. With regards to means of HIV acquisition, all participants had a history of intravenous opioid use. The cascade of care of our cohort is presented in Figure 1. We entered 119/130 (91.5%) DAA applications into the national HCV registry. The 11 patients not entered into the national registry included two patients with undetectable HCV RNA, three who died before the process could be completed, three immigrants who did not have Greek national social security numbers, and three who were not able to complete testing for HCV genotype and viral load.
Response to treatment
Of those registered, 106/119 (89.1%) started treatment with DAAs. Table 1 presents information on people who initiated DAA treatment. With regards to barriers to treatment, people not starting treatment were more likely to be in active injecting drug use (OR 0.25; 95% CI 0.07–0.97, p = 0.045) and recreational benzodiazepine use (OR 0.25; 95% CI 0.07–0.95, p = 0.042). There was a trend for women to be less likely to initiate treatment (93/102 [91.2%] men vs. 13/17 [76.4%] women, p = 0.09). Participants not starting treatment had lower median serum glutamic-oxaloacetic transaminase (24 μ/L vs. 37 μ/L, p = 0.039) and serum glutamate pyruvate transaminase (24 μ/L vs. 42 μ/L, p = 0.009) levels. We did not identify other risk factors predicting failure to start treatment among age, sex, socioeconomic status, HIV, and HCV virological information.
| Characteristic | Total (N = 106) |
|---|---|
| Age | 40.1 ± 7.9 |
| Male sex | 93 (87.7) |
| Greek | 94 (88.7) |
| Education | |
| Primary | 15 (14.2) |
| Lower secondary | 58 (54.7) |
| Upper secondary | 33 (31.1) |
| Opioid substitution | 53 (50) |
| Inpatient drug rehabilitation programme | 7 (6.6) |
| Prison | 11 (10.4) |
| Steady job | 20 (18.9) |
| Alcohol misuse | 19 (17.9) |
| Active opioid use | 48 (45.3) |
| Recreational benzodiazepine use | 48 (45.3) |
| Non-HIV medications | 2 (0–3) |
| Drug interactions requiring change | 18 (17) |
| Years since HCV diagnosis | 6 (4–9) |
| Genotype | |
| 1a | 38 (35.8) |
| 1b | 3 (2.8) |
| 3 | 41 (38.7) |
| 4 | 22 (20.8) |
| 1 + 3 | 1 (0.9) |
| Log10 HCV RNA | 6.15 ± 0.96 |
| Any previous HCV treatment | 18 (17) |
| Previous treatment with interferon | 15 (14.2) |
| Previous treatment with DAA | 4 (3.8) |
| Liver elastography | 73 (68.9) |
| Liver stiffness (kpa) | 6.4 (5.4–7.8) |
| FIB-4 | 1.11 (0.88–1.65) |
| APRI | 0.44 (0.29–0.76) |
| Advanced fibrosis | 14 (13.2) |
| Years since HIV diagnosis | 4.8 (2.6, 5.6) |
| CD4 count at HCV treatment initiation (cells/μL) | 488 (275–721) |
| Log10 HIV RNA | 0.47 ± 1.36 |
| Required HIV treatment change | 27 (25.5) |
| DAA treatment | |
| SOF/LDV | 27 (25.5) |
| SOF/VEL | 47 (44.3) |
| ELB/GRZ | 19 (17.9) |
| 3D/RBV | 2 (1.9) |
| 2D/RBV | 2 (1.9) |
| SOF/VEL/RBV | 2 (1.9) |
| GP | 4 (3.8) |
| SOF/VEL/VOX | 3 (2.8) |
| Completed treatment | 100 (94.3) |
| Poor adherence | 17 (16) |
| Performed SVR test | 86 (81.1) |
| Achieved SVR | 72/86 (83.7) |
| FIB-4 after treatment | 0.98 (0.71–1.38) |
| APRI after treatment | 0.28 (0.2–0.49) |
| CD4 after treatment (cells/μL) | 577 (336–782) |
- Note: Data are presented as n/N (%), mean ± standard deviation, or median (interquartile range).
- Abbreviations: 2D, paritaprevir/ombitasvir/ritonavir; 3D, paritaprevir/ombitasvir/ritonavir + dasabuvir; APRI, aspartate transaminase-to-platelet ratio index; DAA, direct-acting antivirals; ELB, elbasvir; FIB-4, Fibrosis-4 Index for Liver Fibrosis; GP, glecaprevir/pibrentasvir; GRZ, grazoprevir; HCV, hepatitis C virus; LDV, ledipasvir; RBV, ribavirin; SOF, sofosbuvir; SVR, sustained virological response; VEL, velpatasvir; VOX, voxilaprevir.
In 27 106 (25.5%) participants, there were significant drug interactions requiring changes in ART. After changes in ART, we did not record any cases of immunological or HIV virological failure. After treatment completion, patients were maintained on their new regimens. Moreover, in 18/106 (17%), there were significant drug interactions with non-HIV medications, including quetiapine (11/18), rifampicin (2/18), novel anticoagulants (2/18), phenytoin (1/18), and proton pump inhibitors (2/18). In all cases, non-HIV medications were substituted with equivalent medicines without interactions for the duration of DAA treatment without the emergence of complications.
The majority of participants (100/106 [94.3%]) completed treatment. At the time of database lock, one patient was still receiving treatment. One person died during treatment due to opioid overdose. We did not identify risk factors with regards to barriers to treatment completion. SVR data were available for 86/100 (86%) participants who had completed initial treatment. Two people died after having completed treatment but before testing for SVR, and SVR12 date was pending at the time of database lock for one patient who had completed treatment. PWID in opioid substitution programmes tended to be more likely to return for SVR testing (54.7% vs. 30%, p = 0.081). We did not identify any other differences between the two groups.
Successful SVR12 was recorded in 72/86 (83.7%) of those who were tested after treatment. Table 2 presents a comparison between those achieving and not achieving SVR. With regards to investigation of barriers, active opioid use and poor adherence predicted treatment failure with statistical significance. Participants were sometimes tested for SVR at times later than the planned 12 weeks after treatment completion, but there was no significant difference (p = 0.115) between those attaining (median time 16.5 weeks [13–27.5]) and failing SVR (20 weeks [16.5–36]). People retained in HIV care and with a longer duration of HIV infection tended to fare better (OR 1.27, p = 0.055), however, this finding was not statistically significant. Individuals in active intravenous drug use (10/14 [71.4%] vs. 26/72 [36.1%], OR 0.226; 95% CI 0.064–0.793, logistic regression p = 0.02) or with poor adherence (4/14 [28.6%] vs. 5/72 [6.9%], OR 0.187; 95% CI 0.043–0.814, logistic regression p = 0.025) were less likely to achieve SVR.
| Characteristic | No SVR | SVR | p |
|---|---|---|---|
| n = 14 | n = 72 | ||
| Age | 39.6 ± 6.4 | 39.7 ± 6.1 | 0.979 |
| Male sex | 13 (92.9) | 63 (87.5) | 1 |
| Greek | 12 (85.7) | 65 (90.3) | 0.636 |
| Education | 0.126 | ||
| Primary | 4 (28.6) | 7 (9.7) | |
| Lower secondary | 5 (35.7) | 40 (55.6) | |
| Upper secondary | 5 (35.7) | 25 (34.7) | |
| Opioid substitution | 9 (64.3) | 38 (52.8) | 0.561 |
| Inpatient drug rehabilitation programme | 0 (0) | 6 (8.3) | 0.583 |
| Prison | 1 (7.1) | 6 (8.3) | 1 |
| Steady job | 2 (14.3) | 17 (24.3) | 0.508 |
| Alcohol misuse | 3 (21.4) | 14 (19.4) | 1 |
| Active opioid use | 10 (71.4) | 26 (36.1) | 0.019 |
| Recreational benzodiazepine use | 9 (64.3) | 29 (40.3) | 0.142 |
| Non-HIV medications | 1 (0–2) | 2 (1–3) | 0.101 |
| Drug interactions requiring change | 2 (14.3) | 14 (19.4) | 1 |
| Years since HCV diagnosis | 6 (2.5–10) | 6 (4–9) | 0.761 |
| Genotype | 0.819 | ||
| 1a | 6 (42.9) | 24 (33.8) | |
| 1b | 1 (7.1) | 2 (2.9) | |
| 3 | 4 (28.6) | 29 (40.8) | |
| 4 | 3 (21.4) | 15 (21.1) | |
| 1 + 3 | 0 (0) | 1 (1.4) | |
| Log10 HCV RNA | 6.35 ± 0.67 | 6.14 ± 1.02 | 0.455 |
| Any previous HCV treatment | 4 (28.6) | 11 (15.3) | 0.255 |
| Previous treatment with interferon | 3 (21.4) | 11 (15.3) | 0.692 |
| Previous treatment with DAA | 1 (7.1) | 1 (1.4) | 0.301 |
| Liver elastography | 11 (78.6) | 53 (73.6) | 1 |
| Liver stiffness (kpa) | 7 (5.3–8.1) | 6.4 (5.2–8.1) | 0.873 |
| FIB4 | 1.22 (0.88–1.88) | 1.09 (0.94–1.6) | 0.779 |
| APRI | 0.34 (0.25–0.79) | 0.44 (0.31–0.76) | 0.490 |
| Advanced fibrosis | 1 (7.1) | 11 (15.3) | 0.681 |
| Years since HIV diagnosis | 3.5 (0.8–4.8) | 5 (2.6–5.6) | 0.055 |
| CD4 count at HCV treatment initiation (cells/μL) | 412 (254–570) | 547 (297–741) | 0.279 |
| Log10 HIV RNA | 0.34 ± 1.28 | 0.42 ± 1.27 | 0.844 |
| Required HIV treatment change | 4 (28.6) | 19 (26.4) | 1 |
| DAA treatment | 0.252 | ||
| SOF/LDV | 6 (42.9) | 18 (25) | |
| SOF/VEL | 3 (21.4) | 32 (44.4) | |
| ELB/GRZ | 2 (14.3) | 14 (19.4) | |
| 3D/RBV | 1 (7.1) | 1 (1.4) | |
| 2D/RBV | 1 (7.1) | 1 (1.4) | |
| SOF/VEL/RBV | 0 (0) | 2 (2.8) | |
| GP | 0 (0) | 3 (4.2) | |
| SOF/VEL/VOX | 1 (7.1) | 1 (1.4) | |
| Poor adherence | 4 (28.6) | 5 (6.9) | 0.035 |
| FIB-4 after treatment | 0.93 (0.61–1.4) | 0.95 (0.72–1.4) | 0.574 |
| APRI after treatment | 0.3 (0.21–0.57) | 0.26 (0.19–0.43) | 0.393 |
| CD4 after treatment (cells/μL) | 605 (324–925) | 608 (347–792) | 0.924 |
- Note: Data are presented as n/N (%), mean ± standard deviation, or median (interquartile range). p-Values in bold denote statistical significance.
- Abbreviations: 2D, paritaprevir/ombitasvir/ritonavir; 3D, paritaprevir/ombitasvir/ritonavir + dasabuvir; APRI, aspartate transaminase to platelet ratio index; DAA, direct-acting antivirals; ELB, elbasvir; FIB-4, Fibrosis-4 Index for Liver Fibrosis; GP, glecaprevir/pibrentasvir; GRZ, grazoprevir; HCV, hepatitis C virus; LDV, ledipasvir; RBV, ribavirin; SOF, sofosbuvir; SVR12, sustained virological response at 12 weeks; VEL, velpatasvir; VOX, voxilaprevir.
Liver fibrosis tests
Among people attaining SVR, there was a significant improvement in FIB-4 (1.09 to 0.95, Wilcoxon p = 0.004) and APRI (0.44 to 0.26, Wilcoxon p < 0.001) scores, whereas these laboratory parameters did not differ before and after treatment in the non-SVR group (FIB-4 1.22 to 0.93, Wilcoxon p = 0.177; APRI 0.34 to 0.3, Wilcoxon p = 0.198). Both those achieving (547 to 608 c/μL, Wilcoxon p < 0.001) and not achieving (412 to 603 c/μL, Wilcoxon p = 0.035) SVR had a significant increase in CD4 counts before and after treatment.
Long-term follow-up
Overall, individuals have been in follow-up for a median period of 5.8 (4.9–6) years. One person died after achieving SVR, due to non-HIV or liver-related causes. Eight people who did not initially achieve SVR received second-line treatment. After second-line treatment, half attained SVR, one treatment failed, and three have not been tested.
Ten participants (13.9%) who achieved SVR have been subsequently re-infected. Six received treatments anew. Among them, two have achieved SVR again and four have not been tested.
At the end of our study period, 113/119 (95%) treatment-eligible patients remained alive. In those still alive and eligible for treatment, HCV was eliminated successfully in 68/113 (61.1%). Over the follow-up period, no patients have been diagnosed with hepatocellular carcinoma. In the treatment-eligible population, six people died during the study period. The death rate was calculated at 0.95/100 patient-years. Median age at death was 42.7 (37.6–51.8) years. Causes of death included a case of AIDS-related central nervous system toxoplasmosis, a case of community-acquired pneumonia, a case of suicide, a death due to undetermined causes, and two opioid overdose cases.
Safety
We recorded 13 adverse events in 12/106 (11.3%) patients during treatment. Seven patients reported mild gastrointestinal discomfort, nausea, and vomiting during the first few days of treatment; these symptoms resolved on their own. One patient reported sleep disturbances that resolved when they switched medication scheduling to receive treatment in the morning. One patient reported headaches and another fatigue, both of which resolved on their own. One patient reported an allergic reaction (oedema) that was evaluated as not attributed to DAA, and they continued treatment without recurrence. Finally, in one patient, we noted a mild transient increase in liver function tests with subsequent normalization without intervention. Among treated individuals, 46/106 (43.4%) had detectable hepatitis B core antibodies. We did not record any event of HBV reactivation during DAA treatment.
DISCUSSION
We have provided an extensive description of a population of PWID living with HIV and HCV. We present data on various socioeconomic barriers to treatment and HCV and HIV virological information. In our cohort, SVR12 was achieved by 83.7% of participants with a 13.9% reinfection rate and an overall cure of 61.1%. It is worth noting that our lower SVR12 rate can be potentially explained by the delay in SVR12 testing, with median times of 16.5 and 20 weeks for SVR12 successes and failures, respectively. This delay raises the possibility of reinfection during the interval between treatment cessation and testing, especially considering that 45.3% of our treated cohort was in active opioid use.
Micro-elimination of HCV has been considered a viable strategy to eradicate HCV infection. In this regard, specific subpopulations and/or geographical regions are targeted for HCV treatment. A micro-elimination strategy needs to have a plan to overcome barriers, achievable goals, to involve multiple stakeholders, and to report outcomes in order to be successful [35]. In a 2022 review, Lazarus et al reported that 71% of micro-elimination efforts have met SVRs exceeding 80% and 25% have exceeded 90% [36].
Data from Canada and Australia have shown that the initial rapid surge in HCV treatment initiation was followed by a plateau in treatment uptake. This trend has shown that a minority people having difficulty accessing services has not been accessed and successfully treated [37, 38]. Despite health insurance coverage of DAA treatment and decreased prices, further efforts are required to detect and eliminate remaining barriers to treatment and care, with the ultimate goal of achieving universal access.
In our cohort, most system-level barriers and all provider-level barriers had been solved. We should note that, for 12 immigrants who participated in our study, social services were able to provide them with social security registration numbers, but three people remained without social security so we could not apply for treatment. Patient-level barriers were investigated as potential risk factors for not starting treatment or not achieving SVR. Cachay et al studied a cohort from California and noted that co-infected people were significantly less likely to be referred for HCV treatment than mono-infected and, surprisingly, that people with a history of intravenous drug or alcohol abuse were more likely to be referred [39]. Although SVR rates in those tested consistently measured in the 90th percentile, when examined against those eligible for treatment, results are disappointing [25, 39, 40]. In a mono-infected cohort of PWID in San Francisco, 81% of treated patients attained SVR, but only 34% of the initial cohort experienced a cure of HCV. In this study, lack of insurance and access to care was associated with not starting treatment [41].
One of the primary obstacles to achieving an overall cure for HCV within our cohort was active drug and/or benzodiazepine use. Individuals in active use failed to start treatment, whereas (acting as a mitigating factor) those in opioid substitution programmes were more likely to return for SVR testing. These programmes play a crucial role in retaining individuals in active care. These results are reflected in the risk factors for failing SVR: people in active use were more likely to not achieve SVR; conversely, those retained in HIV care for a longer period, which implies adherence, were more likely to succeed. Through these years, a trusting relationship between patients and caregivers possibly assisted in maintaining adherence to treatment and ultimately attaining the SVR goal. Failure to attain the goal of HCV micro-elimination is a direct result of failure to combat the ongoing epidemic of opioid use.
We also compared pre- and post-treatment FIB4 and APRI scores and CD4 counts. Predictably, patients who did not experience SVR did not exhibit improvement in liver function tests. However, it is reassuring that immunological reconstitution seems to not have been impaired. Similar studies have found no associations between HCV cure and CD4 count improvements [42]. However, in immunological non-responders, it seems that DAA treatment was beneficial and led to a continuous CD4 count improvement at a rate of 4.1% per 6-month period [43].
Literature suggests that people living with HIV without HCV infection typically exhibit higher FIB-4 scores, with HIV itself serving as a risk factor for fibrosis [44]. In our cohort, we did not witness any liver-related morbidity or mortality during our follow-up period. Furthermore, there were no recorded cases of HBV reactivation, a rare complication of DAA treatment. In a systematic review from 2021, reactivation was found in 12 (0.8%) hepatitis B surface antibody (HBsAb)-negative and seven (0.6%) HBsAb-positive individuals [45].
Our study offers a concise overview of factors affecting HCV cure in a population of PWID living with HIV–HCV. Among our study's strengths is the inclusion of several socioeconomic factors and that the population included is a well-described vulnerable population that perhaps presents the greatest challenge to retain in care and treat. Our study is limited because it includes data from a single centre, albeit patients were referred to us through the ARISTOTLE programmes, which expanded our reach. Results derived from our analysis pertain to a mainly Greek population of PWID and might not be generalizable to other settings. We were unable to source information on liver stiffness for all participants, and even though we were tracking adherence through prescription pick-ups, we cannot definitively ascertain whether patients were adherent to medication at home.
CONCLUSIONS
PWID living with HIV and HCV present a significant challenge for healthcare systems in the attempt to eliminate HCV infection. Healthcare systems need to address potential barriers at all levels from system to provider to patient. Our findings highlight the importance of prioritizing substance use in people living with HIV to achieve HCV elimination goals. A systematic approach to this population, including efforts to overcome these barriers to receiving and completing treatment and encouraging patients to enrol in opioid substitution programmes if not possible to completely abstain from use, can help increase chances of HCV cure. Combating the opioid use epidemic will help us achieve micro-elimination targets and prevent further HCV spread.
AUTHOR CONTRIBUTIONS
Dimitris Basoulis: Data curation, Formal analysis, investigation, visualization, writing - original draft. Elpida Mastrogianni: Investigation, Witing - review and editing. Irene Eliadi: Investigation, writing - review and editing. Martha Papadopoulou: Data curation, writing - review and editing, Mina Psichogiou: Supervision, Conceptualization, Writing - review and editing.
ETHICS STATEMENT
The study was approved by the Laiko General Hospital Scientific and Ethics Board in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments and national laws.
INFORMED CONSENT
Informed consent for participation in this study was obtained from all patients.
Open Research
DATA AVAILABILITY STATEMENT
Data are available upon acceptance of this article on the Pergamos repository of the National and Kapodistrian University of Athens.




