Impact of tissue sampling on detection of venous invasion in colorectal cancer: a prospective analysis
Abstract
Aims
Venous invasion (VI) is a powerful yet under-reported prognostic factor in colorectal cancer (CRC). Efforts to improve its detection have largely focused upon histological assessment, with less attention paid to tissue-sampling strategies. This study aimed to prospectively determine the number of tumour blocks required to optimise VI detection in CRC resections. In addition, the relationship between linear spiculation (LS) and extramural venous invasion (EMVI) was investigated.
Methods and results
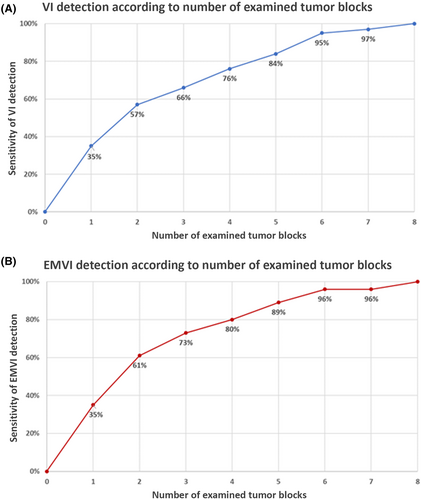
A standardised tissue sampling protocol was developed and applied prospectively to 217 CRC resections [AJCC 8th edition, stage 1 (n = 32); stage 2 (n = 84); stage 3 (n = 87); stage 4 (n = 14); and post-neoadjuvant therapy (n = 46)]. Elastin stains were performed on all tumour blocks. VI was identified in 55% of cases (EMVI = 37%; IMVI alone = 18%). The sensitivity of VI detection increased with increasing numbers of tumour blocks submitted [one block (35%), three blocks (66%), five blocks (84%), six blocks (95%) and seven blocks (97%)]. Similar findings were observed for EMVI [one block (35%), three blocks (73%), five blocks (89%), six blocks (96%) and seven blocks (96%)]. LS was identified macroscopically in 22% of specimens. In cases where no neoadjuvant therapy had been given, EMVI was significantly associated with LS (71% in LS+ cases versus 29% in LS– cases; P < 0.001). In addition, tumour blocks targeting LS were associated with a fivefold higher rate of EMVI compared with blocks that did not (P < 0.001).
Conclusions
Our findings demonstrate the impact of tissue sampling and quality of gross examination on VI detection and may inform practices in future CRC protocols.
Graphical Abstract
Protocols for the pathological examination of colorectal cancer specimens now recommend assessment of a number of microscopic findings that are associated with prognosis, including venous invasion. Here we prospectively identify an approach to gross dissection and sectioning of these cases to maximise sensitivity for detection of this important histological feature.
Introduction
Venous invasion (VI) is a powerful predictor of haematogenous spread and mortality in colorectal cancer (CRC).1-14 This is particularly true for extramural venous invasion (EMVI); prognostic significance of intramural venous invasion (IMVI) alone has been suggested, but is less well-established.2-4, 10, 12, 13, 15, 16 The accurate detection of VI is especially relevant in stage II CRC, where its presence may influence adjuvant treatment decisions. Despite its prognostic importance, the assessment of VI remains one of the most poorly performed aspects of CRC pathology. Population-based studies suggest that VI is under-reported in CRC,17, 18 with wide variation in VI detection rates among published studies.19 Such variation may be multifactorial and probably reflects differences in case mix, tissue sampling techniques, diagnostic thresholds, use of ancillary stains and the diligence and skill of the reporting pathologist.19 VI can be challenging to assess on a routine haematoxylin and eosin (H&E) stain and is therefore easily overlooked, especially when tumour distorts, effaces or obliterates involved vessels. Elastin stains (which highlight residual elastic fibres of the vein wall) have been shown to be remarkably effective in facilitating VI detection in this setting.20 In addition, there is growing evidence that elastin stains not only improve the detection of VI, but also enhance its ability to stratify risk in CRC.11, 21-23 Several international CRC pathology protocols/guidelines now recognise the utility of elastin stains in facilitating VI detection.24-28
To date, most efforts to improve VI detection have focused upon histological assessment, including the recognition of key morphological clues (e.g. ‘orphaned artery’ and ‘protruding tongue’ signs) and the role of elastin stains.11, 19, 20, 29 Less attention has been paid to tissue sampling strategies, with the result that there are currently no evidence-based guidelines specifying the number of tumour-containing blocks that should be submitted to optimise VI detection. While some retrospective studies have reported an association between VI detection rates and the number of tumour-containing blocks submitted,10, 30 prospective studies using standardised tissue sampling protocols are lacking. Thus, the number of tumour blocks that should be submitted to optimise VI detection remains to be determined. In addition to these quantitative considerations, there are also qualitative issues relating to tissue sampling for VI detection that remain to be resolved. For example, some have proposed the use of tangential sectioning along the radial border of the tumour to enhance VI detection by increasing the likelihood of capturing veins that pass at right angles across the bowel wall and into mesocolic tissues.30-32 It has been suggested that this allows for the transverse sectioning of multiple vessels, as opposed to longitudinal sections of individual vessels associated with the conventional perpendicular sampling approach.31 However, others point out that tangential sectioning may compromise the assessment of other key aspects of the pathology assessment, such as assessment of tumour depth, tumour deposits and tumour budding.33 It has also been suggested that a more targeted approach to tissue sampling may enhance VI detection. In particular, areas of linear spiculation (LS), defined as fine pale lines emanating from the base of the tumour generally perpendicular to the infiltrating edge, are considered a possible clue to the presence of EMVI. For this reason, the Royal College of Pathologists of the United Kingdom (RCPathUK) recommends sampling areas of LS at the advancing edge of the tumour,26 a practice also favoured by others.19, 34 Despite such guidance, the relationship between LS and EMVI remains to be studied prospectively.
In this prospective study we developed a standardised tissue sampling protocol to evaluate the impact of tissue sampling on VI detection. Specifically, we aimed to determine the number of tissue blocks required to optimise VI detection and to evaluate the relationship between LS and EMVI in order to inform future sampling practices.
Material and methods
Study Cohort
A search of our laboratory information system identified all consecutive patients who underwent radical surgical resection of CRC at Mount Sinai Hospital, Toronto, Canada, between 1 November 2016 and 30 June 2019 (n = 244). Cases were excluded if complete archival material was not available (n = 12), if there was no residual tumour in the specimen (either post-chemoradiation or post-polypectomy) (n = nine) or if the standardised grossing protocol had not been applied (n = six). Following these exclusions, a total of 217 stage I–IV CRC resections were included in this study. This study was approved by our institutional Research Ethics Board.
Standardised Tissue Sampling Protocol
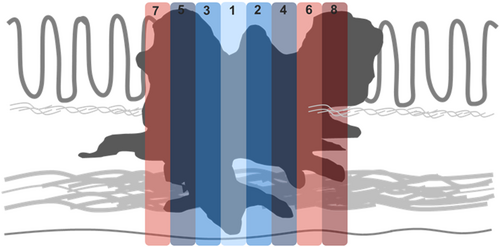
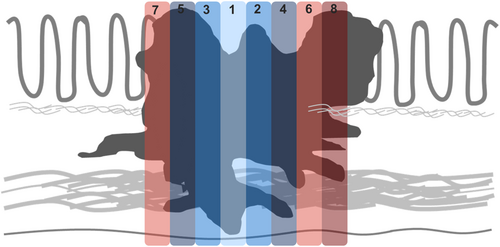
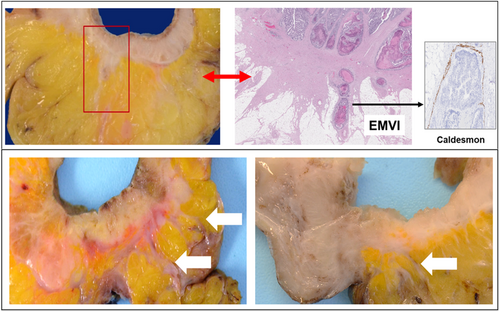
A standardised tissue sampling protocol was developed and applied prospectively to all CRC resection specimens (Figure 1). This included submission of a minimum of eight tumour-containing blocks for histological examination (tumour size permitting), with smaller tumours submitted in their entirety. Each tumour was perpendicularly sectioned and submitted in a consistent order starting from the point of deepest invasion and moving proximally and distally in a stepwise fashion, with the resulting tumour blocks designated 1–8. All specimens were inspected for evidence of LS at the advancing edge of the tumour (Figure 2). All foci of LS were incorporated in the submitted tumour blocks and the presence or absence of LS was documented in the gross description section of the pathology report. Elastin trichrome stains were performed on all tumour blocks (Figure 3).
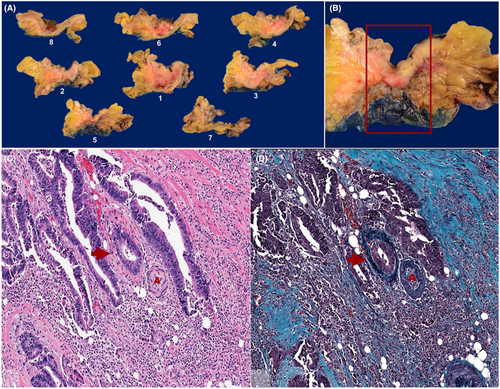
Clinicopathological Data
Pathology reports were reviewed to confirm that the standardised tissue sampling protocol was followed and to document patient demographics, the presence/absence of LS and histopathological data elements included the College of American Pathologists CRC checklist. In addition, all cases underwent a re-review for VI using both H&E and corresponding elastin trichrome stains (K.D.). The presence or absence of VI was documented for each individual tumour block. The location of VI (i.e. EMVI versus IMVI) was also documented. The number of tumour-containing blocks submitted and the number of elastin stains performed were recorded for each case.
Determining the Sensitivity of VI Detection
Based on the assumption that histological examination of eight tumour-containing blocks would identify 100% of VI-positive cases, we determined the sensitivity of VI detection for each incremental number of submitted blocks (1–8). To illustrate, for determining the sensitivity of VI detection associated with submission of four tumour blocks, the number of VI-positive cases identified by histological examination of only blocks 1–4 was divided by the VI detection rate obtained by histological examination all the tumour blocks.
Statistical Analysis
Continuous variables were reported as mean, median and range. Categorical variables were reported as absolute numbers and proportions. Comparison testing was performed using the χ2 test, t-test or analysis of variance (ANOVA) test, as appropriate. Statistical significance was defined as P-value < 0.05. Statistical analyses were performed using the SPSS statistical software program (SPSS for Windows release 19.0; SPSS Inc., Chicago, IL, USA).
Results
Cohort Characteristics
The cohort included 217 patients (116 male, 101 female) with a median age of 67 years (mean = 65 years). AJCC 8th edition stage was as follows: stage 1, n = 32; stage 2, n = 84; stage 3, n = 87; and stage 4, n = 14. Forty-six patients received neoadjuvant therapy while 171 patients did not. Forty-eight cases were right-sided, 85 left-sided and 84 rectal in location. The mean tumour size was 4.9 cm (median = 4.3 cm, range = 0.5–13.7 cm). An average of 7.3 tumour-containing blocks were submitted from each specimen and stained with elastin trichrome (this number falls below the eight blocks specified in the tissue sampling protocol, owing to a small minority of tumours that were entirely submitted in fewer than eight blocks). The cohort characteristics and relationship with VI and EMVI status are summarised in Table 1. Both VI and EMVI were significantly associated with T-stage, N-stage, AJCC stage, tumour deposits, perineural invasion and resection margin status. In addition, EMVI (but not VI) was significantly associated with tumour size ≥ 5.0 cm, tumour grade and number of tumour blocks stained with an elastin stain.
| Whole cohort (n = 217) | VI− (n = 98) | VI+ (n = 119) | P-value | EMVI− (n = 137) | EMVI+ (n = 80) | P-value | |
|---|---|---|---|---|---|---|---|
| Age, years, mean (median, range) | 65.1 (67, 22–98) | 64.3 (66, 24–90) | 65.9 (68. 22–98) | 0.428 | 65.5 (67, 24–96) | 64.6 (66, 22–98) | 0.689 |
| Sex, n (%) | |||||||
| Female | 101 (47) | 46 (47) | 55 (46) | 0.916 | 65 (47) | 36 (45) | 0.729 |
| Male | 116 (53) | 52 (53) | 64 (54) | 72 (53) | 44 (55) | ||
| Site, n (%) | |||||||
| Left colon | 85 (39) | 32 (33) | 53 (45) | 0.167 | 50 (37) | 35 (44) | 0.058 |
| Right colon | 48 (22) | 21 (21) | 27 (23) | 27 (20) | 21 (26) | ||
| Rectum | 84 (39) | 45 (46) | 39 (33) | 60 (44) | 24 (30) | ||
| Tumour size ≥ 5, n (%) | |||||||
| No | 133 (61) | 67 (68) | 66 (56) | 0.052 | 97 (71) | 36 (45) | < 0.001 |
| Yes | 84 (39) | 31 (32) | 53 (44) | 40 (29) | 44 (55) | ||
| T-stage, n (%) | |||||||
| T1 | 13 (6) | 8 (8) | 5 (4) | < 0.001 | 12 (9) | 1 (1) | < 0.001 |
| T2 | 32 (15) | 22 (23) | 10 (8) | 32 (24) | 0 (0) | ||
| T3 | 108 (50) | 50 (52) | 58 (49) | 69 (51) | 39 (49) | ||
| T4 | 63 (29) | 17 (18) | 46 (39) | 23 (17) | 40 (50) | ||
| N-stage, n (%) | |||||||
| N0 | 120 (55) | 70 (71) | 50 (42) | < 0.001 | 96 (70) | 24 (30) | < 0.001 |
| N1/N2 | 97 (45) | 28 (29) | 69 (58) | 41 (30) | 56 (70) | ||
| AJCC TNM stage, n (%)* | |||||||
| I | 32 (15) | 22 (22) | 10 (8) | < 0.001 | 32 (23) | 0 (0) | < 0.001 |
| II | 84 (39) | 47 (48) | 37 (31) | 62 (45) | 22 (27) | ||
| III | 87 (40) | 25 (26) | 62 (52) | 37 (27) | 50 (63) | ||
| IV | 14 (7) | 4 (4) | 10 (8) | 6 (4) | 8 (10) | ||
| Neoadjuvant therapy, n (%) | |||||||
| No | 171 (79) | 72 (73) | 99 (83) | 0.082 | 106 (77) | 65 (81) | 0.502 |
| Yes | 46 (21) | 26 (27) | 20 (17) | 31 (23) | 15 (19) | ||
| Histological type, n (%) | |||||||
| Adenocarcinoma, NOS | 197 (91) | 88 (90) | 109 (92) | 0.632 | 124 (91) | 73 (92) | 0.274 |
| Mucinous adenocarcinoma | 13 (6) | 9 (9) | 4 (3) | 12 (9) | 1 (1) | ||
| Signet ring cell adenocarcinoma | 4 (2) | 1 (1) | 3 (3) | 1 (1) | 3 (4) | ||
| Medullary carcinoma | 1 (1) | 0 (0) | 1 (1) | 0 (0) | 1 (1) | ||
| Neuroendocrine carcinoma | 1 (1) | 0 (0) | 1 (1) | 0 (0) | 1 (1) | ||
| Grade, n (%) | |||||||
| Low | 181 (84) | 86 (88) | 95 (81) | 0.152 | 122 (89) | 59 (75) | 0.006 |
| High | 35 (16) | 12 (12) | 23 (20) | 15 (11) | 20 (25) | ||
| Lymphovascular invasion, n (%) | |||||||
| Absent | 80 (37) | 80 (82) | 0 (0) | < 0.001 | 80 (58) | 0 (0) | < 0.001 |
| Present | 137 (63) | 18 (18) | 119(100) | 57 (42) | 80 (100) | ||
| Perineural invasion, n (%) | |||||||
| Absent | 132 (61) | 77 (79) | 55 (47) | < 0.001 | 103 (75) | 29 (37) | < 0.001 |
| Present | 84 (39) | 21 (21) | 63 (53) | 34 (25) | 50 (63) | ||
| Tumour deposits, n (%) | |||||||
| Absent | 165 (76) | 88 (90) | 77 (65) | < 0.001 | 122 (89) | 43 (54) | < 0.001 |
| Present | 52 (24) | 10 (10) | 42 (35) | 15 (11) | 37 (46) | ||
| Resection margin, n (%) | |||||||
| Uninvolved | 195 (90) | 95 (97) | 100 (85) | 0.002 | 133 (97) | 62 (79) | < 0.001 |
| Involved | 21 (10) | 3 (3) | 18 (15) | 4 (3) | 17 (21) | ||
| Mismatch repair protein status, n (%) | |||||||
| Proficient | 180 (83) | 80 (83) | 100 (84) | 0.655 | 110 (80) | 70 (88) | 0.140 |
| Deficient | 35 (16) | 17 (17) | 18 (15) | 26 (19) | 9 (11) | ||
| Unknown | 2 (1) | 1 (1) | 1 (1) | 1 (1) | 1 (1) | ||
| Tumour blocks with elastin | |||||||
| Mean (median, range) | 7.3 (8, 2–8) | 7.2 (8, 2–8) | 7.4 (8, 3–8) | 0.273 | 7.2 (8, 2–8) | 7.6 (8, 3–8) | 0.035 |
- * All tumours staged according to AJCC 8th edition.
VI Detection and Association with Submitted Tumour Blocks
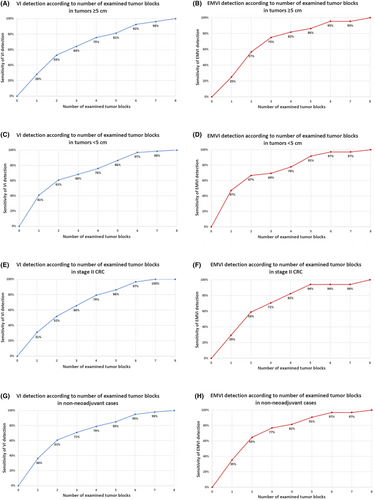
Overall, VI and EMVI were detected in 55 and 37% of cases, respectively. In stage 2 cancers (n = 84), the rates of VI and EMVI were 44 and 26%. There was a stepwise increase in the sensitivity of VI and EMVI detection with the increasing numbers of tumour blocks examined histologically (VI = 35% for one block, 66% for three blocks, 84% for five blocks, 95% for six blocks and 97% for seven blocks; EMVI = 35% for one block, 73% for three blocks, 89% for five blocks and 96% for six and seven blocks; Figure 4.A,B). Similar trends were observed when the analysis was restricted to tumours ≥ 5.0 cm in maximum dimension (n = 84) (Figure 5A,B), tumours < 5.0 cm in maximum dimension (n = 133) (Figure 5C,D), stage 2 CRC (n = 84) (Figure 5E,F) and patients not receiving neoadjuvant therapy (n = 171) (Figure 5G,H).
Relationship between LS and EMVI
LS was identified macroscopically in 47 CRC resections (22%). In tumours that were not treated with neoadjuvant chemoradiation (n = 171), EMVI was identified in 71% of tumours with LS compared to 29% of tumours without (P < 0.001). Moreover, tumour blocks targeting LS were five times more likely to show EMVI than those without LS (P < 0.001). However, among cases with EMVI detected histologically, only 39% showed LS, while only 31% of blocks targeting LS were found to have EMVI on corresponding slides. There was no significant association between LS and EMVI in the post-neoadjuvant group (P > 0.95), although this analysis was limited by small sample size (n = 46).
Discussion
This is the first study, to our knowledge, to evaluate the impact of tissue sampling on VI detection using a prospectively designed tissue-sampling protocol. We found that histological examination of six standard-sized tumour-containing blocks in CRC resection specimens identified approximately 95% of VI- and EMVI-positive cases, while histological examination of fewer than six blocks resulted in significant numbers of cases being missed. For example, when only five tumour blocks were examined histologically, 16% of VI-positive and 11% of EMVI-positive cases were missed, while when only four tumour blocks were examined histologically, 24% of VI-positive and 20% of EMVI-positive cases were missed. Based on these findings, we believe that a minimum of six tumour-containing blocks should be submitted for optimal histological assessment of VI and EMVI. Of note, the blocks used in our study were standard-sized (3.0 × 2.5 cm). The study design did not permit assessment of an optimal number for other sizes, including ‘macro’ or ‘mega’ blocks used in some centres.
VI (especially EMVI) is among the most powerful prognostic factors in CRC, rivalling lymph node status.6, 12-14, 35 Therefore, strategies to ensure its accurate assessment are critical. Such strategies have largely focused upon histological aspects of VI assessment, including recognition of morphological clues and the use of elastin stains. Tissue sampling strategies are less well studied. Adequate tissue sampling is critical, as even the most meticulous histological assessment will not identify a VI focus that is missed due to undersampling. Limited retrospective data point to an association between the number of tumour blocks examined histologically and the rate of VI detection.10, 30 However, prospective studies designed to determine the minimum number of tumour blocks required to optimise VI detection are lacking. In a large retrospective series of rectal cancers, Talbot et al. reported that 3.9% of EMVI-positive cases would be missed if five tumour blocks were submitted, 10.6% if four tumour blocks were submitted, 22.8% if three tumour blocks were submitted and 41.3% if only two were submitted.30 However, their data were calculated retrospectively by a combinatorial analysis of the number of blocks examined in each case broken down according to the number of slides harbouring EMVI in each case, rather than on direct experimental evidence. In a retrospective study of 381 CRC, Betge et al. found a positive association between the number of tumour blocks submitted and the rate of VI detection.10 The VI detection rate was 12% in tumours with three or fewer blocks submitted, 24% in tumours with four to six blocks submitted and 48% in tumours with seven or more blocks submitted. The authors concluded that submission of three to five blocks (which had been recommended in some earlier CRC protocols) was probably insufficient for the detection of VI. However, they cautioned that their cohort was unsuitable for a definitive recommendation, given the retrospective design of their study, and emphasised the need for future prospective studies to define the number of tissue blocks that should be examined for the optimum detection of VI.10
In the absence of prospective data there remain no evidence-based guidelines for tumour block submission to optimise VI detection. Current recommendations rely largely upon expert opinion. The data set for histological reporting of CRC reporting of RCPathUK recommends submission of at least five tumour blocks (or more for large tumours), size permitting, to allow for histological assessment of deepest penetration through the bowel wall, involvement of the peritoneal surface and VI and involvement of adjacent organs, as well as ancillary immunohistochemical and/or molecular tests.26 The CRC structured reporting protocol of the Royal College of Pathologists of Australasia notes that it is not possible to provide an absolute number for tumour blocks that should be routinely examined in CRC resections. Rather, it is recommended that sufficient blocks (generally at least four) should be taken to enable the pathologist to fully assess all the necessary parameters for staging and prognosis.28 Neither the College of American Pathologists CRC protocol nor the International Collaboration on Cancer Reporting CRC data set refer to minimum number of blocks that should be routinely examined in CRC resection specimens.24, 25
In addition to providing important prognostic information, the finding of VI (and particularly EMVI) may prompt oncologists to consider adjuvant chemotherapy in patients with stage 2 CRC. Therefore, missing VI (whether at the tissue sampling or histological assessment stage) may have significant clinical implications. We and others have shown that VI/EMVI-positive cases missed at the time of original pathology assessment but detected on subsequent review have similar adverse outcomes to VI/EMVI-positive cases that were originally detected.23, 36 In addition, Betge et al. found that VI assessed by expert review had a much stronger association with oncological outcomes than VI assessed in the routine setting due, at least in part, to missed VI-positive cases.10 While such misses occurred at the histological level, there is no reason to believe that VI missed due to insufficient sampling carries any less prognostic significance. As such, adequate tissue sampling merits the same attention as accurate histological assessment towards the goal of optimising VI detection.
Our study is the first, to our knowledge, to evaluate the relationship between the macroscopic finding of LS and the histological finding of EMVI. The RCPathUK CRC data set suggests that LS may be a macroscopic marker of EMVI and recommends targeted sampling of such areas,26 a view shared by others.19, 34 This recommendation appears to be largely based upon expert opinion and experience because, to our knowledge, the relationship between LS and EMVI has not been formally studied. We found a positive association between LS and EMVI in patients in the non-neoadjuvant-treated group, with LS-positive specimens showing significantly higher rates of EMVI than LS-negative counterparts (71 versus 29%, respectively). No such association was observed in the neoadjuvant-treated group, possibly due to treatment-associated changes that might obscure or mimic LS, although small sample size probably also contributed. While the rate of EMVI was higher in targeted (LS-positive) than in non-targeted (LS-negative) tumour blocks, most LS-positive blocks did not show EMVI histologically. In addition, the sensitivity of LS for EMVI was only 39%, indicating that its absence does not exclude EMVI. Taken together, our findings provide support for the sampling of LS. On the whole, sampling areas with LS provides a higher yield for EMVI detection than sections without LS, although this effect is modest and LS is neither sensitive nor specific for EMVI detection.
The main strength of our study is that the impact of specimen-handling has been demonstrated prospectively in a ‘real world’ pathology practice setting. Indeed, the prospective nature of our study allowed us to carefully design and apply a standardised protocol for both macroscopic and microscopic assessment of VI in a cohort of consecutive CRC resections. This permitted us to adjust for confounding variables, particularly those during specimen-handling and tissue sampling, that would not have been possible in a retrospective study. Data were also collected consecutively, limiting the potential for selection bias.
Elastin trichrome stains were used together with H&E to facilitate the assessment of VI in this study. This allowed us to determine with a high level of confidence whether VI was present or absent in each individual tissue block. Numerous studies have shown that VI is frequently missed when evaluated on H&E stains alone, with VI detection rates increasing by at least twofold when an elastin stain is used.11, 19-23, 29, 37-40 More importantly, several studies have shown that VI assessed with an elastin stain is associated with superior risk stratification in CRC compared to VI assessed on H&E alone.11, 21-23 This is probably due to the large number of VI-positive cases that are missed on H&E and consequently categorised as ‘VI-negative’, with the effect that overall outcomes appear worse in that group. Although our study does not provide data on the minimum number of tumour blocks required to ‘optimise’ VI detection based on H&E alone, we believe that the number would probably be higher than the minimum number of six blocks proposed in this study. This is because the sensitivity of VI detection by H&E is lower than with an elastin stain, so that more blocks would probably need to be examined to identify a VI-positive case. Irrespective of the stain used, our data suggest that submission of four to five tumour blocks is probably insufficient for the sensitive detection of VI and that, at a minimum, six tumour blocks should be submitted (tumour size permitting). The benefit of elastin stains to optimise VI is well documented and acknowledged in leading CRC protocols, although time restraints and resource limitations may affect individual decisions as to whether these stains are used routinely. Where cost considerations are an issue, the use of an elastin trichrome as an alternative to H&E might be feasible. In a recent study, elastin trichrome performed similarly to H&E in the assessment of College of American Pathologists mandated histological features, while outperforming H&E with respect to VI detection.41
In summary, we believe that is the first study to evaluate the impact of specimen-handling techniques on the detection of VI in a prospective manner. Our findings suggest that a minimum of six tumour containing blocks should be submitted to optimise VI detection and that targeted sampling of areas of LS may be of value. Our findings may inform sampling practices in future CRC protocols.
Conflicts of interest
None of the authors declare any conflicts of interest.
Open Research
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.