Impact of hormonal therapy on HIV-1 immune markers in cis women and gender minorities
Karoline Aebi-Popp, Roger D. Kouyos and Irene A. Abela contributed equally to this work.
Publication history: A previous version of this manuscript was posted to medRxiv: https://doi.org/10.1101/2023.05.09.23289654.
Abstract
Background
Although sex hormones are recognized to induce immune variations, the effect of hormonal therapy use on immunity is only poorly understood. Here, we quantified how hormonal therapy use affects HIV-1 immune markers in cis women (CW) and trans women and non-binary people (TNBP) with HIV.
Methods
We considered CD4, CD8 and lymphocyte measurements from cis men (CM), CW and TNBP in the Swiss HIV Cohort Study. We modelled HIV-1 markers using linear mixed-effects models with an interaction between ‘gender’ (CW, TNBP) and ‘hormonal therapy use’ (yes/no). Models were adjusted on age, ethnicity, education level, time since start of antiretroviral therapy and use of intravenous drugs. We assessed the inflammatory effect of hormonal therapy use in 31 TNBP using serum proteomics measurements of 92 inflammation markers.
Results
We included 54 083 measurements from 3092 CW and 83 TNBP, and 147 230 measurements from 8611 CM. Hormonal therapy use increased CD4 count and CD4:CD8 ratio in TNBP more than in CW (pinteraction = 0.02 and 0.007, respectively). TNBP with hormonal therapy use had significantly higher CD4 counts [median = 772 cells/μL, interquartile range (IQR): 520–1006] than without (617 cells/μL, 426–892). This was similar to the effect of CW versus CM on CD4 T cells. Hormonal therapy use did not affect serum protein concentrations in TNBP.
Conclusion
This study highlights the potential role of hormonal therapy use in modulating the immune system among other biological and social factors, especially in TNBP with HIV.
INTRODUCTION
Sex and gender differences in the immune response have been observed in many settings, and in general women mount more robust immune responses to infection and vaccination compared with men [1-7]. These differences are multifactorial and could be influenced by the immunomodulatory effect of sex hormones [8-10], with oestrogens having a stimulating effect [11] and testosterone having a suppressive effect on immune function [12]. Sex hormones have also shown a multilevel effect on chronic infections such as HIV-1 [13], where women have displayed lower viral loads [14-17] and higher CD4 T-cell counts [18, 19] than men following seroconversion, and higher levels of immune activation [20, 21]. Women also tend to have a lower latent HIV-1 reservoir size [22-24] and oestrogens were found to inhibit HIV-1 transcription [25]. Although sex hormones could play a role in HIV-1 acquisition risk [26], recent evidence suggests that hormonal contraception does not affect the risk of HIV-1 acquisition [27-30] or disease progression [31, 32].
However, the effect of hormonal therapy on HIV-1 immune marker dynamics has been insufficiently explored. Additionally, most of the findings previously mentioned were obtained by studying cisgender women (CW) versus cisgender men (‘cis’ referring to people whose gender identify matches the one assigned at birth) or without specifying this information. Trans women and non-binary people (TNBP, referring here to people who were assigned male at birth but do not identify with being a man) are disproportionally affected by HIV-1 [33, 34] and constitute a distinct epidemiological group from CW and men having sex with men [34-36]. TNBP are still under-represented in HIV-1 research [37]; therefore, little is known about the immune effect of hormonal therapy use in TNBP and more generally about its effect on HIV-1 suppression and longer-term health outcomes [38-41]. Additionally, concerns on drug–drug interactions between antiretroviral treatment (ART) and sex hormones might limit hormone prescription in women and gender minorities with HIV-1 [42, 43].
The aim of this study was to analyse hormonal therapy use in CW and TNBP from the Swiss HIV Cohort Study (SHCS) and to delineate the effect of sex hormones on key HIV-1 markers in CW and TW with or without hormonal therapy use. Furthermore, we compared the effect of sex hormones on HIV-1 markers in CW and TNBP with the difference in HIV-1 markers in CW and cis men (CM). Finally, we aimed to characterize the potential immuno-modulatory effect of sex hormones in TNBP by measuring inflammatory protein levels before and after hormonal therapy use using a high-throughput proteomics assay.
METHODS
Swiss HIV cohort study
The Swiss HIV Cohort Study (SHCS) is a prospective multicentre cohort study enrolling people with HIV in Switzerland [44]. The SHCS was approved by the local ethical committees of the participating centres and written informed consent was obtained from all participants. Participants are followed up biannually with clinical and behavioural data collection. Blood samples are systematically drawn at each visit for laboratory measurements and stored in a biobank. We considered laboratory measurements from CW and TNBP after 1 January 2015 – when systemic report of most co-medications taken by SHCS participants started – and until 1 September 2022. Reports include ATC codes, drug brand, dose and route of administration for both antiretrovirals and co-medication. We excluded samples when participants were not on ART, less than 3 months after the first start of ART intake, during pregnancies and during hormonal therapy for bacterial vaginosis, treatment for endometriosis or breast cancer (as these conditions could themselves be associated with changes in immunity).
We similarly considered laboratory measurements after 1 January 2015, in samples from CM on ART for at least 3 months. In CM we excluded samples when participants were under antiandrogen therapy for prostate cancer.
Cis women and trans women and non-binary people in the SHCS
Trans women were previously identified in the cohort using written comments from study physicians and nurses and indirect data [35]. Given that gender identity is not directly available in the SHCS, we acknowledge that the method previously used could identify trans women but also other gender-diverse people such as non-binary people. We included 86 TNBP and 3165 CW with at least one laboratory measurement after 1 January 2015.
Hormonal therapy use definition
Hormonal therapy use was assessed using the following ATC codes: G02BA03 (hormonal intrauterine contraceptive with progestogens), G02BB (contraceptive vaginal ring with oestrogens and progestogens in combination), G03A (hormonal contraceptive for systemic use), G03C (oestrogens), G03D (progestogens), G03E (androgens and female sex hormone in combination), G03F (progestogens and oestrogens in combination) and G03H (antiandrogens). For each ATC code, reports of the start and end of the corresponding drug are indicated. For each participant, we combined different sex hormones when taken at the same time (Figure S3), then classified the report by the type (e.g. oestrogens, progestogens, oestrogens + antiandrogens) and route (e.g. systemic, local – including both injection and topical) of intake. For example, a participant's report with a simultaneous intake of an ATC code G03CA (transdermal, oestrogens) and another ATC code G03AC (systemic, progestogens) will be classified as systemic oestrogens + progestogens.
Measurements performed on blood samples drawn during the period of hormonal therapy use were labelled as ‘with hormonal therapy’, and others were labelled as ‘without hormonal therapy’.
Smoking, depression and adherence definition
The values used in the analysis for smoking, depression and self-reported adherence to ART are obtained from data up to 6 months before the corresponding laboratory measurement. Sensitivity analyses were performed by adding this information in addition to other confounding factors (detailed in the section ‘Statistical analysis’).
Proteomics measurements
We retrieved plasma samples from the SHCS biobank: we identified 31 TNBP for which plasma samples were available both before the first reported start of hormonal therapy use (between 1 month and 2 years) and after it (between 3 months and 2 years), with no reported interruption between the start of intake and the plasma collection. Proteomics measurements were realized on the 62 retrieved samples using the Olink® Target 96 technology Inflammation panel [45], which enables high-throughput, multiplex immunoassays by measuring up to 92 proteins across 96 plasma samples simultaneously (Olink Bioscience AB, Uppsala, Sweden). Olink® Target 96 is based on the Proximity Extension Assay (PEA), which permits simultaneous assessment of multiple proteins and reports fold change in log2 [normalized protein expression (NPX)] units. The inflammation panel informs on factors involved in cell differentiation and cytokine-mediated signalling pathways.
Statistical analysis
For the fixed effect, we included a ‘group’ variable (CW < 40 years old, CW 40–60 years old, CW > 60 years old, TNBP) and a variable ‘with hormonal therapy’ (yes/no). We considered CW in different age groups due to the correlation between the type of sex hormones used and the age of CW [i.e. CW < 40 mostly take hormonal contraception (HC), CW > 60 mostly menopause hormone therapy (MHT)]. We tested whether hormonal therapy use was differentially associated with the outcome by estimating an interaction between the variables ‘group’ and ‘hormonal therapy’. The reference category was CW 40–60, the group with the biggest sample size. We assessed the significance of the interaction term using a likelihood ratio test between the models with or without the interaction term. We report the effect of hormonal therapy use in the different groups on the scaled outcomes and p-value of the likelihood ratio test for the interaction term. We adjusted the models for the following confounders: age at time of sample (continuous with splines), ethnicity, education level, time since ART start (continuous with splines) and use of intravenous drugs. In sensitivity analyses, we also adjusted for depression, smoking status at time of sampling, self-reported adherence (categories: never missed, missed once a month, and missed more than once every 2 weeks) and viral load (categories: < 50, 50–1000 and >1000 copies/mL). Comparison between CW and CM included CW of all ages, and the model was adjusted for age at time of sample.
Given the left-censored nature of the proteomics data, the analysis was conducted in two steps: we first pre-selected proteins of interest, by excluding those with a proportion of censored values among the 62 samples of more than 75%, and a dispersion value (standard deviation divided by the mean value) less than 5%. We then tested for differences between pre- and post-hormonal therapy use samples using paired t-tests. Paired t-tests in proteins with left-censored data were realized using the function cen_paired from the NADA2 R package. These analyses were conducted on all samples, on the subset of samples in participants taking oestrogens only, and on the subset of samples in participants taking a combination of oestrogens and antiandrogens. Multiple testing was corrected using the false discovery rate method.
RESULTS
Study population
Participants from the SHCS attend biannual visits during which blood samples are drawn and the following laboratory measurements are realized: number of leucocytes, lymphocytes, CD3, CD4 and CD8 count (cells/μL) and HIV-1 viral load (copies/mL). We analysed a total of 54 083 laboratory measurements collected from 954 CW younger than 40 years old (CW < 40), 2365 CW between 40 and 60 years old (CW 40–60), 573 CW older than 60 years-old (CW > 60) and 83 TNBP (Figure 1). We also considered similarly selected 147 230 laboratory measurements from 8611 cis men (CM).
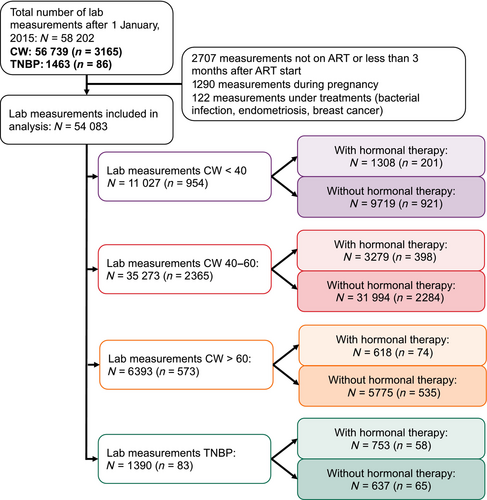
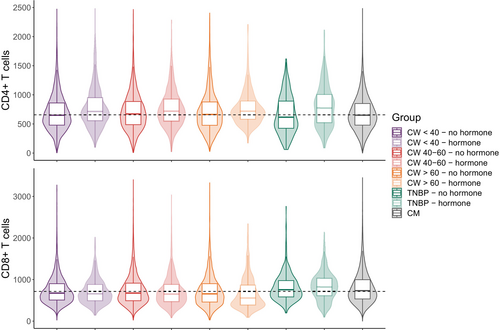
As previously described [35], TNBP in the SHCS were more likely to be of Asian and Hispano-American ethnicity compared with CW (Table 1). A higher proportion of TNBP had ever used hormonal therapy compared with CW (69.9% vs. 18.9%). We observed the highest CD4, CD8 and lymphocyte counts in TNBP with hormonal therapy use [median (IQR) values of 772 (520–1006), 822 (609–1033), and 2288 (1768–2866) cells/μL, respectively], while the CD4:CD8 ratio was the lowest in TNBP without hormonal therapy use (median = 0.84, IQR: 0.56–1.12) (Table 2; Figure 2, Figure S1). The CW > 60 group with hormonal therapy use had the lowest proportion (0.2%) of samples with detectable viral load (RNA > 50 copies/mL), and the TNBP group without hormonal therapy use had the highest (8.7%). Of note, only a small proportion of viral load measurements in TNBP without hormonal therapy use were > 1000 copies/mL (1.3%; Table S1).
| CW | TNBP | Overall | |
|---|---|---|---|
| Sample size | n = 3092 | n = 83 | n = 3175 |
| Age in 2022 | |||
| Median (min–max) | 53 (18–95) | 45 (21–75) | 53 (18–95) |
| Ethnicity [n (%)] | |||
| White | 1642 (53.1%) | 36 (43.4%) | 1678 (52.9%) |
| Black | 1103 (35.7%) | 5 (6.0%) | 1108 (34.9%) |
| Hispano-American | 104 (3.4%) | 16 (19.3%) | 120 (3.8%) |
| Asian | 227 (7.3%) | 25 (30.1%) | 252 (7.9%) |
| Other/unknown | 14 (0.5%) | 1 (1.2%) | 15 (0.5%) |
| Missing | 2 (0.1%) | 0 (0%) | 2 (0.1%) |
| Education [n (%)] | |||
| Less than bachelor | 2329 (75.3%) | 63 (75.9%) | 2392 (75.3%) |
| Bachelor and more | 635 (20.5%) | 18 (21.7%) | 653 (20.6%) |
| Other/unknown | 47 (1.5%) | 2 (2.4%) | 49 (1.5%) |
| Missing | 81 (2.6%) | 0 (0%) | 81 (2.6%) |
| IVD use [n (%)] | |||
| No | 2549 (82.4%) | 80 (96.4%) | 2629 (82.8%) |
| Yes | 518 (16.8%) | 3 (3.6%) | 521 (16.4%) |
| Missing | 25 (0.8%) | 0 (0%) | 25 (0.8%) |
| Has taken sex hormones at least once since 2015 [n (%)] | |||
| No | 2509 (81.1%) | 25 (30.1%) | 2534 (79.8%) |
| Yes | 583 (18.9%) | 58 (69.9%) | 641 (20.2%) |
| CW < 40 years old | CW 40–60 years old | CW > 60 years old | TNBP | CM | |||||
|---|---|---|---|---|---|---|---|---|---|
| Hormonal therapy use | No | Yes | No | Yes | No | Yes | No | Yes | |
| Total sample size (N) | 9719 | 1308 | 31 994 | 3279 | 5775 | 618 | 637 | 753 | 147 230 |
| Age at sample time (years) [median (Q1–Q3)] | 36 (32–38) | 35 (30–38) | 51 (46–55) | 51 (45–55) | 66 (63–71) | 67 (62–76) | 45 (38–52) | 42 (37–50) | 52 (44–59) |
| CD4 (cells/μL) (N) | 8107 | 1108 | 27 360 | 2805 | 4996 | 509 | 532 | 631 | 124 765 |
| Median (Q1–Q3) | 650 (480 862) | 715 (557 954) | 672 (488 887) | 720 (548 929) | 664 (477 878) | 720 (580 893) | 617 (426 892) | 772 (5 201 006) | 651 (481 851) |
| CD8 (cells/μL) (N) | 8053 | 1103 | 27 159 | 2793 | 4991 | 494 | 531 | 616 | 123 627 |
| Median (Q1–Q3) | 674 (507 900) | 656 (489 880) | 675 (491 913) | 651 (468 882) | 656 (460 900) | 557 (387 866) | 755 (583 975) | 822 (6 091 033) | 732 (534 997) |
| CD4:CD8 ratio (N) | 8053 | 1103 | 27 155 | 2793 | 4991 | 494 | 531 | 616 | 123 621 |
| Median (Q1–Q3) | 1.00 (0.70–1.35) | 1.16 (0.81–1.54) | 1.01 (0.71–1.39) | 1.14 (0.82–1.59) | 1.03 (0.67–1.50) | 1.25 (0.92–1.76) | 0.84 (0.56–1.12) | 0.94 (0.69–1.22) | 0.89 (0.62–1.24) |
| Lymphocytes (cells/μL) (N) | 7963 | 1096 | 26 635 | 2663 | 4802 | 487 | 525 | 618 | 121 505 |
| Median (Q1,Q3) | 1874 (1500–2332) | 1908 (1529–2398) | 1900 (1500–2400) | 1937 (1569–2400) | 1900 (1476–2395) | 1870 (1558–2364) | 2082 (1700–2581) | 2288 (1768–2866) | 1949 (1548–2421) |
| HIV-1 VL (copies/mL) (N) | 9575 | 1288 | 31 234 | 3212 | 5678 | 612 | 612 | 745 | 144 168 |
| Detectable VL (>50) [n (%)] | 698 (7.3%) | 45 (3.5%) | 1499 (4.8%) | 94 (2.9%) | 256 (4.5%) | 1 (0.2%) | 53 (8.7%) | 12 (1.6%) | 6063 (4.2%) |
- Abbreviations: CM, cis men; CW, cis women; TNPB, trans women and non-binary people; VL, viral load.
Diversity of hormonal therapy use in participants from the SHCS
Using information contained in the ATC codes, we further differentiated the type of hormonal therapy use and their route of administration (Table 3, Table S2; Figure S2). The CW < 40 group mostly used systemic progestogens (35.1%) or combined oestrogens and progestogens (36.9%), in line with the use of HC. The CW > 60 group mostly used local oestrogens (63.2%) or systemic combined oestrogens and progestogens (21.1%), corresponding to the use of MHT. In the group of CW 40–60, we observed more heterogeneity, with reports of HC use [systemic combined oestrogens and progestogens (38.4%) and systemic progestogens (21.1%)] and MHT [systemic and local oestrogens (17.6% and 14.5%, respectively)], reflecting the potential overlap of contraception with the need for managing symptoms during perimenopause. Finally, TNBP mostly reported the use of oestrogens (53.2%) or combined oestrogens and antiandrogens (32.9%). Our descriptive analysis highlighted the diversity of hormonal therapy use in SHCS participants and the complexity of the data when assessing and comparing different age groups.
| Hormone type | Route | CW < 40 | CW 40–60 | CW > 60 | TNBP |
|---|---|---|---|---|---|
| n = 390 | n = 620 | n = 76 | n = 158 | ||
| Oestrogens | Systemic | 13 (3.3%) | 109 (17.6%) | 8 (10.5%) | 84 (53.2%) |
| Local | 8 (2.1%) | 90 (14.5%) | 48 (63.2%) | 0 | |
| Systemic and local | 0 | 2 (0.3%) | 2 (2.6%) | 0 | |
| Progestogens | Systemic | 137 (35.1%) | 131 (21.1%) | 2 (2.6%) | 4 (2.5%) |
| Local | 55 (14.1%) | 28 (4.5%) | 0 | 0 | |
| Systemic and local | 3 (0.8%) | 0 | 0 | 0 | |
| Oestrogens + progestogens | Systemic | 144 (36.9%) | 238 (38.4%) | 16 (21.1%) | 2 (1.3%) |
| Local | 8 (2.1%) | 3 (0.5%) | 0 | 0 | |
| Systemic and local | 3 (0.8%) | 7 (1.1%) | 0 | 0 | |
| Antiandrogens | Systemic | 0 | 1 (0.2%) | 0 | 7 (4.4%) |
| Oestrogens + antiandrogens | Systemic | 17 (4.4%) | 9 (1.5%) | 0 | 52 (32.9%) |
| Oestrogens + androgens | Systemic | 0 | 2 (0.3%) | 0 | 5 (3.2%) |
| Oestrogens + antiandrogens + androgens | Systemic | 0 | 0 | 0 | 3 (1.9%) |
| Oestrogens + progestogens + antiandrogens | Systemic | 2 (0.5%) | 0 | 0 | 1 (0.6%) |
- Note: Sex hormone reports are defined as explained in Figure S2. Total number of sex hormone reports in each group are displayed (n). Discrepancies between total number of sex hormone reports with number of included participants (see Table 1) can be explained by two reasons: one person can have more than one sex hormone report (see Figure S2); and as we consider repeated measurements over time, one person can also contribute to different age categories.
- Abbreviations: CW, cis women; TNPB, trans women and non-binary people.
Hormonal therapy use is associated with distinct effects on HIV-1 markers in CW and TNBP with HIV
We assessed the effects of hormonal therapy use on HIV-1 markers by using linear mixed-effect models adjusted for time since ART start, ethnicity, intravenous drug use, education level and age (Figure 3, Figure S3). We found significantly different effects of hormonal therapy use on CD4 and CD4:CD8 ratio between the studied groups (CW < 40, CW 40–60, CW > 60, and TNBP) (interaction pCD4 = 0.02, pratio = 0.007). Interestingly, we found a significant increase in CD4 count, CD4:CD8 ratio and lymphocyte count associated with hormonal therapy use in TNBP [effect on the scaled outcomes were 0.19 95% confidence interval (CI): 0.10–0.28; 0.08, 95% CI: 0.02–0.15; and 0.15, 95% CI: 0.04–0.25, respectively].
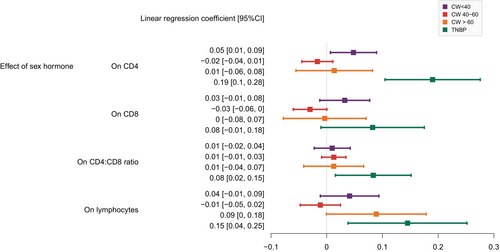
Chronic viral and bacterial infections and lifestyle factors such as smoking could have an impact on HIV-1 markers [46-48]. Sensitivity analyses adjusted on smoking status and co-infection with hepatitis C and/or syphilis showed consistent results (Figure S4). We further investigated whether changes in HIV-1 markers could be linked to viral control, adherence to ART and/or mental health problems. Hormonal therapy use was significantly correlated with undetectable or suppressed viral load in both CW and TNBP (χ2 test: p < 0.001 for CW < 40, CW > 60 and TW, and p = 0002 for CW 40–60; Table S1), with better self-reported adherence in CW < 40 (χ2 test: p < 0001) and CW 40–60 (χ2 test: p < 0.001), but with more depression in CW < 40 (χ2 test: p = 0.026). When adjusting for depression, self-reported adherence and viral load, the increase in CD4 count in TNBP upon hormonal therapy use remained significant [effect on the scaled outcome = 0.14 (95% CI: 0.05–0.23)] (Figure S5), suggesting that this increase cannot be explained solely by better adherence to treatment and control of the virus.
We further investigated whether the effects of sex hormones on HIV-1 markers in CW and TNBP were comparable to the differences in HIV-1 marker levels between CM and CW (Figure S6). Interestingly, the size of the effect of sex hormones on CD4 counts in TW was similar to the difference of CD4 counts in CW (without hormonal therapy use) versus CM [effect on the scaled outcome = 0.19 (95% CI: 0.14–0.24)]. However, the effect on CD8 count and lymphocytes was different, suggesting that differences in HIV-1 markers between CM and CW cannot be fully compared with the effect of exogenous hormonal therapy use in CW and TNBP.
Finally, we investigated the effect of different types and routes of hormones among the defined groups (Figure S7). Among TNBP taking sex hormones, we found trends for lower CD4, CD8 and lymphocyte counts in TNBP with combinations of oestrogens and antiandrogens than in TNBP taking oestrogens only (pCD4 = 0.075, pCD8 = 0.052 and plymphocytes = 0.090). Among CW < 40 taking sex hormones, we observed significantly lower CD8 and lymphocyte counts and higher CD4:CD8 ratio (pCD8 = 0.050, plymphocyte = 0.015 and pratio = 0.004, respectively) among those taking progestogens only than among those taking oestrogens and progestogens in combination.
No evidence for inflammatory pathways activation upon hormonal therapy use in TNBP
We further sought to identify whether hormonal therapy use had an anti-inflammatory effect in TNBP [49]. We assessed the effect of hormonal therapy use on immunity in 31 TNBP (19 taking oestrogens only and 12 taking a combination of oestrogens and antiandrogens) by undertaking a targeted proteomic analysis of individual soluble inflammation-associated proteins in two sequential samples: one taken up to 2 years before the start of hormonal therapy use (median 176 days, 1Q–3Q: 58–240) and one taken between 1 and 18 months after the start of hormonal therapy use (median 175 days, 1Q–3Q: 155–225). Using an unsupervised hierarchical clustering strategy, we found no distinct immune signature between the samples taken before and after hormonal therapy use (Figure S8). After adjustment for multiple testing, we found no significant differential protein concentration in TNBP before and after hormonal therapy use (Table S3). Only two proteins displayed significant changes when considering the p-value unadjusted for multiple testing, namely neurotrophin-3 (NT-3, p = 0.019) and STAMBP (p = 0.032), for which we observed a decreased concentration upon hormonal therapy use (Figure S9).
DISCUSSION
We report here on the first large cohort study analysing the impact of exogenous hormonal therapy use on the immune system in women with HIV. Our study explicitly included TNBP in the analysis to gain insights into the effects of hormonal therapy use on HIV-1 markers in a population often understudied in HIV-1 research.
Although rates of MHT intake in menopausal CW were previously reported in the SHCS [50], we were now able to extensively describe the diversity of sex hormones taken by SHCS participants. Notably, our analysis highlighted the complexity of the menopausal transition, during which the need for HC and MHT are likely to overlap.
Based on several studies partially attributing sex and gender differences in immunity to the role of sex hormones [1-5, 18], we investigated how exogenous hormonal therapy use affects major HIV-1 immune markers. Observed differences of hormonal therapy use on HIV-1 markers (CD4, CD4:CD8 ratio) in CW and TNBP could be due to differences in the combination of hormones taken: TNBP mostly used oestrogens and/or antiandrogens and CW mostly used oestrogens and/or progestogens, depending on their age. Additionally, within each group, we observed significant (in CW < 40), or trends (in TNBP) for, differential effects on HIV-1 markers by type of sex hormone. Differences in dosing, baseline physiological concentrations and mechanisms of actions of sex hormones on the immune system in CW and TW could also explain our results and should be further investigated.
Our most robust result was the significant association between hormonal therapy use and increased CD4 counts in TNBP. This increase was similar in magnitude to the difference in CD4 counts between CW (not taking sex hormones) and CM, but this similarity was not identified for the other markers (CD8, CD4:CD8 ratio, lymphocytes). In line with previous work [51-53], this highlights the complexity and variety of factors (chromosomal, hormonal and social) playing a role in immune modulation.
Additionally, we explored how factors such as adherence and mental health could play a role in our analyses. Hormonal therapy use in TNBP could be associated with lower adherence to ART, driven by concerns of drug–drug interactions [41, 42, 54]. However, access to gender-affirming hormone therapy in TNBP is also associated with overall better mental health [55-57] – a good predictor for adherence in people with HIV [58-61] – and retention in care [62]. We did not find any association in TW between hormonal therapy use and self-reported adherence to ART or depression. Even after adjusting for these variables, we still found a significant increase in CD4 count with hormonal therapy use in TNBP, suggesting that other social and biological factors are potentially responsible for this increase. Although previous results showed that mental health was not associated with adherence to ART or viral suppression during menopausal transition [63], more studies are needed in CW and TNBP with HIV to investigate the interplay among hormonal therapy use, mental health and immunity and to include additional confounding factors such as homelessness or access to care.
Using a high-throughput serum proteomics approach, we aimed to identify the inflammatory pathways potentially modulated with hormonal therapy use in TW. We could not identify any proteins with significant concentration change before and after hormonal therapy use in TW after adjusting for multiple testing. This negative result could be due to our low sample size or to the timing of the sampling before and after the start of hormonal therapy use. Indeed, a previous report found that inflammatory markers were only transiently modified in trans women receiving oral oestrogens, with a return to baseline levels between 2 and 6 months after the start of oestrogen intake [64]. As the studied samples were obtained with a median of around 6 months after the start of hormonal therapy use, we cannot exclude that this timeframe did not allow us to identify transient inflammatory changes happening earlier after the start of hormonal therapy use.
Our study has several limitations. First, as gender identity is not directly available for all participants in the SHCS, the identification of trans women and non-binary people relies on comments from study physicians and nurses and indirect data, as previously described [35]. Therefore, we cannot fully characterize the group of identified trans women and non-binary people, nor consider the potential dynamic aspect of gender. Given the potential heterogeneity of this group, it might also be interesting in the future to further divide it into sub-groups. However, we currently lack the statistical power to conduct these analyses. Additionally, although all our statistical analyses were adjusted for confounders and we conducted several sensitivity analyses, this study is observational by design and it is therefore not possible to draw conclusions on the causality between hormonal therapy use and increase in CD4 counts in TW. Our analysis did not include longitudinal modelling of immune trajectories, preventing us from drawing final conclusions on the time between the start of hormonal therapy use and potential modulations of HIV-1 markers. However, measurements at more regular and specific time points would be required to address this question. Additionally, the cohort design does not include any phenotyping of cells, which prevents us from drawing conclusions as to the cell subtypes affected by hormonal therapy use. Finally, given the large sample size of the cohort, only information on hormonal therapy use is available, but we do not have measures of sex hormone levels, which would be interesting to investigate in further analyses.
In conclusion, our results present new insights into the impact of hormonal therapy on HIV-1 immune markers in CW and gender minorities with HIV and highlights the need to consider the potential role of sex hormones in modulating the immune system among other biological and social factors.
AUTHOR CONTRIBUTIONS
CP, RDK: conception and design of the study, analysis and interpretation of data, drafting and revision of the article. IAA: conception and design of the study, acquisition of data, analysis and interpretation of data, drafting and revision of the article. KAP: acquisition of data, analysis and interpretation of data, drafting and revision of the article. All other co-authors: acquisition of data, drafting and revision of the article.
ACKNOWLEDGEMENTS
We thank the participants of the SHCS, the physicians and study nurses for excellent patient care, and the SHCS data centre for excellent data management. Open access funding provided by Universitat Zurich.
FUNDING INFORMATION
This work has been financed within the framework of the Swiss HIV Cohort Study, supported by the Swiss National Science Foundation (grant no. 201369), by SHCS project no. 882 and by the SHCS research foundation. The data were gathered by the five Swiss university hospitals, two cantonal hospitals, 15 affiliated hospitals and 36 private physicians (listed at http://www.shcs.ch/180-health-care-providers). IAA is supported by a research grant of the Promedica Foundation. CP is supported by a Fellowship from the Collegium Helveticum.
CONFLICT OF INTEREST STATEMENT
IAA has received honoraria from MSD and Sanofi, a travel grant from Gilead Sciences, and a grant from the Promedica foundation. RDK has received research fundings from Gilead unrelated to this work. AH's institution has received travel grants, congress and advisory fees from MSD, Viiv and Gilead, unrelated to this work. KAP's institution has received travel grants and advisory fees from MSD, Gilead and ViiV healthcare unrelated to this work. HFG has received grants from the SNF; SHCS; Yvonne Jacob Foundation; University of Zurich's Clinical Research Priority Program, viral disease; Zurich Primary HIV Infection; Systems.X; National Institutes of Health; Gilead Sciences; and Roche; and personal fees from Merck, Gilead Sciences, ViiV, GSK, Janssen, Johnson & Johnson and Novartis, for consultancy or DSMB membership and a travel grant from Gilead.
APPENDIX A: MEMBERS OF THE SWISS HIV COHORT STUDY
Abela I, Aebi-Popp K, Anagnostopoulos A, Battegay M, Bernasconi E, Braun DL, Bucher HC, Calmy A, Cavassini M, Ciuffi A, Dollenmaier G, Egger M, Elzi L, Fehr J, Fellay J, Furrer H, Fux CA, Günthard HF (President of the SHCS), Hachfeld A, Haerry D (deputy of ‘Positive Council’), Hasse B, Hirsch HH, Hoffmann M, Hösli I, Huber M, Jackson-Perry D (patient representatives), Kahlert CR (Chairman of the Mother & Child Substudy), Kaiser L, Keiser O, Klimkait T, Kouyos RD, Kovari H, Kusejko K (Head of Data Centre), Labhardt N, Leuzinger K, Martinez de Tejada B, Marzolini C, Metzner KJ, Müller N, Nemeth J, Nicca D, Notter J, Paioni P, Pantaleo G, Perreau M, Rauch A (Chairman of the Scientific Board), Salazar-Vizcaya L, Schmid P, Speck R, Stöckle M (Chairman of the Clinical and Laboratory Committee), Tarr P, Trkola A, Wandeler G, Weisser M, Yerly S.
Open Research
DATA AVAILABILITY STATEMENT
The individual-level datasets generated or analysed during the current study do not fulfil the requirements for open data access: (1) the SHCS informed consent states that sharing data outside the SHCS network is only permitted for specific studies on HIV infection and its complications, and to researchers who have signed an agreement detailing the use of the data and biological samples; and (2) the data are too dense and comprehensive to preserve patient privacy in persons living with HIV. According to Swiss law, data cannot be shared if data subjects have not agreed or data are too sensitive to share. Investigators with a request for selected data should send a proposal to the respective SHCS address (www.shcs.ch/contact). The provision of data will be considered by the Scientific Board of the SHCS and the study team and is subject to Swiss legal and ethical regulations, and is outlined in a material and data transfer agreement.




